Abstract
Direct calorimetry is used to study the heats of dissolution of crystalline glycyl-phenylalanine in water and solutions of potassium hydroxide at 298.15 K. The standard enthalpy of formation of glutathione in the crystalline state is calculated according to additive groups, based on group systematics with fragments classified in a manner similar to Benson’s approach, which considers the influence of the primary environment for atoms. The standard enthalpies of formation are calculated for glycyl-phenylalanine and products of its dissociation in an aqueous solution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
It is important to obtain new data on the enthalpies of dissolution of amino acids and dipeptides in water, since features of the solvation (hydration) of simple structural elements (amino acids and oligopeptides) largely determine the behavior of complex biosystems. It is not surprising that these compounds are of great interest to numerous researchers who consider them from various points of view [1–4].
There are reliable data in the literature on the constants of ionization of glycyl-phenylalanine (Table 1) [5–10]. These works were performed at different ionic strengths of the solution, against backgrounds of supporting electrolytes that differ in nature. We recalculated the values of pK1 and pK2 to zero ionic strength in order to compare constants obtained by different authors for the stepwise dissociation of the peptide.
Constants of the dissociation of glycyl-phenylalanine to zero ionic strength were recalculated using the Davis equation [11] (for I < 0.5)
and Eq. (2) (for I > 0.5):
where \({{\text{p}}{{K}^{c}}}\) and \({{\text{p}}{{K}^{o}}}\) are the negative logarithms of the constants of concentration and thermodynamic dissociation; \({{{\Delta }}{{Z}^{2}}}\) is the difference between the squares of the charges of the reaction products and initial materials; \(A\) is the constant of the limiting Debye law, equal to 0.5107 at 25°C; \(\delta \) is an empirical coefficient equal to 0.05; and \(I\) is the ionic strength of the solution (mol/L). The thermodynamic constants of stepwise dissociation were also determined graphically [12]:
After processing the literature data, we may assume the constants of stepwise dissociation are the most probable values of the constants of thermodynamic dissociation at 298.15 K: \({\text{p}}K_{1}^{^\circ }\) = 2.98 ± 0.03, \({\text{p}}K_{2}^{^\circ }\) = 8.13 ± 0.03.
The aim of this work was to determine the standard enthalpies of formation of glycyl-phenylalanine and products of its dissociation in an aqueous solution according to the thermal effects of dissolving the peptide in water and in aqueous solutions of KOH at 298.15 K.
EXPERIMENTAL
ReaChem crystalline glycyl-phenylalanine was used in this work. The content of the main component was 98.8% with no additional purification. Before use, the crystalline peptide was dried to constant weight at 353 K. The operation of the calorimetric setup [13, 14] was tested against a generally accepted calorimetric standard (the heat of dissolution of crystalline potassium chloride in water). The KCl preparation was purified via double recrystallization of the chemically pure reagent from bidistillate. Before sampling, the potassium chloride was dried to constant weight in an oven at 393.15 K. The match between experimentally obtained heats of dissolution KCl(cr.) in water ΔsolH(∞H2O) = 17.25 ± 0.06 kJ/mol and the most reliable published data [15] showed there was no notable systemic error in the operation of the calorimetric setup. Samples of the solutions were weighed on a VLR-200 balance with an accuracy of 2 × 10−4 g.
The confidence interval of the mean ΔH was calculated with a probability of 0.95. The equilibrium composition of the solutions was calculated using the RRSU program, allowing for the simultaneous occurrence of several processes of acid–base interaction and the dissociation of water [16].
RESULTS AND DISCUSSION
The dissolution of glycyl-phenylalanine in water can be described by the scheme
Standard enthalpies of formation of glycyl-phenylalanine in solution at different dilutions were calculated using the equation
where ΔfH°(HL±, cr., 298.15 K) is the standard enthalpy of formation of crystalline glycyl-phenylalanine, and ΔsolH(HL±, 298.15 K) is the heat of dissolution of the peptide (Table 2).
