Abstract
The results of studying the hydrochloric acid decomposition of the niobium–rare-earth slag produced by reducing roasting of the high-iron rare-earth ore from the Chuktukon deposit are discussed. The slag is presented by four main phases: a glassy phase, a phase with a perovskite structure, a phase with a loparite structure, and a MnAl2O4-based spinel phase. Niobium and rare-earth metals in the slag are distributed between the first three phases. The hydrochloric acid leaching of the slag is carried out in two stages: leaching under atmospheric pressure and the solid residue is then leached in an autoclave at high temperatures. The slag starts to decompose at very low acid concentrations (pH 4–2.5), but the maximum development of slag (70%) is reached for leaching with 20% HCl at ~100°C. Under these conditions, only the glassy phase of the slag decomposes. The phases with loparite and perovskite structures decompose at high temperatures in an autoclave. The completeness of their decomposition is achieved at 20% HCl and 200°C for 2 h. According to X-ray diffraction analysis data, the spinel phase weakly decomposes by pressure leaching and remains in the solid phase. The yield of the solid phase is ~12% of the slag weight. Niobium, titanium, and zirconium are nearly completely concentrated in the form of oxides in the solid phase along with the spinel phase. The spinel phase can be removed from the solid residue using magnetic separation. The collective niobium–titanium–zirconium concentrate isolated to a nonmagnetic fraction can further be processed using the chloric method to produce the corresponding metals. The hydrochloric solution formed upon pressure leaching is proposed to be directed to the first leaching stage of the slag at ~100°C. This procedure makes it possible to decrease the hydrochloric acid consumption during leaching to the maximum extent and to substantially facilitate the further recovery of rare-earth metals and manganese by precipitation from weakly acidic solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Rare-earth metals (REMs) are applied in many high-tech areas of industry, in particular, in the production of diverse types of electronics, magnets, high-temperature semiconductors, optical devices, and modern samples of military technology [1, 2].
The Russian Federation possesses 10% world resources of REMs (about 12 mln t) [1]. However, according to the data of the United States Geological Survey, only 250 t REM (1.3% of the world volume) were produced in 2019 in Russia, while the demand is 1100 t. The REM deficiency is compensated due to the import from China. The development of the rare-earth industry reaching a parameter of 10% world volume in 2030 is planning in Russia, which would allow Russia to take the second place after China in the volume of REM production [1].
The following three large Russian deposits are considered for the development of the mineral sources of rare and rare-earth metals as the most priority trend: Tomtor (Sakha, Yakutia), Chuktukon (Krasnoyarsk krai), and Zashikhinsk (rare metal deposit in the Irkutsk oblast) [1, 3]. The Tomtor deposit is situated in the poorly available district, and its development is associated with transport problems [3]. The development of the Zashikhinsk deposit would not solve the problem of providing the industry with REMs because of the low contents of these metals in the ore, whereas the consumption of REM should be 6000 t annually according to the state program “Development of Industry and Enhancement of Its Competitive Ability for the Period until 2020” [4].
Therefore, the Chuktukton deposit of niobium–rare-earth metal ores turns out to be the most promising object due to both the resource volume and qualitative characteristics of the ore for the modernization and development of the rare-metal industry in Russia. The resources of rare-earth ores asserted by the State Commission on Mineral Resources of the Russian Federation (2007) are 6 639 000 t (category S2), the resources of niobium pentoxide are 39 800 t (content 0.6%), and those of REM oxides are 486 000 t (content 7.3%) [3]. The ore is characterized by high dispersion and contains up to 50% Fe, 12% Mn, and more than 7% REM oxides. The deposit is situated near the Boguchansk hydroelectric power station, which solves the problem of power supply. It is assumed to mine the ore using open-cut mining.
The combined pyrohydrometallurgical scheme seems to be promising for the efficient processing of the rare-earth ores from the Chuktukon deposit. At the first stage (reducing melting), the scheme makes it possible to isolate iron as cast iron [5–8], and REM can be concentrated in a slag for further recovery by hydrometallurgical methods. The preliminary removal of iron substantially facilitates the conditions of hydrometallurgical slag processing with a high degree of recovery of niobium and REM.
