Abstract
The interphase distribution of lanthanide chlorides in ternary aqueous–organic systems containing DEHPA has been studied as a function of the compositions of the aqueous and organic phases. It is found that, in a 1 : 1 : 1 ternary system (aqueous LaCl3 solution + CH3COONa + CH3COOH–DEHPA in hexane–isopropanol), one can achieve the separation of lanthanides, which depends on the ratio between sodium acetate and acetic acid in the aqueous phase and the DEHPA concentration in the organic phase. The separation coefficients of lanthanides with respect to lanthanum in the extraction of rare-earth metal chlorides from acetic acid–acetate solutions are calculated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Rare-earth metals (REMs) are used in many fields of science and industry. Along with their conventional use in the ceramic and glass industry and as catalysts in oil refining, REMs find application in the production of magnets, TV phosphors, electronics, batteries, precision-guided munitions, and others. Lanthanides, the main group of REMs, have similar chemical and physical properties because of the similarity of their ionic radii. These metals are difficult to separate by conventional adsorption or ion-exchange processes; therefore, the recovery and separation of lanthanides have been investigated in numerous works using more effective methods, among which a special place is held by extraction and chromatographic techniques. Extraction is used to recover lanthanides, remove impurities, and isolate high-purity individual metals. Efficient extractants for the recovery and separation of REMs are various organophosphoric acids, among which di-(2-ethylhexyl)phosphoric acid is most known and well studied [1, 2].
For separating substances, new extraction–chromatographic methods were proposed, which combine the advantages of liquid–liquid extraction and liquid–liquid chromatography (solid support-free liquid chromatography) [3–12]. Unlike classical chromatography, in liquid–liquid chromatography, the stationary phase is actually also mobile, because it is not immobilized to a solid support, but is held in place in a chromatograph by centrifugal forces [3, 9–11] or by viscosity and surface tension forces [6, 8]. The efficient extraction–chromatographic separation of components of mixtures is reached by choosing a two-phase system of solvents. For this purpose, a heptane–ethyl acetate–methanol–water group is often used; using it, any system can be composed, including the most hydrophilic (0 : 1 : 0 : 1) and most hydrophobic (1 : 0 : 1 : 0) ones [9–12].
Previously, we investigated the interphase distribution of lanthanide salts in ternary aqueous–organic systems to the organic phase of which a binary extractant was added. We showed that the distribution of lanthanide salts in these systems differs from that in extraction systems [13]. In this work, we studied 1 : 1 : 1 ternary systems containing a hydrophilic component (water), a hydrophobic component (hexane), and a component exhibiting both hydrophilic and hydrophobic properties (isopropanol). To extract lanthanide chlorides into the organic phase (hexane), a cation-exchange extractant—di-(2-ethylhexyl)phosphoric acid (DEHPA)—was added.
It is known from literature sources that complexing agents may favor the more efficient separation of REMs in extraction systems. For example, the extraction of Pr and Nd from chloride–acetate media with 8-hydroxyquinoline solutions in heptane showed that the acetate ion concentration affects the distribution coefficients of these metals because of complexation processes in the aqueous phase [14]. The effect of complexing agents such as lactic and citric acids on the synergistic extraction and separation of lanthanides was detected [15, 16]. It was of interest to investigate the distribution of lanthanide chlorides in ternary systems of aqueous solutions in the presence of a complexing agent (sodium acetate).
EXPERIMENTAL
The initial solutions of lanthanum and ytterbium chlorides were prepared by the dissolution of samples of chemically pure salts LaCl3 ⋅ 7H2O and YbCl3 ⋅ 6H2O in distilled water. The initial solutions of chlorides of Nd, Sm, Gd, Dy, and Er were produced by the dissolution of chemically pure oxides of these metals in concentrated HCl with the subsequent multiple boiling down of solutions on a water bath to remove the excess of the acid.
The initial solutions of lanthanide chlorides were used for preparing aqueous solutions of metal chlorides of various compositions by adding buffer solutions, sodium acetate, and solutions of acetic and hydrochloric acids.
The extractant was a hexane solution of di-(2-ethylhexyl)phosphoric acid (Aldrich). The solvent was chemically pure hexane.
The characteristics of the distribution in ternary systems were investigated mainly by the example of lanthanum chloride and isopropanol as the third component.
The phases were stirred at 20°C in ground-glass-stoppered test tubes for 15 min, which was sufficient for reaching constant values of the lanthanide distribution coefficients. In 1 : 1 : 1 systems, the addition of a third component with both hydrophilic and hydrophobic properties leads to the fact that the aqueous phase may contain organics, and the organic phase may contain water; therefore, after the phase separation, the volumes of the upper (organic) and lower (aqueous) phases were determined.
