Abstract
Using affinity capillary electrophoresis, the stability constants of inclusion complexes of a number of betulin ester derivatives with randomly methylated β-cyclodextrin in 0.0100 M sodium tetraborate (pH 9.18, ionic strength 0.0200 M) at 25°С were determined for the first time. The decimal logarithms of the stability constants of 1 : 1 complexes of betulin 3,28-diphthalate, betulin 3,28-disulfate, and betulin 3,28-disuccinate in a 95% confidence interval were 4.64 ± 0.05, 4.78 ± 0.02, and 4.94 ± 0.02, respectively. The logarithms of the stability constants of 1 : 2 complexes of betulin 3,28-diphthalate and betulin 3,28-disulfate were 7.91 (6.66–8.19) and 8.02 (7.63–8.23), respectively. It was determined that betulin 3,28-disuccinate forms only 1 : 1 complex. The obtained stability constants of 1 : 1 complexes are generally higher than those of the complexes of the same compounds with β-cyclodextrin (β-CD) and lower than those for dimethyl-β-CD, γ-CD, and (2-hydroxypropyl)-γ-CD complexes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In recent decades, there has been an increase in the number of studies on the development and application of drug delivery systems. One of the most frequently used nanocontainers are cyclodextrins (CDs), which are natural macrocycles based on α-1,4-linked D-glucopyranoses [1]. Molecules with six, seven, and eight glucose residues are called α-, β-, and γ-CDs, respectively. Such systems are most often used to increase the solubility and bioavailability of compounds with poor water solubility. Betulin and its derivatives, pentacyclic triterpenoids of the lupane series, are no exception due to their high natural abundance (the content of betulin in birch bark reaches 30%), a number of useful properties (antiviral, anticancer, etc.), and poor solubility in water [2]. The complex formation of betulin and its derivatives with CDs was studied by various methods, such as UV spectrophotometry [3], high-performance liquid chromatography [4, 5], affinity capillary electrophoresis (ACE) [6–16], and combinations of the solubility method with UV spectrophotometry [17, 18] and with capillary zone electrophoresis [6, 19–21].
Complexes of ester derivatives of betulin with CDs were studied predominantly in a medium of 0.0100 M sodium tetraborate with pH 9.18 at 25°С (ionic strength 0.0200 M) by ACE [8–16]. The most widely used variant of ACE is the recording of electropherograms of the compounds under study using a number of background electrolytes with a variable content of CD [12, 22, 23]. The stability constants are calculated from the following equations, which take into account the formation of only 1 : 1 complex or both 1 : 1 and 1 : 2 complexes:
Here, νi is the correction for the change in the viscosity of the background electrolyte; μeff,i is the effective electrophoretic mobility; µB, µ11, and µ12 are the ionic mobilities of the ester derivative of betulin and 1 : 1 and 1 : 2 complexes, respectively; β11 and β12 are the stability constants of 1 : 1 and 1 : 2 complexes, respectively; [CD] is the concentration of CD in the background electrolyte (which is equated with the total concentration CCD in the background electrolyte, since it is typically much higher than the concentration of the compounds under study, see our previous work for details [10]); and \(t_{i}^{'}\) and t0 are the DMSO peak migration times at 0 kV under the application of a hydrodynamic pressure of 100 mbar in background electrolytes with and without the addition of CD, respectively. There are three ways to linearize Eq. (1) [23]; however, it was previously shown [24] that the most accurate method for calculating the stability constants is the method of nonlinear regression directly from Eq. (1). In addition, it was shown [12, 25] that the y-reciprocal and double-reciprocal linearization methods cannot identify the presence of 1 : 2 complexes, and these methods are not recommended. The decimal logarithms of the stability constants of CD complexes are typically in the range of 1–4; the features of the application of ACE for determining higher values of the stability constants were considered in detail before [10]. Some of these features are the following: the concentration of analytes in samples should be the lowest possible (e.g., such that gives rise to electrophoretic peaks with a signal-to-noise ratio of about 10); the electrophoretic mobilities should be calculated using not the migration time at the maximum of the peak, but the parameter a1 of the Haarhoff–Van der Linde (HVL) function; and the electrophoretic mobility of peaks of a distorted triangular shape should not be calculated (and thus these data should not be used in the calculations).
