Abstract
The results of studying the reducing roasting of the high-iron (65% Fe2O3) niobium–rare earth ore of the Chuktukon deposit with the production of high-phosphorous cast iron and niobium–rare earth slag are discussed. The optimum reducing roasting conditions are found, and fairly complete separation of the roasting product into metal and slag phases is shown to be achieved at 1400°C. Under these conditions, 88% of phosphorus passes into cast iron, and 95% of niobium and 85% of manganese are concentrated in a rare-earth slag. The yield of phosphorous cast iron (up to 3.5% P) is about 45% and that of slag is 25% of the ore mass. The main slag phases are glass and, crystals with perovskite, loparite and spinel structures. Niobium and rare earth metals are concentrated in the first three phases, and the spinel phase is represented by an MnAl2O4–MgAl2O4 solid solution. After the preliminary removal of iron and phosphorus from the ore in the form of a metallic product, the material flows in the hydrometallurgical stage of processing are found to be reduced fourfold. Moreover, the entire process can become environmentally friendly with high technical and economic indicators.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The world production of rare earth metals (REM) is about 135 ths t per year. China is the monopolist in the REM production and Brazil is the monopolist in the niobium production [1]. About 8.5 ths t per year of REMs based on their oxides were produced in the Soviet Union. The difficult situation in the country in the 1990s led to a sharp decline in the production of these metals. The current consumption of REMs (individual oxides and metals) in Russia is very small and amounts to 2 ths t per year, or 2.5–3% of the global consumption [1]. The great importance of the development of the rare-earth industry in Russia requires the development of new highly efficient technologies to solve the problem of developing domestic deposits.
A significant increase in the world prices for rare metals and REMs in recent years and the impossibility of increasing the volume of production at the existing facilities make the creation of new enterprises in Russia particularly important, which is provided for by the state program “Development of industry and increasing its competitiveness on the period until 2020” adopted by the Government of the Russian Federation in 2013 (subprogram 15 “Development of the industry of rare and rare-earth metals”) [2].
Two large Russian deposits, namely, Tomtor (Sakha Yakutia) and Chuktukon (Krasnoyarsk Territory), are considered as the priority areas for the development of the mineral resource base of rare metals and REMs. The Tomtor deposit is located in a difficult-to-reach area; i.e., the problems of its development are associated with the complexity of product transportation [3].
The Chuktukon deposit of niobium–rare earth ores located in the Boguchansky district of the Krasnoyarsk Territory can become an important object for the modernization and development of the rare-metal industry in Russia in the ore reserve and the quality characteristics of the ore. The reserves of rare-earth ores in this deposit, which where approved by the State Reserves Committee of the Russian Federation (2007), amount to 6639 ths t (category C2); the reserves of niobium pentoxide are 39.8 ths t (0.6% niobium in ore); and the reserves of REM oxides are 486 ths t (7.3% in orfe). This deposit belongs to the ore-formation type of rare-metal weathering crust of carbonatites. The ore zone of the deposit is a powerful cloaklike deposit 3.5 km long, 800–1400 m wide, and 200 m thick. The ore is highly dispersed and contains up to 50% Fe, 12% Mn, and >7% REM oxides. The mineral composition of the ores is as follows: goethite, hydrogoethite, hematite, psilomelan, pyrolusite, bariopyrochlor, strontiopyrochlor, ceriopyrochlor, pyrochlor, florencite, monazite, and cerianite. The deposit is located relatively close to the Boguchanskaya HPP, which solves the problem of energy supply. Ore is proposed to be open-pit mined.
One of the decisive arguments about the necessity of developing the Chuktukon deposit is the economic factor determined by the natural share of the yttrium group metals in the REM composition (5–8%), which corresponds to the world level of consumption; i.e., it will contribute to a more complete sale of products. Therefore, the creation of REM production on the basis of the Chuktukon deposit will allow Russia to modernize the rare-metal industry and to take the leading place on the world market of rare metals at a high production efficiency [3].
The processing of complex iron-containing rare-earth ores is a big problem due to the fact that these ores cannot be beneficiated because of their fine dispersion and the close coalescence of the mineral components.
When choosing a ore processing technology for the Chuktukon deposit, it is necessary to take into account a high iron content in it and the characteristic features of the behavior of rare metals and REMs. At 65% Fe2O3, purely hydrometallurgical processing methods, in particular, pressure leaching with nitric, sulfuric and hydrochloric acids, become unacceptable.
During nitric acid leaching, iron and niobium weakly dissolve, and about half of manganese is concentrated in a solid iron-containing residue [4]. This circumstance greatly complicates niobium recovery.