The lanthanide concentrations in the initial solutions and the aqueous phases after extraction were determined by trilonometric titration with the indicator xylenol orange. The lanthanide concentrations in the organic phases were found from the difference between the concentrations in the initial solution and the aqueous phase after extraction with account for the change in the volumes of the aqueous and organic phases.
RESULTS AND DISCUSSION
The object of investigation was the interphase distribution of REM chlorides from solutions of an acetate buffer solution (pH 4) in 1 : 1 : 1 multicomponent aqueous–organic systems containing DEHPA with isopropanol as the third component. The extraction was performed from buffer solutions for maintaining constant values of the pH of the aqueous phase under the conditions of the cation-exchange process in the system with di-(2-ethylhexyl)phosphoric acid. In the experiments, equal volumes of DEHPA solutions in hexane of various concentrations and isopropanol were added to a solution of a metal chloride in the acetate buffer solution. With correction for the change in the volume ratio between the aqueous and organic phases in this system, the lanthanide distribution coefficients were calculated as
where Cin and C(aq) are the metal concentrations in the initial solution and the aqueous phase, respectively, after extraction; Vaq and Vor are the volumes of the aqueous and organic phases, respectively, after extraction.
The experimental results (Table 1) showed a high distribution coefficients of lanthanide chlorides from acetate buffer solution in the DEHPA-containing ternary systems. If the extractant is in a deficit (Cext(in) < CLn(in)), the lanthanide distribution coefficients differ slightly, probably because of the saturation of the organic phase (Cext(in)/CLn(or) = 1.13–1.60). If the extractant is in a double excess, the distribution coefficients are high for all lanthanides, which can be used, e.g., for concentrating these metals from aqueous solutions.
A comparison of the lanthanum chloride extraction from acetate buffer solution in the 1 : 1 : 1 systems and in the 1 : 1 systems containing DEHPA at equal ratios between the aqueous and organic phases (Table 2) demonstrated that the lanthanum extraction in the DEHPA-containing ternary systems is more efficient than that in multicomponent systems containing trioctylmethylammonium dialkyl phosphate [13]. In the extraction of lanthanum chloride from neutral aqueous solutions in the extraction system and the ternary systems, the distribution coefficients do not exceed 0.5.
The lanthanum chloride distribution in the 1 : 1 : 1 ternary system from aqueous solutions in the presence of a complexing agent (sodium acetate) was studied. Figure 1 and Table 3 illustrate the results of the LaCl3 extraction as a function of the sodium acetate concentration at various initial concentrations of lanthanum and DEHPA. Note that sodium acetate not only participates in the complexation of REMs [14], but it also influences the acidity of the aqueous phase because of involving in hydrolysis processes. The data (Fig. 1) show that an increase in the sodium acetate concentration increases the lanthanum recovery into the organic phase, which is particularly noticeable at lower metal concentrations in the solutions and higher extractant concentrations (curve 3). The increase in the lanthanum recovery into the organic phase in the presence of sodium acetate can be due both to an increase in pH and to the formation of lanthanum complexes with the acetate ion in the aqueous phase. The ratios between the extractant and lanthanum concentrations in the organic phase in the lanthanum chloride extraction in the 1 : 1 : 1 system containing DEHPA were calculated (Table 3). These data demonstrate that, with increasing sodium acetate concentration, the organic phase is saturated to a Cext : CLa(or) ratio of about 1. It is known from the literature data that, in DEHPA-containing extraction systems, the extraction of REMs from weakly acidic chloride solutions occurs by a cation-exchange mechanism to form extractable complexes LnA3 ⋅ 3HA [1, 2] and the extraction from solutions with elevated acidity occurs by a solvation mechanism to form complexes LnCl3 ⋅ qHA (YF–DEHPA) [17]. The data in Table 3 indicate changes in the compositions of the extracted lanthanide compounds in the studied DEHPA-containing 1 : 1 : 1 systems.