The results of studying the reducing roasting of the high-iron niobium–rare-earth ore from the Chuktukon deposit with the production of high-phosphorus cast iron and niobium–rare-earth slag were presented in [8]. The yield of the slag is 23–26% of the ore weight, which makes it possible to increase the content of REM and niobium in the oxide phase by four times on the average. The slag is presented by four main phases: a glassy phase, a phase with the perovskite structure, a phase with the loparite structure, and spinel. The glassy phase consists of barium, calcium, and manganese aluminosilicates and contains a significant amount of rare and rare-earth elements. The Nb2O5 content reaches 4%, and the content of lanthanum, cerium, and neodymium oxides exceeds 3%. The phase with the perovskite structure is a solid solution of titanium perovskite (CaTiO3) with niobium and REM oxides of the general formula (ABO3). The Nb2O5 content ranges from 11.3 to 16.5%, and the content of REM oxides reaches 30%, about 5.5% of which fall on the fraction of Nd2O3 9% fall on La2O3, and 15% fall on Ce2O3. The phase with the loparite structure has a very high MnO content (49–51%) and contains up to 10% Nb2O5. The contents of titanium and REM are insignificant. The spinel phase of the slag represents a solid solution based on MnAl2O4. The content of niobium and REM in spinel is insignificant and totally does not exceed 0.3–0.4%.
In this work, we discuss the behavior of the slag phases during slag leaching with hydrochloric acid solutions, the knowledge of which is important for the development of a technology for complex hydrometallurgical processing of a niobium–rare-earth slag with high technical economical parameters.
EXPERIMENTAL
For studies of hydrochloric acid leaching, a pilot batch of the slag was produced under the earlier determined optimum conditions for reducing roasting of the rare-earth ore from the Chuktukon deposit [8]. The ore had the following chemical composition (%): 0.17 Na2O, 0.17 MgO, 3.06 Al2O3, 1.71 SiO2, 4.14 P2O5, 2.42 CaO, 1.20 TiO2, 0.39 V2O5, 7.1 MnO, 64.8 Fe2O3, 1.04 ZnO, 1.49 BaO, 0.055 ZrO2, 1.54 Nb2O5, 0.54 SrO, 0.12 Y2O3, 0.79 La2O3, 1.51 CeO2, 0.13 PrO3, 0.45 Nd2O3, and 0.039 Gd2O3 (loss on ignition of about 10%). Prior to roasting, the ore with divided coke additives (~0.1 mm) was briquetted. The coke consumption was 13% of the ore weight. Reducing roasting was carried out with coke on a coal bed in the temperature range from 1300 to 1400°C. The heating time in the 1000–1400°C range was 10 min followed by holding for 5 min. After cooling and crushing, the reducing roasting products were divided into two fractions, namely, a metal and a slag.
The slag was milled to a size of ~0.05 mm, and fine metallic iron particles were removed by magnetic separation. The chemical composition of the slag by the main components is shown in Table 1. The total content of rare and rare-earth metal oxides in the slag exceeded 20%. The slag contained 21% manganese oxide. The content of residual iron was Fetot = 3.3%. The main impurities were the following (%): 10.3 CaO, 7.8 SiO2, 7.4 Al2O3, and 2.2 P2O5.
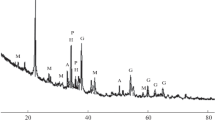
The X-ray diffraction (XRD) pattern of the slag (CuKα radiation) is shown in Fig. 1. The microstructure of the slag is shown in Fig. 2. The slag is finely crystalline, and its main phases are the glassy matrix, light skeletal crystals with the perovskite structure, large gray grains of cubic habitus (spinel phase), and fine crystals as asterisks with the loparite structure arranged in the glassy matrix. As found by electron probe microanalysis, niobium and rare-earth elements are distributed between all phases except for spinel.
The hydrochloric acid leaching of the slag was carried out in two stages: at first, leaching was conducted under atmospheric pressure, and the solid residue was then leached in an autoclave at high temperatures. The first stage of the process was carried out at ~100°C for 2 h using the solid to liquid (S : L) ratio equal to 1 : (5–6). The hydrochloric acid concentration was varied from 15 to 24%. After leaching, the pulp was filtered in vacuo, and the residue was washed and dried to determine the mass loss during leaching. To determine the development of slag and the content of the phase decomposed during leaching, the residue after drying was subjected to desiliconization with a 10% solution of NaOH at 100°C and S : L = 1 : 5 for 1 h to remove amorphous silica having precipitated upon leaching.
