Abstract
A study is performed of the electrical conductivity of a number of ionic liquids based on 1-alkyl-3-methylimidazolium quaternary salts with inorganic anions in acetonitrile. The Lee–Wheaton approach is used to calculate the ion association constants Ka, limiting molar electrical conductivity (λ0), and the Gibbs energy of association (ΔG) in solutions. It is shown that the nature and size of the anion has a decisive influence on the association of the studied ionic liquids. It is found that the limiting molar electrical conductivity of bromides is lower than that of hexafluorophosphates and tetrafluoroborates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The number of studies on ionic liquids (IL) is constantly growing. In the search for new areas of application for ionic liquids, existing applied aspects of the use of ILs are being expanded and improved with new fundamental knowledge about the relationship between the structure of ILs and their properties. Ionic liquids are already being used in such areas of science as electrochemistry, separation, processes of synthesis and catalysis, and in pharmaceutical industry [1–5]. Interest in this class of compounds is due to ILs having a wide range of such properties as low saturated vapor pressure, high electrical conductivity, incombustibility, and good solvating ability for a number of polar and nonpolar compounds [6–8]. ILs are increasingly used in the field of electrochemical research and applied aspects for their use as new generation electrolytes in, e.g., storage batteries and solar panels [9–12]. Conductometric analysis can be used to obtain valuable information on the ionic association and solvation of electrolytes [13]. Data on the molar conductivity and ionic association of ionic liquids are required for extensive conductometric studies of ILs, mainly in mixtures with molecular solvents in a wide range of concentrations. Results show that the structures of the cations and anions in the composition of ionic liquids strongly affects ionic association and molar conductivity at infinite dilution [13–17]. It is therefore an important task to study the dependences of electrical conductivity (EC) on concentration for solutions of ionic liquids in molecular solvents, along with the behavior of ions in solutions.
The aim of this work was to study the electrical conductivity of imidazolium ionic liquids with inorganic anions, and the processes of their association in acetonitrile solutions.
EXPERIMENTAL
All compounds were synthesized and purified according to the procedure described in [18]. The structure of the compounds was confirmed by IR spectroscopy, while their composition was determined via elemental analysis. IR spectra were recorded on an ALPHA spectrometer in a thin film between KBr glasses for liquids and in KBr pellets for solid compounds. Elemental analysis was performed on a PerkinElmerCHNS/O PE 2400-II unit. Electrical conductivity of the IL solutions in acetonitrile at 25 ± 0.1°C was measured on a Seven Go Pro MettlerToledeo conductometer. Acetonitrile (analytical grade) was preliminarily dried by refluxing with subsequent distillation over P4O10. A series of solutions were prepared for each ionic liquid in the 10−4–10−2 mol/L range of concentrations. The EC of each solution was measured 5 times, and the average value was determined. The specific EC was recalculated into its equivalent according to the familiar formula.
The Lee–Wheaton approach was used to calculate the ion association constants, the limiting molar electrical conductivity (λ0), and the Gibbs energy of association (ΔG). The dependence of molar electrical conductivity on the concentrations of the electrolyte and solvent can generally be expressed as [19]
To determine the ionic association constant (Ka) and the limiting molar electrical conductivity (λ0) from the experimental conductometric data, we used the Lee–Wheaton equation [20–22] modified by Petibridge [23] and a second approximation of the Debye–Hückel theory:
In expression (2), λ is the molar electrical conductivity of the electrolyte, S cm2/mol; λ0 is the limiting molar EC; and α is the degree of dissociation of the electrolyte;
q is Bjerrum’s critical distance, m. The value of the Debye parameter for the I–I electrolyte is determined by the expression [23]
where c is the molar concentration of the electrolyte solution, mol/L; ε is the relative dielectric constant of the medium; and T is the absolute temperature, K:
where F is the Faraday number, C/mol; e is the electron charge, C; η is the solvent’s viscosity, Pa s; and Cn = f(k, R) denotes coefficients expressed by nonlinear dependences [23]. When deriving Eq. (2) using parameter R of the closest approach of ions, we considered the possibility of solvation shells (Gurney cospheres) forming around the ions [21].
The processing of experimental data was reduced to minimizing function F and finding unknown parameters Ka and λ0 [20]:
The problem of finding unknown parameters (Ka and λ0) consists of solving a system of nonlinear equations: the concentration dependence of molar electrical conductivity, the law of mass action, the material balance, and average ionic activity coefficients.