When leaching with sulfuric acid, a significant part of iron oxides is retained in a solid phase due to poor solubility of goethite and hematite in sulfuric acid. Manganese behaves similarly. Niobium is distributed between the solid phase and the solution, which leads to its inevitable loss.
When hydrochloric acid is used, niobium, silicon and titanium concentrate in a solid phase, and almost all iron and manganese pass to a solution, together with REMs. Moreover, the consumption of concentrated hydrochloric acid is very high (about 3–3.5 tons per 1 ore t). To extract REMs, it is first necessary to remove iron from the solution by neutralization. High REM losses are inevitable because of the very high iron content in the solution and the formation of large sediments during neutralization. In addition, serious problems are associated with the disposal of the large volumes of iron-containing residues. Therefore, the hydrochloric acid method can become effective when iron is preliminarily removed preferably in the form of a commercial product or semiproduct.
For efficient processing of the niobium–rare earth ores of the Chuktukon deposit, a combined pyrohydrometallurgical scheme, in which major part of iron is separated in the form of metal at the first stage (reduction smelting), is more promising. In [4–6], reduction ore smelting was considered in the temperature range 1550–1600°C for ~2 h with the transfer of manganese, phosphorus, titanium, and niobium along with iron into a metallic phase (cast iron) and with REM concentration in a slag. The cast iron is proposed to be subjected to stage-by-stage conversion to extract niobium and manganese into a slag and, then, to remove phosphorus, and the slag from ore smelting is proposed to be subjected to nitric acid decomposition followed by the extraction of REMs from solutions. Despite the fact that ore reduction smelting allows a fourfold reduction in the material flows in hydrometallurgical processing, the proposed version needs high energy, and it is very difficult to achieve the desired degree of niobium and manganese recovery into a metallic phase under industrial conditions. Therefore, it is reasonable to reduce the ores under moderate temperature conditions, where phosphorus is transferred to a metal and niobium, manganese, and REMs are retained in a slag phase. Preliminary removal of iron will significantly facilitate further hydrometallurgical extraction of niobium, REMs, and manganese from the slag at high technological parameters.
In this work, we discuss the results of studying the reducing roasting of the niobium–rare earth ore of the Chuktukon deposit with the formation of high-phosphorous cast iron and a niobium–rare earth slag.
EXPERIMENTAL
The studies were conducted on a representative sample of ore crushed to <0.16 mm. In the initial sample, the fraction of the fine fraction (<0.16 mm) was 55%. The moisture content of the ore was 0.83%. The chemical composition of the ore was as follows (%): 0.17 Na2O, 0.17 MgO, 3.06 Al2O3, 1.71 SiO2, 4.14 P2O5, 2.42 CaO, 1.20 TiO2, 0.39 V2O5, 7.1 MnO, 64.8 Fe2O3, 1.04 ZnO, 1.49 BaO, 0.055 ZrO2, 1.54 Nb2O5, 0.54 SrO, 0.12 Y2O3, 0.79 La2O3, 1.51 CeO2, 0.13 PrO3, 0.45 Nd2O3, 0.039 Gd2O3, and ~10 others. The ore is characterized by a high content of iron and manganese. The content of rare metals in the form of their oxides is 5.2%, of which 1.54% is Nb2O5.
Reducing roasting was carried out with coke in a coal bed to prevent contact of the slag with the crucible material. The main problem was to determine the optimal reducing agent consumption necessary to minimize the reduction of niobium and manganese into a metal phase. For roasting, the ore with crushed coke additives (<0.1 mm) was briquetted. The amount of coke was calculated from the iron oxide content. In experiments, the coke consumption was varied within 11–15% of the ore mass. The weight of the pellets made from a roasting mixture was 17–23 g (15–20 g based on ore). Reducing roasting was carried out in the temperature range 1200–1400°C. The time of heating from 1000 to 1200–1400°C was ~10 min. When the maximum temperature was reached, the process continued after holding for 5 min. The products of reducing roasting after cooling and crushing were divided into the following two fractions: metal and slag. The metal was analyzed to determine the impurity composition, and the slag was examined using optical microscopy, electron-probe microanalysis (EPMA), and X-ray diffraction (XRD).
RESULTS AND DISCUSSION
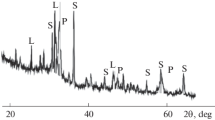
According to XRD data (Fig. 1), the main minerals of the Chuktukon niobium–rare earth ore are hydroxides and iron oxides, namely, goethite FeOOH and hematite Fe2O3. Manganese is in psilomelan (Ba,H2O)2Mn5O10, niobium is in pyrochlore CaNaNb2O6F or in similar minerals, and REMs are mainly in monazite (Ce,La)PO4. Along with these minerals, a significant amount of apatite Ca5(PO4)3(OH), silica, and clay minerals is also present in the ore.