Standard enthalpies of the combustion and formation of glycyl-phenylalanine were calculated using the additive group approach [17–19], based on group systemics with a classification of fragments similar to Benson’s, which considers the influence of the initial environment for atoms. The enthalpies of combustion and formation of the test compound were calculated using the formula
where \({{\Delta }_{{{\text{c(f)}}}}}H_{i}^{^\circ }\) is the energy contribution to the heat of combustion and formation of a certain atomic group, \(H_{i}^{^\circ }\) is the number of such atomic groups in a molecule, and n is the number of types of atomic groups in a molecule.
The initial data for calculating \({{\Delta }_{{\text{f}}}}H_{{({\text{s}})}}^{^\circ }\)(C11H14N2O3) = −690.4 ± 1.9 kJ/mol and \({{\Delta }_{{\text{c}}}}H_{{({\text{s}})}}^{^\circ }\)(C11H14N2O3) = −5678.8 ± 1.9 kJ/mol are presented in Table 3.
Heat of combustion \({{\Delta }_{{\text{c}}}}H_{{({\text{s}})}}^{^\circ }\)(C11H14N2O3) = −5645.1 kJ/mol of the given dipeptide was determined experimentally in [20]. The energies of the combustion of the compound were associated with the combustion reaction, described by the general scheme
The enthalpy of formation of crystalline glycyl-phenylalanine was calculated using the formula
Standard enthalpies of formation of CO2 and H2О were taken from [21]: −ΔfH°(CO2, g, 298.15 K) = 393.51 ± 0.05 kJ/mol and −ΔfH°(H2O, l, 298.15 K) = 285.83 ± 0.04 kJ/mol. As a result, \({{\Delta }_{{\text{f}}}}H_{{({\text{s}})}}^{^\circ }\)(C11H14N2O3) = −684.3 kJ/mol.
Table 2 shows that the heat of formation of glycyl-phenylalanine in an aqueous solution in the considered range of concentrations is virtually independent of the degree of dilution, which is not surprising for such high values.
The standard enthalpy of formation of a glycyl-phenylalanine zwitterion in a hypothetically undissociated state upon final dilution in an aqueous solution was found using the equation
where α(H2L+), α(L−) are fractions of particles H2L+ and L−, respectively, calculated using the RRSU universal program [16]. The value of α(H2L+) varied from 0.00144 to 0.00234; that of α(L−), from 0.000534 to 0.000323 at the given concentrations. The thermal effects of the stepwise dissociation of particle H2L+ were ΔdisH(H2L+) = 1.7 ± 0.25 kJ/mol and ΔdisH(HL±) = 43.9 ± 0.32 kJ/mol.
Values of ΔdisH°(H2L+) and ΔdisH°(HL±) were determined in [21]. The total contribution from the second and third terms on the right side of Eq. (9) did not exceed 0.25 kJ/mol or change in the considered range of concentrations.
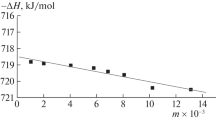
The standard enthalpy of formation of glycyl-phenylalanine in a hypothetical non-dissociated state at infinite dilution was found by extrapolating values obtained with Eq. (9) to the zero value of molality m of the solution (Fig. 1).
Least squares were used to find
The standard enthalpy of formation for particle L− in an aqueous solution was determined using data on the heat of dissolution of the peptide in alkali solutions at an equivalent ratio of at least 1 : 2 (Table 4). The dissolution of the peptide in a solution of KOH is described by the scheme
Calculations showed that reaction (10) was at least 99.9% complete.
Since Δz2 = 0 in reaction (10), the thermal effects of peptide dissolution at zero ionic strength were calculated using the equation [12]
where ΔrH(10) and \({{\Delta }_{{\text{r}}}}H_{{(10)}}^{^\circ }\) are thermal effects of process (10) at finite and zero values of the ionic strength.
We calculated the standard enthalpy of anion formation using the obtained values of \({{\Delta }_{{\text{r}}}}H_{{(10)}}^{^\circ }\) and definition ΔfH°(OH–, sol., H2O std. s., 298.15 K), ΔfH°(H2O, l, 298.15 K) recommended in [22]:
The standard enthalpy of formation of particle HL± in std. s., hip. non-diss. was also calculated using the equation
The standard enthalpy of formation of the peptide’s zwitterion agrees satisfactorily with the value obtained earlier. The weighted average value based on results from two independent determinations was assumed to be the one most likely: ΔfH°(HL±, sol., H2O std. s., hyp. undiss., 298.15 K) = −698.1 ± 1.9 kJ/mol.