It was found that the stability constants of 1 : 1 complexes of betulin ester derivatives with CDs depend weakly on the type of the ester derivative and depend significantly on the type of CD; i.e., it is the betulin fragment of the molecule that determines the stability of the complex. The decimal logarithms of the binding constants for these complexes with γ-CD and (2‑hydroxypropyl)-γ-CD (HP-γ-CD) reach 7 [9, 10, 14], whereas those for β-CD [11], HP-β-CD [12], and dimethyl-β-CD (DM-β-CD) are only 4–5 [15]. It was also found that ester derivatives of betulin with β-CD and its derivative form not only 1 : 1, but also 1 : 2 complexes (with the exception of betulin 3,28‑disuccinate with DM-β-CD). In this regard, it was interesting to determine the stability constants of complexes of betulin ester derivatives with randomly methylated β‑cyclodextrin (RAMEB): we failed to find any information in the literature about the study of these complexes by any method.
This work aimed at determining the stability constants of the inclusion complexes of a number of betulin ester derivatives with RAMEB by ACE (Fig. 1). The measurements were also carried out in a medium of 0.0100 M sodium tetraborate with pH 9.18 at 25°C, since it was shown [14] that, at pH 6 and below, BDP and BDSc, which are amphiphilic compounds and weak acids, occur in solutions mainly as micelles. This leads to a strong broadening of the electrophoretic peaks and complicates the determination of the stability constants of the complexes of these betulin derivatives with cyclodextrins by ACE. By the example of β‑CD complexes of BDP, BDSс, and BDS, it was shown [15] that the stability constants are independent of which background electrolyte is used: 10 mM sodium tetraborate or a phosphate buffer with the same ionic strength (0.0200 M). However, the first background electrolyte is more convenient to use in practice due to the shorter measurement time and longer shelf life.
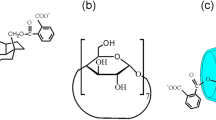
Structural formulas of (a) RAMEB, (b) the studied betulin derivatives, and (c) a possible scheme of the supramolecular 1 : 1 complex (betulin derivative–RAMEB). R = H or CH3. R' = –COC6H4COOH for betulin 3,28-diphthalate (BDP), ‒COCH2CH2COOH for betulin 3,28-disuccinate (BDSc), and –SO3H or –SO3Na for betulin 3,28-disulfate (DBS).
EXPERIMENTAL
Betulin derivatives were obtained according to published procedures [26–28]. Methyl-β-cyclodextrin (average molecular weight 1310) was purchased from Acros Organics (Belgium). The reagents used in the work were of analytical grade or higher. Solutions were prepared using deionized water produced by a Direct Q3 water purification system (Millipore, France) and filtered through 0.45-µm filters, unless otherwise indicated. The background electrolyte was 10 mM sodium tetraborate with pH 9.18 (ionic strength 0.0200 M [10]) supplemented with 0–10 mM RAMEB. The marker of electroosmotic flow (EOF) was 0.001% dimethyl sulfoxide (DMSO). A 1 g/L BDS solution was prepared by dissolving an exactly weighed amount of BDS sodium salt in 10 mM sodium tetraborate without subsequent filtration. A 0.1 g/L BDP solution and a 0.7 g/L BDSc solution were prepared by stirring weighed amounts of these compounds in 10 mM sodium tetraborate for 3 days [20] with subsequent filtration through 0.45-µm filters. The accurate concentrations of BDP and BDSc in the solutions obtained after filtration were determined using electrophoretic peak areas and calibration dependences. The latter were constructed as concentration dependences of electrophoretic peak areas of samples obtained by diluting ethanol solutions. The ethanol solutions of BDP and BDSc were prepared by dissolving their exactly weighed amounts in ethanol without subsequent filtration. Samples for introduction into a capillary were prepared by diluting concentrated solutions with the corresponding background electrolyte. When determining the stability constants, the concentration of analytes in the samples was chosen so that the signal-to-noise ratio for the electrophoretic peaks recorded using a background electrolyte without the addition of CD was about 10, i.e., 0.002, 0.02, and 0.07 mM for BDP, BDS, and BDSc, respectively.