The pressure leaching was carried out using 20% HCl at 140, 160, and 200°C and at S : L = 1 : 5 for 2 h. After leaching, the pulp was filtered, and the residue was washed with water and dried at 105°C. Silica precipitated upon hydrochloric acid leaching was removed with a 5% solution of NaOH using the procedure described above. The phase compositions of the residues formed under both leaching conditions were studied by XRD.
RESULTS AND DISCUSSION
The results of slag leaching with hydrochloric acid under atmospheric conditions are given in Table 2. It is seen that a change in the acid concentration within 15–24% exerts no appreciable effect on the development of slag. The maximum development of slag is achieved at an acid concentration of 20–24% and is equal to 70%, and the development is slightly lower (68%) at 15%, which is probably related to acid deficiency for complete decomposition of the easily soluble phases of the slag. According to XRD data (Fig. 3), under the conditions of maximum slag decomposition, all crystalline phases are retained in the solid residue, namely, perovskite, loparite, and spinel. This means that only the glassy phase (matrix) of the slag decomposes on leaching with hydrochloric acid, and other phases remain nearly uninvolved. Thus, the content of the glassy phase in the slag is approximately 70%, and remained 30% fall on the fraction of other three phases: perovskite, loparite, and spinel.
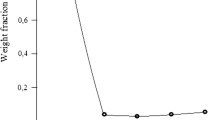
The slag was leached in a pH medium with the gradual addition of 20% HCl to determine the minimum concentration of hydrochloric acid at which the glassy phase began to decompose. A change in the pH of the pulp was monitored with a pH meter. An aqueous pulp consisting of the slag (90 g) and water (350 mL) was prepared. The pulp was sufficiently strongly alkaline: pH 9.8. The pulp was permanently stirred for 1 h with the gradual addition of 20% HCl, and the pH values of the solution changed. The total consumption of the acid was 220 mL. The initial temperature of the solution was 40°C. The temperature of the solution increased with the addition of the acid and reached 52°C. The results of the leaching are graphically shown in Fig. 4.
It is seen that at the initial stage for an acid consumption of 20 mL, the pH of the solution decreased sharply to 4.7 and then slowly decreased to 2.65 with the addition of the acid up to its consumption of 120 mL. This is accompanied by a noticeable increase in the temperature of the solution (from 40 to 52°C), and finally the pH decreased to 1.0 at the end of leaching when the acid consumption reached 220 mL. The yield of the solid residue (after desiliconization) was 37.3% (33.5 g from 90 g of the slag); that is, the development of slag was 62.7%. It follows from this that about 90% glassy phase of the slag decomposed upon slag leaching in a weakly acidic medium without heating of the solution. The intense decomposition occurs in a pH range of 4–2.5 and is accompanied by increasing temperature of the solution. If leaching occurs at higher temperatures (about 100°C), the process can be substantially accelerated to complete decomposition of the glassy phase of the slag.
The possibility of decomposition of the residue with hydrochloric acid at elevated temperatures under pressure (in an autoclave) was studied further. Some experiments were carried out in the presence of oxidants (chloric and nitric acids) for comparison. For this purpose, a pilot batch of the residue consisting of perovskite, loparite, and spinel was formed by slag leaching with 20% HCl at 100°C. The yield of the residue was 31% of the slag weight. The results of the pressure leaching of the residue at different temperatures are given in Table 3.
The data obtained indicate that the maximum development of the residue treated with hydrochloric acid is achieved at 200°C. In this case, 57.7% are extracted from the residue to the solution, whereas 32.2% remain in the solid phase, which is 13.1% of the slag weight (or 3.4% of the ore weight). After the desiliconization of the solid phase, the yield decreases from 13.1 to 11.6%. A temperature decrease results in a substantial deterioration of the results. In the temperature range 160–180°C, the introduction of chloric acid as an oxidant gave no positive results. This is probably associated with the fact that the oxidation ability of chloric acid manifests itself at higher temperatures (200°C or higher). When using a 25% solution of nitric acid, the decomposition of the residue at 160°C is substantially lower (23.9%) than for leaching with 20% HCl (39.1%). Additional studies are needed to improve the parameters of pressure leaching.
