Abstract
An investigation is performed of the electrical conductivity of a number of dicationic ionic liquids based on 3‑methylpyridinium and inorganic anions in acetonitrile. The Lee–Wheaton procedure is used to calculate constants Ka of ion association, the limiting molar electrical conductivity (λ0), and the Gibbs energy of association (ΔG) in solutions. It is shown that the nature and size of the anion are a key influence on the association of the studied ionic liquids. It is established that dicationic 3-methylpyridinium salts with bromide anions are more associated in solution than corresponding hexafluorophosphates or tetrafluoroborates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The great interest in such classes of compounds as ionic liquids (ILs) is due to their having a wide range of properties (e.g., low saturated vapor pressure, high ionic conductivity, incombustibility, and good solvating ability) [1–3]. There has recently been a sharp rise in interest in using ionic liquids in the field of electrochemical research, as electrolytes of a new generation in storage batteries, as solar panels, and so on [4–6].
The tendency toward aggregation in solutions of ionic liquids depends on the molecular volume, the structure and nature of both the cation and the anion, and the concentration of the electrolyte [7]. The properties of ILs and solutions of them are also influenced by supramolecular structures that can form because of intermolecular interaction. It is not easy to predict the physicochemical properties of a specific IL, since they are influenced by an entire range of factors. The importance of knowing the molar conductivity and ionic association of ionic liquids drives extensive conductometric studies of IL, mainly in mixtures with molecular solvents in a wide range of concentrations. Results show that the structure of the cations and anions in the composition of ionic liquids strongly affects ionic association and molar conductivity at infinite dilution. Studying the dependences of electrical conductivity on concentration for IL solutions in molecular solvents therefore remains highly relevant.
The aim of this work was to study the electrical conductivity of solutions of dicationic pyridinium ionic liquids with inorganic anions, along with processes of their association in acetonitrile solutions.
EXPERIMENTAL
All of our compounds were synthesized and purified according to the procedure described in [8]. The synthesis scheme is as follows:

The structure of the compounds was confirmed by data from IR spectroscopy; their compositions, by data from elemental analysis. IR spectra were recorded on an ALPHA spectrometer in a thin film between KBr glasses for liquids, and in KBr pellets for solid compounds. Elemental analysis was performed on a PerkinElmerCHNS/O PE 2400-II analyzer. The electrical conductivity of IL solutions was measured on a Seven Go Pro MettlerToledeo conductometer at 25 ± 0.1°C in acetonitrile. The reagent grade acetonitrile was preliminarily dried by refluxing and subsequent distillation over P4O10. The purity of acetonitrile was monitored according to its specific electrical conductivity (κ25 = (1–3) × 10−8 S cm−1). The absence of halide ions, the most common impurities in ionic liquids, was monitored with a negative Beilstein test. All ionic liquids were dried to constant mass at 60°C in a vacuum. A series of solutions was prepared for each ionic liquid in the 10−4–10−2 mol/L range of concentrations. The EC of each solution was measured 5 times, and the average value was found. The specific EC was recalculated into an equivalent one according to a familiar formula.
The Lee–Wheaton procedure was used to calculate the constants of ion association, the limiting molar electrical conductivity (λ0), and the Gibbs energy of association (ΔG). The dependence of molar electrical conductivity on the concentration of the nature of the electrolyte and solvent in can generally be expressed as [9]
where c is the molar concentration of the electrolyte, mol/L; ε is the dielectric constant of the medium; R is the parameter of the closest approach of ions in the solution, Å; and Ka denotes constants of ionic association, L/mol.
In simplified form, the association of an ionic liquid in an acetonitrile solution is expressed by the equilibria
The constant of association is thus equal to the sum \(K_{{\text{a}}}^{1}\) + \(K_{{\text{a}}}^{2}\) + \(K_{{\text{a}}}^{3}\).