Measurements were performed with a capillary electrophoresis system with a diode array detector Agilent 7100 (Agilent Technologies, Waldbronn, Germany). An unmodified quartz capillary 50 µm i.d., 80.5 mm in total length and 72 cm in effective length was used. The temperature of the capillary was maintained at 25 ± 0.04°C. Detection was carried out in the UV region at 200 nm. A voltage of +30 kV was used. The samples were introduced into the capillary hydrodynamically at a pressure of 50 mbar for 5 s. All experiments were made in 3–5 replicates. At the beginning of each day, the capillary was washed with 0.1 M NaOH for 10 min, twice with water for 5 min, and then with the background electrolyte for 10 min. Between the recordings, the capillary was washed with the background electrolyte for 3 min.
The effective electrophoretic mobility was calculated from the experimental data by the formula
where l and leff are the total and effective capillary lengths, respectively; U is the voltage; a1,i is the center of the peak of the HVL function for the ith peak [29]; tcor is the time correction to take into account that the voltage is applied neither instantly, nor at zero time; tEOF is the migration time of the EOF marker (neutral compound, DMSO); tin is the time from which the voltage begins to be applied (0.01 min); and tfin is the time at which the voltage reaches the specified value (0.18 min).
The stability constants of complexes, ionic mobilities, and 95% confidence intervals were found by nonlinear regression using MS Excel and OriginPro 8.1 software (OriginLab Corporation, Northampton, USA). The parameter a1 of the HVL function for electrophoretic peaks was determined using the CEval program (Prague, Czech Republic) [30].
RESULTS AND DISCUSSION
Electropherograms of BDP, BDSc, and BDS were recorded using background electrolytes supplemented with 0–10 mM RAMEB. Figure 2 presents examples of the recorded electropherograms. Since acidic groups completely dissociate at pH 9.18 (Fig. 1b), the studied betulin derivatives were represented as doubly charged anions and were recorded on electrophoregrams at positive polarity after the peak of the neutral compound (DMSO). At a low RAMEB content in the background electrolyte, the electrophoretic peaks of the studied betulin derivatives had a triangular shape, and the electrophoretic mobility was calculated not using the migration time at the peak maximum, but using the parameter a1 of the HVL function [10]. Figure 2 by the example of the BDS peak at 0.01 mM RAMEB content in the background electrolyte illustrates that the difference between these time parameters can be quite significant (the vertical dashed line shows the time equal to the parameter a1). The BDP peak looks more symmetric than the BDS and BDSc peaks, since the BDP concentration in the sample is lower due to the higher detection sensitivity, and the triangularity of the peaks, in turn, depends on the analyte concentration [10].
Based on the obtained electropherograms, the electrophoretic mobilities were calculated and the concentration dependences of these mobilities corrected for the change in viscosity were constructed (Fig. 3). Within the framework of 1 : 1 interaction, only the data on BDSc are well described (Fig. 3a). This situation may be due to the fact that the location of the DCBP molecule inside the cavity of methylated β-CD derivatives is unfavorable for the formation of 1 : 2 complexes. For BDP and BDS, there is a clear (greater than the experimental error in the measurement of electrophoretic mobilities) deviation of the experimental points from the theoretical dependences that take into account the formation of only 1 : 1 complexes. The deviation reaches 1.5 and 5% for BDP and BDS, respectively, whereas the measurement error of electrophoretic mobilities does not exceed 0.4–0.7%. Therefore, for BDP and BDS, the experimental data were processed taking into account the formation of 1 : 1 and 1 : 2 complexes. Table 1 presents the calculated stability constants and 95% confidence intervals. Figure 4 presents the pCCD distribution of the species of the studied betulin derivatives, where pCCD is the negative logarithm of the CD concentration. It can be seen that, for both BDP and BDS, the fractions of both 1 : 1 and 1 : 2 complexes are quite significant at RAMEB concentrations above 0.1 mM.
Dependences of the effective electrophoretic mobility of (a) BDSc, (b) BDP, and (c) BDS, corrected for the change in viscosity on the RAMEB concentration in the background electrolyte. The dashed and solid lines are the theoretical curves that take into account the formation of only 1 : 1 complexes (Eq. (1)) and the formation of both 1 : 1 and 1 : 2 complexes (Eq. (2)), respectively.