According to the data of microscopic analysis (Fig. 2), the coarsening of metallic iron particles in reducing roasting begins at 1200°C, and the slag phase contains many fine (1–10 μm) iron particles. At 1250°C, the fraction of fine iron particles in the slag decreases noticeably and new phases solidify in the slag. A further increase in temperature leads to acceleration of these processes, and the formation of metal and slag phases is completed at 1400°C. In this case, large metallic iron shot is separated from the slag, and the slag is almost completely free of fine metallic particles. The results of reducing roasting of the ore at 1400°C at various coke contents are given in Table 1. Photographs of the reducing roasting products are shown in Fig. 3. It should be noted that the slag structure is most dense at 15% coke. As follows from Table 1, the metal yield increases slightly (from 44.8 to 46.7%) and the slag yield decreases (from 26.2 to 22.6%) when the coke consumption increases from 11 to 15%, which is associated with the complete reduction of iron into cast iron, its carburization, and a slight increase in the fraction of extraction of other components into the metal. Under the optimum reducing roasting conditions, the iron recovery from the ore into the metal phase reaches 98%.
Appearance of the products of reducing roasting of pellets made of a mixture of the niobium–REM ore with coke at 1400°C: (1–3) experiments 1–3 in Table 1. M, metal; S, slag.
The results of X-ray photoelectron spectroscopy (GDS 850A glow discharge atomic emission spectrometer, LECO) of metal samples formed at various solid reducer contents are given in Table 2.
According to the analysis data, the synthesized metal is low-silicon high-phosphorus cast iron containing 0.01–0.033% Si and about 3.5% P. The extraction of phosphorus into cast iron is 84–88%; i.e., its content in slag is 16–12%, respectively. The presence of phosphorus improves the fluidity of cast iron, which is explained by the formation of a low-melting point triple (phosphide) eutectic melting at 950°C in cast iron [7]. Therefore, the coarsening and coalescence of metallic particles at relatively low reducing roasting temperatures (1400°C) are significantly facilitated. According to the results of microscopic analysis, the slag is almost completely free of fine metallic particles.
The reduction of niobium into a metal under these conditions is weak. The niobium content in the metal is 0.04–0.1%. Taking into account these data, the extraction of niobium into a metal is 1.7–4.3% depending on the amount of a reducing agent, more than 95% of niobium concentrates in slag. The extraction of manganese into cast iron is 10–15%, the rest of manganese concentrates in the slag phase along with REMs.
The fields of application of high-phosphorus cast iron strongly depend on the volume of processing of the niobium-rare earth ore. At small processing volume (up to 100–200 ths t per year), the production of phosphorous cast iron is 45–90 ths t per year. In this case, cast iron containing about 3.5% P can be successfully used as a valuable material for the manufacture of brake pads for high-speed locomotives [8, 9]. The high technological properties of phosphorous cast iron for the manufacture of brake pads are achieved when it contains <1.9% Si and <3.0% C. The high-phosphorous cast iron formed by processing the niobium–rare earth ore of the Chuktukon deposit contains 2.1–2.4% C and 0.01–0.033% Si, which fully meets the quality requirements imposed on cast iron for the production of brake pads for high-speed locomotives.
At large volume of processing the niobium–rare earth ore (up to 0.5–1 mln t per year), the produced high-phosphorous cast iron (225–450 ths t per year) can be cost-effectively processed in open-hearth furnaces (or other units) with the formation of medium- or high-carbon steel. At the same time, the associated production of phosphate slags will significantly increase the profitability of this stage of processing. In this regard, the processing of the phosphorous cast iron should be divided into two stages, which will allow the process to be conducted at each of them at a low slag content. In addition, dephosphorization can be performed at the first stage after casting.
The second stage, namely, the oxidation of metal impurities, is carried out for retreating, as in the scrap–ore process. If necessary, an additional operation can be introduced because of too high contents of manganese (0.9–1.65%) and vanadium (0.12–0.16%). As a result, the melt of phosphorous cast iron is initially blown by oxygen to form a manganese–vanadium slag and is then subjected to dephosphorization in two periods.