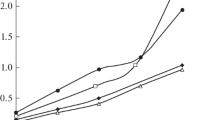
The results showed that lanthanum chloride from CH3COONa-containing solutions in the DEHPA-containing 1 : 1 : 1 ternary systems is also recovered at high distribution coefficients in the presence of an excess of the extractant (Table 3), much as from the acetic acid–acetate buffer solution (Tables 1, 2). Therefore, the LaCl3 extraction from sodium acetate solutions in the presence of acetic and hydrochloric acids was investigated to reach lower lanthanum distribution coefficients. Figure 2 presents data on the lanthanum chloride extraction in the 1 : 1 : 1 ternary system as a function of the composition of the isomolar (0.07 mol/L) mixtures of CH3COONa and CH3COOH (curve 1) and CH3COONa and HCl (curve 3). The experimental results (Fig. 2) showed that, with increasing mineral acid content, the lanthanum distribution coefficients decrease, and this decrease is more significant in the HCl-containing system because of an increase in the acidity of the aqueous phase. The lanthanum chloride distribution from an acetic acid–acetate solution (0.02 M CH3COONa + 0.05 M CH3COOH) and a chloride–acetate solution (0.04 M CH3COONa + 0.03 M HCl) was investigated in comparison with the LaCl3 recovery from aqueous salt-free solutions as a function of the DEHPA concentration (Fig. 3). An increase in the DEHPA concentration increases the lanthanum chloride extraction on all the systems, but this effect is most pronounced in the recovery from the acetic acid–acetate media.
Effect of the DEHPA concentration on the lanthanum chloride extraction in the 1 : 1 : 1 ternary system (1) aqueous LaCl3 solution–DEHPA in hexane–isopropanol, (2) aqueous LaCl3 solution + 0.02 M CH3COONa + 0.05 M CH3COOH–DEHPA in hexane–isopropanol, and (3) aqueous LaCl3 solution + 0.02 M CH3COONa + 0.03 M HCl–DEHPA in hexane–isopropanol at CLa(in) = 0.019 mol/L and Vaq : Vor = 5.5 : 3.5.
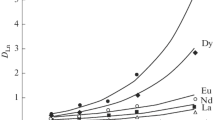
The extraction of lanthanide chlorides from acetic acid–acetate solutions containing 0.018 M CH3COONa and 0.05 M CH3COOH was studied at the DEHPA concentrations, ensuring relatively low and medium lanthanum distribution coefficients (Fig. 3). The experimental data (Table 1) suggest that, in the extraction from acetic acid–acetate solutions in the case of a deficit of the extractant, the lanthanide distribution coefficients differ insignificantly, but if DEHPA is in an excess, the separation coefficients βLn/La of lanthanide chlorides with respect to LnCl3 increase with increasing lanthanide atomic number and are within the range 1.22–2.62. Thus, in the 1 : 1 : 1 ternary system (aqueous LaCl3 solution + CH3COONa + CH3COOH–DEHPA in hexane–isopropanol), separation of lanthanides is reached, which depends on the ratio between sodium acetate and acetic acid in the aqueous phase and the DEHPA concentration in the organic phase. Thus, by varying the compositions of the aqueous and organic phases in a DEHPA-containing multicomponent system, the separation coefficients of lanthanides can be affected.
CONCLUSIONS
The interphase distribution of lanthanide chlorides was studied in DEHPA-containing ternary aqueous–organic systems with isopropanol as the third component. It was shown that the LaCl3 extraction from acetate buffer solution in multicomponent aqueous–organic systems is more efficient than that in DEHPA-containing extraction systems. The lanthanum chloride extraction from acetic acid–acetate and chloride–acetate solutions was investigated as a function of the compositions of the aqueous and organic phases. It was found that, in the 1 : 1 : 1 ternary system (aqueous LaCl3 solution + CH3COONa + CH3COOH–DEHPA in hexane–isopropanol), the separation of lanthanides can be reached at certain ratios between sodium acetate and acetic acid in the aqueous phase and a certain DEHPA concentration in the organic phase. The separation coefficients of lanthanides with respect to lanthanum in the extraction of rare-earth metal chlorides from acetic acid–acetate solutions were calculated.
REFERENCES
Michelsen, O.B. and Smutz, M., Separation of yttrium, holmium, and erbium with di-(2-ethylhexyl) phosphoric acid in chloride and nitrate systems, J. Inorg. Nucl. Chem., 1971, vol. 33, no. 1, pp. 265–278. https://doi.org/10.1016/0022-1902(71)80028-3
Minagawa, Y. and Yamaguchi, K., Relative extraction rates of rare earth ions from weakly acidic solution of binary mixture of rare earth chlorides by di-(2-ethyl-hexyl) phosphoric acid/kerosine, Hydrometallurgy, 1990, vol. 24, no. 3, pp. 333–350. https://doi.org/10.1016/0304-386X(90)90097-L
Kostanyan, A.E., On the description of liquid–liquid chromatography processes with a free stationary phase, Khim. Tekhnol., 2004, vol. 5, no. 8, pp. 39–42.