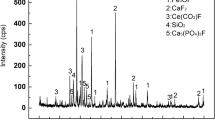
According to XRD data (Fig. 5), the solid phase, which does not decompose under the pressure leaching conditions with 20% HCl at 200°C, consists of manganese spinel (galaxite MnAl2O4). There are no reflections of perovskite and loparite on the XRD pattern; that is, these phases decompose completely under the specified conditions to precipitate titanium and niobium oxides. Other elements, including REM, go to the solution. The XRD pattern exhibits broadened reflections of titanium dioxide with the anatase structure. No lines of niobium oxides were identified. Probably, when the slag phases decompose, niobium is isolated in the form of hydrate oxides amorphous to X-rays.
After pressure leaching, it seems reasonable to direct the hydrochloric acid solution to the first stage of slag leaching at the temperature about 100°C. This would allow one, first, to decrease the hydrochloric acid consumption for leaching to a maximum extent and, second, to substantially facilitate the further recovery of REM and manganese by the precipitation (neutralization) method from weakly acidic solutions (pH 2–2.5). In addition, when the slag decomposes via the two-stage scheme (first the slag decomposes under atmospheric pressure, and then the formed residue is leached in an autoclave), it is unnecessary to achieve the complete decomposition of the glassy phase at the first leaching stage.
Thus, niobium and titanium oxides and the spinel phase MnAl2O4 are concentrated in the solid residue upon the hydrochloric acid decomposition of the niobium–rare-earth slag. It seems interesting to apply physical beneficiation methods for the separation of these products. The synthesized spinel phase was found to have weak magnetic properties. Therefore, wet magnetic separation was used as one of probable variants. Separation was carried out manually at the magnetic field strength about 4–5 kOe. After drying at 105°C, the yield of the magnetic fraction (dark gray-colored) was 51.9%, and the yield of the nonmagnetic fraction (light gray) was 48.1%. The images of the formed fractions are shown in Fig. 6. It is seen that the manual magnetic separation does not provide distinct phase separation: the nonmagnetic fraction contains a sufficiently high amount of dark gray crystals (probably of the spinel phase), and the magnetic fraction contains a light gray substance consisting of niobium and titanium oxides. This is confirmed by the chemical analysis results of these fractions (Table 4). The nonmagnetic fraction consists of niobium, titanium, and zirconium oxides by 63% and also contains 16% Al2O3, 8.7% MnO, and 1.7% MgO as the spinel phase, as well as about 7% residual SiO2, probably, in the form of quartz, which is insoluble in dilute alkaline solutions. The magnetic fraction consists of the spinel phase by 75%, and its composition (according to the chemical analysis data) corresponds to the formula (Fe,Mg,Mn)(Al,V)2O4. This fraction contains 4.46% Nb2O5 and 2.65% TiO2 as the main impurities.
The results of the study showed the principal possibility of using magnetic separation to divide the solid residue from the hydrochloric acid decomposition of the niobium–rare-earth slag into two products: an oxide niobium–titanium–zirconium concentrate and a manganese–aluminum spinel. However, to achieve a distinct division of these products, it is necessary to continue studies on the refinement of the magnetic separation conditions.
The complex niobium–titanium–zirconium concentrate formed upon magnetic separation can be successfully processed by the chloric method to form NbCl5, TiCl4, and ZrCl4, which are important intermediates for the production of metallic niobium, titanium, and zirconium by magnesium-thermic reduction. The nonmagnetic fraction (spinel phase) is also of interest for processing by the soda method in a closed cycle with recovery of aluminum, manganese, and vanadium as oxide products. In this case, soda is readily regenerated and returns to the roasting process.
CONCLUSIONS
(1) The hydrochloric acid decomposition of the niobium–rare-earth slag, which was produced by reducing roasting of the rare-earth ore from the Chuktukon deposit, was studied. The hydrochloric acid leaching of the slag occurred via the following two-stage scheme: leaching under atmospheric pressure (~70% developed slag) and pressure leaching of the solid residue with 20% HCl at 140–200°C.