The chemical composition of the slags formed reducing roasting of the ore is given in Table 3. These slags are rich artificial raw materials of rare metals and REMs. The total content of their oxides in the slag exceeds 20%. The slag contains 21% MnO, and content Fetot is 1.8–3.6%. The increase in the iron content in the slags with increasing coke consumption is caused by the presence of small metallic iron particles in the slag. The main impurities are CaO (9.6–10%), SiO2 (7.3–7.8%), Al2O3 (7.1–7.4%) and P2O5 (2.0–2.65%). When the solid reducing agent content increases during reduction roasting of the ore, the phosphorus content in the slag decreases slightly.
The diffraction patterns of the slags are shown in Fig. 4 and their microstructures are shown in Fig. 5. The slags have a fine crystalline structure. According to XRD and microscopic analysis data, the phase ratio in the slag changes as a function of the amount of the reducing agent. All slags have the following four main phases: a glassy matrix, bright skeleton crystals with a perovskite structure, coarse grains with a cubic habit plane (spinel phase), and fine crystals in the glassy matrix in the form of stars with a loparite structure.
In the case of hydrometallurgical processing, the achievement of high parameters for the extraction of the target components mainly depends on the completeness of decomposition of slag phases, which, in turn, is determined by the chemical compositions of the phases and the form of the elements in these phases. For an example, Table 4 gives the results of EPMA analysis of the mineral phases in slag 3, and Fig. 6 shows the microstructure of this slag.
The glassy phase of the slag consists of barium, calcium and manganese aluminosilicates. It contains a significant amount of rare metals and REMs. The Nb2O5 content reaches 4%, and the content of lanthanum, cerium, and neodymium oxides exceed 3%. In mass, the glassy phase is the main phase of the slag.
The phase with a perovskite structure is a solid solution of titanium perovskite (CaTiO3) with niobium and REM oxides with the general formula ABO3 [10]. The Nb2O5 content in this phase is very high, 11.3–16.5%. The REM oxide content reaches 30%: ~5.5% is the fraction of Nd2O3; 9%, La2O3; and 15%, Ce2O3. This phase also contains up to 11% Al2O3 and 2.5–3% SrO.
The phase with a loparite structure is characterized by a very high manganese content (49–51% based on MnO). The Nb2O5 content is ~10%. It contains an insignificant amount of REM and ~1% TiO2. The total content of lanthanum, cerium, and neodymium oxides does not exceed 0.5–0.6%. This phase has a relatively high content of aluminum (16.5–17.5% Al2O3), silicon (4–4.5% SiO2), and magnesium (2.2–3% MgO).
It is important to note that the phases with perovskite and loparite structures, which are the solid solutions of titanium, niobium, and REM oxides, formed under reducing conditions. They belong to the cubic titanium and niobium bronzes solidifying in the structure type of perovskite [10]. The titanium and niobium bronzes containing low-valence elements are difficult to decompose in mineral acids in the absence of oxidizing agents. This fact should be taken into account in developing the processes of hydrometallurgical processing of such slags.
The spinel phase of the slag consists of an MnAl2O4–MgAl2O4 solid solution, which contains ~1% TiO2. The content of niobium and REM oxides is insignificant and does not exceed 0.3–0.4% in total. Spinel weakly dissolves in the solutions of mineral acids even at elevated temperatures and pressures.
Thus, niobium and REMs in the slags formed upon high-temperature reducing roasting of the ore from the Chuktukon deposit are distributed over all phases except for spinel.
For processing of such slags, the most appropriate solution can be hydrochloric acid or nitric acid pressure leaching. As a result, all niobium should be concentrated in a solid phase and REMs should be completely transferred to a solution and then extracted in the form of a collective concentrate.
In an industrial implementation of the proposed process, reducing roasting of a high-iron niobium–rare earth ore should to be carried out in a ring furnace with a rotating hearth according to the ITmk3 technology (Ironmaking Technology Mark Three) [11–13]. In this case, the ore production per furnace can be 100–500 ths t per year. A mixture of ore with fluxing additives and a carbon-containing reducing agent is briquetted, dried, and subjected to reducing roasting when the process temperature is increased to 1400°C, at which the coalescence of the resulting metallic particles leads to the formation of cast iron granules and slag. The entire process of reducing roasting is completed within 13–15 minutes. After cooling, the roasting product is crushed and directed to magnetic separation to separate metal and slag. The consumed energy is significantly reduced due to a short process time and a low roasting temperature. In addition, reducing roasting in a rotary hearth furnace makes it possible to use cheap power-generating coal with a moderate ash content as a solid reducing agent.