To determine the constant of ionic association and the limiting molar electrical conductivity (λ0) according to experimental conductometric data, we used the Lee–Wheaton equation [10–12] modified by Petibridge [13], and the second approximation of the Debye–Hückel theory:
where λ is the molar electrical conductivity (EC) of the electrolyte, S cm2 mol−1; λ0 is the limiting molar EC; α is the degree of dissociation of the electrolyte; and β = 2q, where q is Bjerrum’s critical distance, m;
The Debye parameter for electrolyte II–I is determined by the expression [13]
where c is the molar concentration of the electrolyte solution, mol/L; ε is the relative dielectric constant of the medium; ε0 is an electrical constant equal to 8.85419 × 10−12 m−3 K g–1s4 A2; k is the Boltzmann constant equal to 1.38065 × 10−23 J/K; and T is absolute temperature, K:
where F is the Faraday number, C/mol; e is the electron charge, C; η is the viscosity of the solvent, Pa s; and Cn = f(k, R) denotes coefficients expressed by nonlinear dependences [13]. In deriving Eq. (2) using the parameter of the closest approach of ions R we considered the possibility of solvation shells (Garney’s cosphers) forming around the ions [11].
The processing of experimental data was reduced to minimizing function F and finding unknown parameters Ka and λ0 [10]:
The problem of finding unknown parameters (Ka and λ0) requires us to solve a system of nonlinear equations: the concentration dependence of molar electrical conductivity, the law of mass action, the material balance, and the coefficients of average ionic activity.
For the maximum average ionic concentration proposed in [12] for the electrolyte, we can write \(C_{{{\text{max}}}}^{i}\) = 9.1 × 10−15(εT)3. Based on this, we used conductometric data on the 10−4–10−2 mol/L range of concentrations in our calculations. The experimental data were processed according to the procedure described in [10]. The Gibbs energy of the association was calculated using the formula \(\Delta G = - RT\ln {{K}_{{\text{a}}}}\). Our calculated results are presented in Table 1. Quantum-chemical calculations of the structure of cations in ionic liquids were made by optimizing all parameters at the B3LYP level in the 6-31G basis (d,p) using the GAMESS/Firefly program [14, 15].
RESULTS AND DISCUSSION
In ionic association, new charged particles emerge during the interaction of ions and participate in charge transfer, so the ratio between different ions is no longer stoichiometric and multiple. Considering the separate distribution of the ionic environment with respect to the central ion incorporated into the Lee–Wheaton equation when calculating the relaxation and electrophoretic effects of inhibition allows us to determine their individual parameters in describing nonstoichiometric mixtures of different ions in accordance with the accepted theoretical model of electrical conductivity. If the theoretical description is satisfactory, the optimized parameters should correspond to the values found in other ways, particularly by using experimental ion transport numbers. To study the processes of association in solutions, we must first establish the dependences of the equivalent EC on concentration.
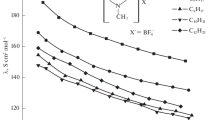
The equivalent electrical conductivity of dilute solutions of tetrafluoroborates of 3-methylpyridinium-based dications in acetonitrile falls as the concentration rises (Fig. 1). Ions of the opposite sign in a solution of an ionic liquid apparently form relatively stable aggregates (neutral subsystems) that cannot be charge conductors. A similar dependence is observed for aqueous solutions of electrolytes. 1,4-Bis(3-methyl-pyridinium-1-yl)butane tetrafluoroborate has the highest electrical conductivity of the studied tetrafluoroborates in the given range of concentrations, while 1,3-bis(3-methylpyridinium-1-yl)propane tetrafluoroborate has the lowest. The values of the equivalent EC are in the range 150–310 S cm2 mol−1.
As for tetrafluoroborates of 3-methylpyridinium-based dications, a similar dependence of the equivalent EC of solutions on concentration is characteristic of hexafluorophosphates (Fig. 2). It should be noted, however, that the electrical conductivity of solutions of hexafluorophosphates with the same cations is not much higher than that of tetrafluoroborates. At a concentration of 6 mmol/L, the equivalent EC values are in the range of 190–285 S cm2 mol−1. This effect could be due to the electrostatic interactions of the organic cation with the hexafluorophosphate anion being hindered by the fairly large radius of the latter. The formation of hydrogen bonds upon an increase in the size of the anion is also difficult, which agrees with the known effect the size of the anion has on the strength of the formation of hydrogen bonds with the cation in ionic liquids [16, 17]. The energy of hydrogen bonds falls as the size of the anion grows. For comparison, we studied the electrical conductivity of solutions of bromides of 3-methylpyridinium-based dications (Fig. 3), which were precursors for the preparation of ionic liquids. The electrical conductivity of their solutions in this range of concentrations is much lower than those of the corresponding hexafluorophosphates and tetrafluoroborates.