The RAMEB used is also an almost dimethylated derivative: the degree of hydrogen substitution in the hydroxyl groups of β-CD that was calculated from the manufacturer’s declared molecular weight of RAMEB (1310) and the molecular weight of β-CD (1135) is about 1.8 per glucose residue or 12.5 per molecule of this CD. As can be seen from Table 1, the stability constants of these 1 : 1 complexes are almost half as high as (logβ11 is 0.2–0.3 logarithmic unit lower than) the stability constants of 1 : 1 complexes of DM-β-CD (heptakis(2,6-di-O-methyl)-β-CD with a degree of substitution of 14.5 methyl groups per CD molecule or 2.1 per glucose residue). The binding constants for 1 : 2 complexes are determined with a large error and do not differ within the error for complexes of methylated CD derivatives.
The obtained stability constants of 1 : 1 complexes are by a factor of 2–5 higher than those of β-CD complexes of the same compounds (Table 1). A comparison with the data on HP-β-CD gives a more complex picture: the β11 obtained in this work for BDP is by a factor of 1.6 lower than the constant for HP-β-CD, whereas β11 for BDS is 1.5 times higher, and the β11 for BDSc is the same within the error. Interestingly, it is the RAMEB derivative that is characterized by the fact that the binding constants of all three studied betulin derivatives differ within the error. Moreover, the value for BDSc is the highest, the value for BDS is the second highest, and the value for BDP is the third highest. The β- and HP-β-CD complexes of BDS have the lowest β11, whereas the values for BDP and BDSc of these CD complexes are the same within the error. As for β12, their values for various CD complexes of for BDP are the same within the error, and β12 for RAMEB complexes of BDS are higher than β12 for β‑CD and HP-β-CD complexes by almost two and one orders of magnitude, respectively.
Thus, by the ACE method, the stability constants of 1 : 1 and 1 : 2 complexes of a number of betulin ester derivatives with RAMEB were determined for the first time. In general, the stability constants of 1 : 1 complexes of these betulin derivatives are higher than those of the β-CD complexes and lower than the stability constants of DM-β-CD, γ-CD, and HP-γ-CD complexes. The results obtained are of interest for optimizing the processes of microencapsulation of betulin derivatives by obtaining inclusion complexes (host–guest complexes) with randomly methylated β-CD to increase the bioavailability of these pharmacologically active compounds.
REFERENCES
G. Crini, Chem. Rev. 114, 10940 (2014). https://doi.org/10.1021/cr500081p
G. A. Tolstikov, O. B. Flekhter, E. E. Shul’ts, et al., Khim. Inter. Ustoich. Razvit. 13, 1 (2005).
L. Sbârcea, A. Ledeţi, L. Udrescu, et al., J. Therm. Anal. Calorim. 138, 2787 (2019). https://doi.org/10.1007/s10973-019-08359-6
B. Claude, Ph. Morin, M. Lafosse, and P. Andre, J. Chromatogr., A 1049, 37 (2004). https://doi.org/10.1016/j.chroma.2004.06.133
S. Fontanay, F. Kedzierewicz, R. E. Duval, and I. Clarot, J. Incl. Phenom. Macrocycl. Chem. 73, 341 (2012). https://doi.org/10.1007/s10847-011-0063-y
O. V. Popova, V. V. Sursyakova, G. V. Burmakina, V. A. Levdansky, and A. I. Rubaylo, Dokl. Chem. 461, 67 (2015). https://doi.org/10.1134/S0012500815030039
T. Ren and Z. Xu, Electrophoresis 39, 1006 (2018). https://doi.org/10.1002/elps.201700408
V. V. Sursyakova, V. A. Levdansky, and A. I. Rubaylo, J. Mol. Liq. 283, 325 (2019). https://doi.org/10.1016/j.molliq.2019.03.092