(2) Only the glassy phase (matrix) of the slag was found to decompose by atmospheric leaching of the slag with hydrochloric acid, and other phases (perovskite, loparite, spinel) remained almost virgin. The glassy phase decomposed easily in the dilute solutions of hydrochloric acid even at lowered temperatures (about 40°C). The maximum development of slag (about 70%) was achieved at 20% HCl and ~100°C.
(3) Under the conditions of pressure leaching with hydrochloric acid, the completeness of decomposition of the slag phases with perovskite and loparite structures was achieved at 200°C and an acid concentration of 20%. The spinel phase remained unchanged. After desiliconization and drying, the yield of the solid residue was ~11.6% of the slag weight or 3.0% of the ore weight. The total development of slag reached 88.4%. Niobium, titanium, and zirconium were almost completely concentrated in the form of oxides along with the spinel phase in the solid residue. It seems reasonable to direct the hydrochloric acid solution of REM formed after pressure leaching to the first stage of slag decomposition.
(4) Magnetic separation was shown to divide the solid residue formed upon acidic decomposition of the slag into the following two products: a niobium–titanium–zirconium oxide concentrate and a spinel solid solution of the general formula (Fe,Mg,Mn)(Al,V)2O4. The niobium–titanium–zirconium concentrate can then be processed by the chloric method to produce these metals.
REFERENCES
Military Review. Production of Rare-Earth Metals: Russia Searches for Ways of Solution of the Problem. https://topwar.ru/175209-v-rossii-obratili-vnimanie-na-proizvodstvo-redkozemelnyh-metallov.html.
J. H. L. Voncken, The Rare Earth Elements. An Introduction (Springer, 2016).
V. G. Lomaev and S. S. Serdyuk, “Chuktukon deposit of niobium–rare-earth ores as a priority object for the modernization of the rare-metal industry in Russia,” Zh. Sib. Federal. Univ., Ser. Tekhn. Tekhnol. 4 (2), 132–154 (2011).
V. V. Perfil’ev, A. O. Seleznev, V. D. Sokolov, and A. V. Koznov, “Prospects of the Zashikhinsk deposit,” Redkie Zemli, No. 1 (8), 142–151 (2017).
V. I. Kuzmin, G. L. Pashkov, V. N. Kuzmina, S. N. Kalyakin, L. I. Dorokhova, V. G. Pavlov, and V. G. Lomaev, “Technological aspects of processing of the rare-metal ores from the Chuktukon deposit,” Khim. Interes. Uspeshn. Razvit., No. 18, 331–338 (2010).
V. I. Kuzmin, G. L. Pashkov, V. G. Lomaev, E. N. Voskresenskaya, and V. N. Kuzmina, “Combined approaches for comprehensive processing of rare earth metal ores,” Hydrometallurgy 129–130, 1–6 (2012).
M. V. Pavlov, I. V. Pavlov, V. F. Pavlov, O. V. Shabanova, and A. V. Shabanov, “Specific features of pyrometallurgical processing processes of the polymetallic ores from the Chuktukon deposit (Krasnoyarsk krai),” Khim. Interes. Uspeshn. Razvit., No. 23, 263–266 (2015).
G. B. Sadykhov, D. Yu. Kop’ev, D. G. Agafonov, T. V. Olyunina, K. G. Anisonyan, and E. N. Levchenko, “Reducing roasting of the niobium–REM ores of the Chuktukon deposit with the production of phosphorus cast iron and niobium–REM slag,” Russ. Metall. (Metally), No. 5, 507–516 (2020).
Funding
This work was supported in terms of state assignment no. 075-00328-21-00.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Sadykhov, G.B., Kop’ev, D.Y., Agafonov, D.G. et al. Hydrochloric Acid Decomposition of the Niobium–Rare-Earth Slag Produced by Reducing Roasting of the Rare-Earth Ore from the Chuktukon Deposit. Russ. Metall. 2021, 809–815 (2021). https://doi.org/10.1134/S0036029521070119
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029521070119