Based on the results from molecular dynamics studies [18], it was found that such aprotic solvents as acetonitrile interact more strongly with readily polarizable ions (e.g., PF6) by means of ion–dipole interactions. In contrast, bromide ions will have weaker interactions with solvent molecules, due to their smaller size and higher charge density, and exhibit stronger ion–ion interactions with an organic cation. The values of the constants of ion association for halides are therefore higher than for tetrafluoroborates and hexafluorophosphates with the same cation. This pattern is clearly seen in Table 1. Stronger ion–dipole interactions are characteristic of hexafluorophosphates, where the anion is most polarizable. This reduces the association constants in a solution, along with the tendency to association for the compounds with the same cation in the series Br− > \({\text{BF}}_{4}^{ - }\) > \({\text{BF}}_{6}^{ - }\) . Similar results have been obtained in studying the electrical conductivity of dilute solutions of imidazolium ionic liquids in acetonitrile [7, 19] and ammonium ILs in other solvents [20, 21]. As noted above, higher values of the association constants for bromides could be a consequence of the formation of stronger intermolecular hydrogen bonds between the organic cation and the bromide anion, along with stronger electrostatic interaction. We recorded the IR spectra of 1,3-bis(3-methyl-pyridinium-1-yl)propane bromide, hexafluorophosphate, and tetrafluoroborate to confirm this hypothesis (Fig. 4). A shift of the absorption band to longer wavelengths is observed in the region of stretching vibrations of the C–H bonds of the aromatic ring (3100–3020 cm−1), due to an increase in C–H bond lengths as a result of H atoms participating in hydrogen bonding with the bromide anion. This displacement is greatest for 1,3-bis(3-methylpyridinium-1-yl)propane bromide, indicating the high energy of hydrogen bonding. The authors of [22] came to similar conclusions.
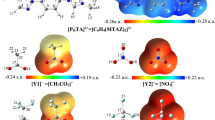
It should also be noted that regardless of the nature of the anion, compounds with a linker of three carbon atoms in the organic cation have the lowest equivalent EC in the studied range of concentrations. This effect could be associated with the specific molecular structure of the 1,3-bis(3-methylpyridinium-1-yl)propane cation, which reduces its mobility in solution. Our quantum-chemical calculations of the geometry of the cations (Fig. 5) of the studied ionic liquids show that the 1,3-bis(3-methylpyridinium-1-yl)propane cation has a different spatial structure than others (the arrangement of two cyclic 3-methyl-pyridinium fragments at an angle of 115° to each other; i.e., in a cis-configuration). This structure of the cation favors a closer approach with the anion, which increases the energy of interaction for the ion pair, due to electrostatic interaction and the formation of hydrogen bonds. The data in Table 1 show that 1,3-bis(3-methylpyridinium-1yl)propane bromide has the highest association constant of the studied compounds.
The range of the Gibbs energies of association was from −9.9 to –15.3 kJ/mol for all of the studied compounds. Comparing the association constants and the Gibbs energies of association, we may conclude the most associated ionic liquid of those we studied was 1,3-bis(3-methylpyridinium-1-yl)propane bromide.
CONCLUSIONS
An investigation was performed on the electrical conductivity of the solutions a number of 3-methyl-pyridinium-based dicationic ionic liquids with inorganic anions in acetonitrile. The electrical conductivity of solutions of hexafluorophosphates with the same cations was found to be higher than that of tetrafluoroborates and bromides.
It was shown that the nature and size of the anion have a key effect on the association of the studied ionic liquids. The tendency to associate in acetonitrile solutions falls in the series Br− > \({\text{PF}}_{4}^{ - }\) > \({\text{PF}}_{6}^{ - }\) for the salts with the same cation.