V. V. Sursyakova, V. A. Levdansky, and A. I. Rubaylo, Electrophoresis 41, 112 (2020). https://doi.org/10.1002/elps.201900347
V. V. Sursyakova, V. A. Levdansky, and A. I. Rubaylo, Anal. Bioanal. Chem. 412, 5615 (2020). https://doi.org/10.1007/s00216-020-02777-4
V. V. Sursyakova, V. A. Levdansky, and A. I. Rubaylo, J. Sib. Fed. Univ. Chem. 13, 534 (2020). https://doi.org/10.17516/1998-2836-0203
V. V. Sursyakova, V. A. Levdansky, and A. I. Rubaylo, Electrophoresis 42, 700 (2021). https://doi.org/10.1002/elps.202000189
V. V. Sursyakova and A. I. Rubaylo, J. Sep. Sci. 44, 4200 (2021). https://doi.org/10.1002/jssc.202100507
V. V. Sursyakova, V. A. Levdansky, and A. I. Rubaylo, Electrophoresis 43, 535 (2022). https://doi.org/10.1002/elps.202100173
V. V. Sursyakova, V. A. Levdansky, and A. I. Rubaylo, J. Sep. Sci. 45, 3745 (2022). https://doi.org/10.1002/jssc.202200453
V. V. Sursyakova, N. G. Maksimov, V. A. Levdansky, and A. I. Rubaylo, J. Incl. Phenom. Macrocycl. Chem. 103, 109 (2023). https://doi.org/10.1007/s10847-023-01182-5
V. Buko, I. Zavodnik, B. Palecz, et al., J. Mol. Liq. 309, 113118 (2020). https://doi.org/10.1016/j.molliq.2020.113118
C. A. Dehelean, C. Soica, C. Peev, et al., Farmacia 59, 51 (2011).
O. V. Popova, V. V. Sursyakova, G. V. Burmakina, et al., J. Sib. Fed. Univ. Chem. 9, 171 (2016). https://doi.org/10.17516/1998-2836-2016-9-2-171-176
V. V. Sursyakova, N. G. Maksimov, V. A. Levdansky, and A. I. Rubaylo, J. Pharm. Biomed. Anal. 160, 12 (2018). https://doi.org/10.1016/j.jpba.2018.07.038
V. V. Sursyakova, V. A. Levdansky, and A. I. Rubaylo, Electrophoresis 40, 1656 (2019). https://doi.org/10.1002/elps.201800516
M. Olabi, M. Stein, and H. Wätzig, Methods 146, 76 (2018). https://doi.org/10.1016/j.ymeth.2018.05.006
P. Dubský, M. Dvořák, and M. Ansorge, Anal. Bioanal. Chem. 408, 8623 (2016). https://doi.org/10.1007/s00216-016-9799-y
M. T. Bowser and D. D. Y. Chen, J. Phys. Chem. A 103, 197 (1999). https://doi.org/10.1021/jp982917e
M. T. Bowser and D. D. Y. Chen, Anal. Chem. 70, 3261 (1998). https://doi.org/10.1021/ac9713114
V. A. Levdanskii, A. V. Levdanskii, and B. N. Kuznetsov, Chem. Nat. Compd. 53, 310 (2017). https://doi.org/10.1007/s10600-017-1976-5
V. A. Levdanskii, A. V. Levdanskii, and B. N. Kuznetsov, RF Patent No. 2614149 (2017).
V. A. Levdanskii, A. V. Levdanskii, and B. N. Kuznetsov, RF Patent No. 2638160 (2017).
T. le Saux, A. Varenne, and P. Gareil, Electrophoresis 26, 3094 (2005). https://doi.org/10.1002/elps.200500029
P. Dubský, M. Ördögová, M. Malý, and M. Riesová, J. Chromatogr., A 1445, 158 (2016). https://doi.org/10.1016/j.chroma.2016.04.004
Funding
This work was conducted within the framework of the budget project no. FWES-2021-0012 (registration no. 121031500209-6 in Unified State Accounting Information System of Research, Development, and Technological Work for Civil Purposes (EGISU NIOKTR)) for Institute of Chemistry and Chemical Technology SB RAS using the equipment of Krasnoyarsk Regional Research Equipment Centre of FRC KSC SB RAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Glyanchenko
Rights and permissions
About this article
Cite this article
Sursyakova, V.V., Levdansky, V.A. & Rubaylo, A.I. Determination of the Stability Constants of Supramolecular Complexes of Ester Derivatives of Betulin with Randomly Methylated β-Cyclodextrin by Affinity Capillary Electrophoresis. Russ. J. Phys. Chem. 97, 2225–2230 (2023). https://doi.org/10.1134/S0036024423100230
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423100230