The standard enthalpy of formation of particle H2L+ was calculated using the equation
CONCLUSIONS
Standard enthalpies of formation of glycyl-phenylalanine and products of its dissociation in an aqueous solution (Table 5) were obtained for the first time. They are the key quantities in the thermochemistry of the peptide and allow us to perform rigorous thermodynamic calculations for systems with glycyl-phenylalanine.
REFERENCES
A. Aragón-Muriel, M. Camprubí-Robles, E. González-Rey, et al., Polyhedron 80, 117 (2014).
I. Y. Váquiro-Reyes, A. Aragón-Muriel, and D. Polo-Cerón, Rev. Colomb. Cie. Quim.-Farm. 48, 557 (2019).
A. Aragón-Muriel, Y. Upegui, J. A. Muñoz, et al., Malaria Trypanosomias. Av. Quim. 11, 53 (2016).
C. Agoston, Z. Miskolczy, Z. Nagy, and I. Sovago, Polyhedron, No. 3, 2607 (2003).
M. Shoukry, E. Khairy, and A. El-Sherif, Trans. Met. Chem. 27, 656 (2002).
M. Nair and G. Subbalakshmi, Indian J. Chem. 39A, 468 (2000).
C. Agoston, T. Jankowska, and I. Sovago, J. Chem. Soc., Dalton Trans., 3295 (1999).
A. Kufelnicki, Pol. J. Chem. 66, 1077 (1992).
M. Jezowska-Bojczuk, H. Kozlowski, et al., Polyhedron 10, 2331 (1991).
G. Brookes and L. Pettit, J. Chem. Soc., Dalton Trans., 2106 (1975).
C. Davies, J. Chem. Soc., 2093 (1938).
V. P. Vasil’ev, Thermodynamic Properties of Electrolyte Solutions (Vyssh. Shkola, Moscow, 1982) [in Russian].
A. I. Lytkin, V. P. Barannikov, V. G. Badelin, and O. N. Krutova, J. Therm. Anal. Calorim. 139, 3683 (2020).
A. I. Lytkin, V. V. Chernikov, O. N. Krutova, and D. K. Smirnova, Russ. J. Phys. Chem. A 93, 1266 (2019).
D. G. Archer, J. Phys. Chem. Ref. Data 28, 1 (1999).
V. A. Borodin, V. P. Vasil’ev, and E. V. Kozlovskii, Mathematical Problems of Chemical Thermodynamics (Nauka, Novosibirsk, 1985), p. 219 [in Russian].
V. P. Vasil’ev, V. A. Borodin, and S. B. Kopnyshev, Zh. Fiz. Khim. 65, 55 (1991).
A. N. Kizin and Yu. A. Lebedev, Dokl. Akad. Nauk SSSR 262, 914 (1982).
A. V. Takhistov and D. A. Ponomarev, Organic Mass Spectrometry (VVM, St. Petersburg, 2002), p. 346 [in Russian].
V. V. Ponomarev, T. A. Alekseeva, and L. N. Akimova, Zh. Fiz. Khim. 36, 872 (1962).
T. Kiss and Z. Szucs, J. Chem. Soc., Dalton Trans., 2443 (1986).
Thermal Constants of Substances, Reference Book, Ed. by V. P. Glushko (VINITI, Moscow, 1965–1971), No. 3 [in Russian].
ACKNOWLEDGMENTS
This work was performed on equipment at the Ivanovo State Chemical Engineering University’s shared resource center with the support of the RF Ministry of Science and Higher Education, grant no. 075-15-2021-671.
Funding
This work was performed at the Research Institute of the Thermodynamics and Kinetics of Chemical Processes, Ivanovo State University of Chemistry and Technology, as part of State Task no. FZZW-2020-0009 (basic part).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krutova, O.N., Chernikov, V.V., Bychkova, S.A. et al. Standard Enthalpies of Formation of Glycyl-Phenylalanine and Products of Its Dissociation in an Aqueous Solution. Russ. J. Phys. Chem. 96, 1963–1967 (2022). https://doi.org/10.1134/S0036024422090187
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422090187