It was found that the solutions of the compounds with 1,3-bis(3-methylpyridinium-1-yl)propane cation had the lowest electrical conductivity, due to features of its molecular structure.
REFERENCES
J. P. Hallett and T. Welton, Chem. Rev. 111, 3508 (2011). https://www.doi.org/10.1021/cr1003248
T. Torimoto, T. Tsuda, K. I. Okazaki, and S. Kuwabata, Adv. Mater. 22, 1196 (2010). https://www.doi.org/10.5772/52597
D. D. Patel and J. M. Lee, Chem. Rec. 12, 329 (2012). https://www.doi.org/10.1002/tcr.201100036
A. P. Abbott and K. J. McKenzie, Phys. Chem. Chem. Phys. 8, 4265 (2006). https://www.doi.org/10.1039/B607329H
L. Gre, E. Paillard, G. T. Kim, et al., Int. J. Mol. Sci. 15, 8122 (2014). https://www.doi.org/10.3390/ijms15058122
H. Sakaebe, H. Matsumoto, and K. Tatsumi, Electrochim. Acta 53, 1048 (2007). https://www.doi.org/10.1016/j.electacta.2007.02.054
O. N. Kalugin, Iu. V. Voroshylova, A. V. Riabchunova, et al., Electrochim. Acta 105, 188 (2013). https://doi.org/10.1016/j.electacta.2013.04.140
O. E. Zhuravlev, L. I. Voronchikhina, and K. P. Gerasimova, Russ. J. Gen. Chem. 86, 2606 (2016). https://www.doi.org/10.1134/S1070363216120069
V. L. Chumak, M. R. Maksimyuk, T. V. Neshta, et al., Vost.-Evrop. Zh. Pered. Tekhnol. 62 (2/5), 59 (2013).
W. H. Lee and R. J. Wheaton, J. Chem. Soc. Faraday Trans. II 74, 743 (1978). https://www.doi.org/10.1039/F29787400743
W. H. Lee, and R. J. Wheaton, J. Chem. Soc. Faraday Trans. II 74, 1456 (1978). https://www.doi.org/10.1039/F29787401456
W. H. Lee, and R. J. Wheaton, J. Chem. Soc. Faraday Trans. II 75, 1128 (1979). https://www.doi.org/10.1039/f29797501128
A. D. Pethybridge and S. S. Taba, J. Chem. Soc. Faraday Trans. I 76, 368 (1980). https://www.doi.org/10.1039/F19807600368
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, et al., J. Comput. Chem. 14, 1347 (1993). https://www.doi.org/10.1002/jcc.540141112
A. A. Granovsky, PC GAMESS/Firefly version 8.1. http://classic.chem.msu.su/gran/gamess/index.html.
N. V. Sastry, N. M. Vaghela, P. M. Macwan, et al., J. Colloid Interface Sci. 137, 52 (2012). https://www.doi.org/10.1016/j.jcis.2011.12.077
R. Jan, G. M. Rather, and M. A. Bhat, J. Solution Chem. 42, 738 (2013). https://www.doi.org/10.1007/s10953-013-9999-4
C. G. Hanke, N. A. Atamas, and R. M. Lynden-Bell, Green Chem. 4, 107 (2002). https://doi.org/10.1039/b109179b
O. E. Zhuravlev, Russ. J. Phys. Chem. A 95, 298 (2021). https://www.doi.org/10.1134/S0036024421020308
B. Ramsauer, M. M. Meier, R. Neueder, et al., J. Acta Chim. Slov. 56, 30 (2009).
D. Das, B. Das, and D. K. Hazra, J. Solut. Chem. 32, 77 (2003). https://www.doi.org/10.1023/A:1022648916138
Y. Gao, L. Zhang, Y. Wang, et al., J. Phys. Chem. B 114, 2828 (2010). https://www.doi.org/10.1021/jp910528m
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhuravlev, O.E. Effect of the Structure of Dicationic Pyridinium Ionic Liquids on Processes of Ionic Association and the Electrical Conductivity of Their Solutions in Acetonitrile. Russ. J. Phys. Chem. 95, 2503–2508 (2021). https://doi.org/10.1134/S0036024421120244
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421120244