Kostanyan, A.E., Erastov, A.A., and Shishilov, O.N., Multiple dual mode counter-current chromatography with variable duration of alternating phase elution steps, J. Chromatogr. A, 2014, vol. 1347, pp. 87–95. https://doi.org/10.1016/j.chroma.2014.04.064
Kostanyan, A.E., Modeling of closed-loop recycling liquid–liquid chromatography: Analytical solutions and model analysis, J. Chromatogr. A, 2015, vol. 1406, pp. 156–164. https://doi.org/10.1016/j.chroma.2015.06.010
Kostanyan, A.E. and Erastov, A.A., Steady state preparative multiple dual mode counter-current chromatography: Productivity and selectivity. Theory and experimental verification, J. Chromatogr. A, 2015, vol. 1406, pp. 118–128. https://doi.org/10.1016/j.chroma.2015.05.074
Chollet, S., Marchal, L., Meucci, J., Renault, J.-H., Legrand, J., and Foucault, A., Methodology for optimally sized centrifugal partition chromatography columns, J. Chromatogr. A, 2015, vol. 1388, pp. 174–183. https://doi.org/10.1016/j.chroma.2015.02.043
Kostanyan, A.E., Erastov, A.A., and Shishilov, O.N., Separation of liquid mixtures by dynamic countercurrent cyclic extraction, Theor. Found. Chem. Eng., 2015, vol. 49, no. 4, pp. 560–566. https://doi.org/10.1134/S0040579515040119
Friesen, J.B., Ahmed, S., and Pauli, G.F., Qualitative and quantitative evaluation of solvent systems for countercurrent separation, J. Chromatogr. A, 2015, vol. 1377, pp. 55–63. https://doi.org/10.1016/j.chroma.2014.11.085
Ito, Y., Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography, J. Chromatogr. A, 2005, vol. 1065, pp. 145–168. https://doi.org/10.1016/j.chroma.2004.12.044
Hewitson, P., Sutherland, I., Kostanyan, A.E., Voshkin, A.A., and Ignatova, S., Intermittent counter-current extraction—Equilibrium cell model, scaling and an improved bobbin design, J. Chromatogr. A, 2013, vol. 1303, pp. 18–27. https://doi.org/10.1016/j.chroma.2013.06.023
Ren, D.B., Yi, L.Z., Qin, Y.H., Yun, Y.H., Deng, B.C., Lu, H.M., Chen, X.Q., and Liang, Y.Z., Systematic and practical solvent system selection strategy based on the nonrandom two-liquid segment activity coefficient model for real-life counter-current chromatography separation, J. Chromatogr. A, 2015, vol. 1393, pp. 47–56. https://doi.org/10.1016/j.chroma.2015.03.016
Belova, V.V. and Martynova, M.M., Interphase distribution of lanthanide salts in multicomponent aqueous-organic two-phase systems, Khim. Tekhnol., 2017, vol. 18, no. 12, pp. 557–561.
Wu, D., Zhang, Q., and Bao, B., Solvent extraction of Pr and Nd(III) from chloride-acetate medium by 8-hydroquinoline with and without 2-ethylhexyl phosphoric acid mono-2-ethylhexyl ester as an added synergist in heptane diluent, Hydrometallurgy, 2007, vol. 88, pp. 210–215. https://doi.org/10.1016/j.hydromet.2007.05.009
Zhang, F., Wu, W., Bian, X., and Zeng, W., Synergistic extraction and separation of lanthanum(III) and cerium(III) using a mixture of 2-ethylhexylphosphonic mono-2-ethylhexyl ester and di-2-ethylhexyl phosphoric acid in the presence of two complexing agents containing lactic acid and citric acid, Hydrometallurgy, 2014, vol. 149, pp. 238–243. https://doi.org/10.1016/j.hydromet.2014.09.002
Yin, S., Wu, W., Bian, X., and Zhang, F., Effect of complexing agent lactic acid on the extraction and separation of Pr(III)/Ce(III) with di-(2-ethylhexyl) phosphoric acid, Hydrometallurgy, 2013, vols. 131–132, pp. 133–137. https://doi.org/10.1016/j.hydromet.2012.11.005
Hirashima, Y., Yamamoto, Y., Takagi, S., Amano, T., and Shiokawa, J., Extraction of lanthanides from hydrochloric and nitric acid solutions by di(2-ethylhexyl) phosphoric acid, Bull. Chem. Soc. Jpn., 1978, vol. 51, no. 4, pp. 2890–2893.
Funding
This work was supported in part by the Russian Foundation for Basic Research (project no. 17-03-00263) and performed within the framework of a state assignment for basic scientific research for the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Glyanchenko
Rights and permissions
About this article
Cite this article
Belova, V.V., Martynova, M.M. Interphase Distribution of Lanthanide Chlorides in Multicomponent Aqueous–Organic Two-Phase Systems Containing DEHPA. Theor Found Chem Eng 54, 775–780 (2020). https://doi.org/10.1134/S0040579519050038
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579519050038