CONCLUSIONS
(1) The great importance of the development of the rare-earth industry in Russia requires the development of new highly efficient technologies to solve the problem of developing domestic deposits. The reducing roasting of the high-iron niobium–rare earth ore from the Chuktukon deposit with the production of high-phosphorous cast iron and slag was studied. The optimum reducing roasting parameters of the ore were determined; they made it possible to achieve the maximum extraction of niobium (>95%) and manganese (85%) into slag at a high degree (up to 88%) of phosphorus reduction to cast iron. Here, the extraction of iron from the ore into cast iron was 98%.
(2) Under these conditions, the yield of phosphorous cast iron was ~45% and that of slag was 26% of the ore mass. Along with the manufacture of a commercial metal product, this process makes it possible to increase the REM and niobium content in an oxide phase by a factor of 4 and to decrease the material flows in a hydrometallurgical process in the same proportion. The low iron content in the resulting slag can significantly facilitate the choice of a technological process for the hydrometallurgical extraction of niobium, REM, and manganese from slag at high technological parameters.
(3) The phase composition of the slag and the distribution of REM, niobium, and other elements over phases were studied. The slag was found to have the following four phases: glassy phase and phases with perovskite, loparite, and spinel structures. Niobium and REM are concentrated in the first three phases, and the spinel phase is an MnAl2O4–MgAl2O4 solid solution. The total content of niobium and REM oxides in the spinel phase does not exceed 0.3–0.4%.
REFERENCES
S. S. Serdyuk, V. G. Lomaev, V. I. Kuzmin, G. L. Pashkov, V. F. Shabanov, V. F. Pavlov, and S. N. Mamonov, “Krasnoyarsk cluster—a strategic priority for the development of the rare-metal industry in Russia,” Zh. SFU. Tekhnika Tekhnologii, No. 7, 816–834 (2015).
“State program of the Russian Federation ‘Development of industry and increasing its competitiveness for the period until 2020’,” Regulation no. 328 of the Government of the Russian Federation of April 15, 2014.
V. G. Lomaev and S. S. Serdyuk, “Chuktukon deposit of niobium–REM ores—a priority object for the modernization of the rare-metal industry in Russia,” J. Sib. Fed. Univ., Ser. Tekhnika Tekhnologii 4 (2), 132–154 (2011).
V. I. Kuzmin, G. L. Pashkov, V. N. Kuzmina, S. N. Kalyakin, L. I. Dorokhova, V. G. Pavlov, and V. G. Lomaev, “Technological aspects of processing of the rare-earth metal ores in the Chuktukon deposit,” Khim. Int. Usp. Razv., No. 18, 331–338 (2010).
V. I. Kuzmin, G. L. Pashkov, V. G. Lomaev, E. N. Voskresenskaya, and V. N. Kuzmina, “Combined approaches for comprehensive processing of rare earth metal ores,” Hydrometallurgy 129–130, 1–6 (2012).
M. V. Pavlov, I. V. Pavlov, V. F. Pavlov, O. V. Shabanova, and A. V. Shabanov, “Features of the pyrometallurgical processing of polymetallic ores of the Chuktukon deposit (Krasnoyarsk Territory),” Khim. Int. Usp. Razv., No. 23, 263–266 (2015).
A. P. Gulyaev, Physical Metallurgy (Metallurgiya, Moscow, 1977).
V. I. Marshev, “High-phosphorus cast iron for the brake pads of high-speed locomotives,” Extended Abstract of Cand. Sci. Dissertation, Vseross. Nauch.-Issled. Inst. Zh.-D. Transp. MPS RF, Moscow, 2006.
GOST 30249–97. Cast Iron Brake Pads for Locomotives. Specifications (Standartinform, Moscow, 2006).
G. V. Bazuev, “Oxide niobium and titanium bronzes,” in Oxide Bronzes (Nauka, Moscow, 1982), pp. 104–121.
I. Kobayashi, Y. Tanigaki, and A. Uragami, “A new process to produce iron directly from fine ore and coal,” Iron and Steelmaker 28 (9), 19–22 (2001).
K. Fuji, H. Tanaka, et al., “Method for producing reduced iron,” US Patent 6602320, 2001.
“Industrial operation of the ITmk3 plant,” Inzh. Resh., Chern. Metall., No. 2, 15 (2012).
Funding
This work was performed in terms of state assignment no. 075-00746-19-00.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by K. Shakhlevich
Rights and permissions
About this article
Cite this article
Sadykhov, G.B., Kop’ev, D.Y., Agafonov, D.G. et al. Reducing Roasting of the Niobium–REM Ores of the Chuktukon Deposit with the Production of Phosphorus Cast Iron and Niobium–REM Slag. Russ. Metall. 2020, 507–516 (2020). https://doi.org/10.1134/S0036029520050122
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029520050122