We can write \(C_{{{\text{max}}}}^{i}\) = 9.1 × 10–15(εT)3 for the maximum average ionic concentration of the electrolyte proposed in [22]. We then use the conductometric data in the 10−4–10−2 mol/L range of concentrations in subsequent calculations. The experimental data were processed as in [20]. The results from calculations are presented in Table 1. The Gibbs energy of association was calculated using the formula ∆G = −RT ln Ka.
RESULTS AND DISCUSSION
To study the behavior of ionic liquids in solutions, we performed a conductometric study of solutions of imidazolium ionic liquids in acetonitrile. Based on conductometric measurements, the dependences of the equivalent electrical conductivity on the concentration were plotted.
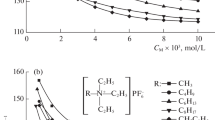
Similar behavior in a solution was characteristic of the 1-alkyl-3-methylimidazolium hexafluorophosphates we studied (Fig. 2). It should be noted that the electrical conductivity of hexafluorophosphates was slightly lower than that of tetrafluoroborates with the same cations.
For comparison, we studied the electrical conductivity of solutions of 1-alkyl-3-methylimidazolium bromides (Fig. 3), precursors for the preparation of ionic liquids. The equivalent EC of their solutions in acetonitrile fell monotonically as the concentration rose. It should be noted that all of the studied compounds, which included the 1-butyl-3-methylimidazolium cation, had the highest equivalent EC in this range of concentrations. This effect was apparently due to the smaller size and higher symmetry of the 1‑butyl-3-methylimidazolium cation, compared to the others studied. This increased its mobility in a solution and thus its electrical conductivity.
When comparing the association constants (Table 1) for ILs with the same cation, there was a decline in the constants upon moving from bromide to hexafluorophosphate anion. Tetrafluoroborates and hexafluorophosphates of 1-alkyl-3-methylimidazolium had close values of the association constants, which coincided within the permissible error for most of the studied compounds. This agrees with the effect the size of the anion has on the formation of hydrogen bonds with the cation [24, 25]: the energy of hydrogen bonds falls as the anion grows. The simple bromide anion is held more strongly by the cation, so compounds containing the bromide anion will dissociate less in a solution. In contrast, the bulkier BF\(_{4}^{ - }\) and PF\(_{6}^{ - }\) are more difficult for the cation to retain; as a result, ILs that contain them will dissociate more. Using IR spectroscopy and quantum-chemical calculations, the authors of [26] established that strong electrostatic interactions predominate for such simple anions as halogens and OH−, while hydrogen bonding is observed for more complex ones (particularly PF\(_{6}^{ - }\) and BF\(_{4}^{ - }\)). This explains the lower limiting EC of 1-alkyl-3-methylimidazolium bromides, relative to hexafluorophosphates and tetrafluoroborates. This is also confirmed by the limiting molar ECs for 1-alkyl-3-methylmidazolium bromides being lower than for hexafluorophosphates and tetrafluoroborates.
CONCLUSIONS
Comparing the values of the association constants for ILs with the same name PF\(_{6}^{ - }\) anion, we can see that the values for all studied compounds coincide within the permissible error. The nature and structure of the anion thus have a decisive influence on the process of ionic association. The authors of [27], who studied the association of a number of imidazolium and pyridinium ILs in acetonitrile, came to a similar conclusion.
The range of Gibbs energies of association was −9.0 to −10.7 kJ/mol for all of the studied compounds. Comparing the association constants and the Gibbs energies of association, we may conclude that the most associated ionic liquid of those studied was 1-decyl-3-methylimidazolium bromide. The highest electrical conductivity of the studied tetrafluoroborates (Fig. 1) in this range of concentrations was found for 1-butyl-3-methylimidazolium tetrafluoroborate; the lowest, for 1-decyl-3-methylimidazolium tetrafluoroborate. The equivalent EC values were 115–190 S cm2 mol−1. The equivalent electrical conductivity of dilute solutions of tetrafluoroborates in acetonitrile fell as their concentrations rose. Ions of opposite signs in a solution of an ionic liquid apparently form relatively stable aggregates (neutral subsystems) that cannot be charge conductors. A similar dependence was observed for aqueous solutions of electrolytes.
REFERENCES
J. P. Hallett and T. Welton, Chem. Rev. 111, 3508 (2011). https://doi.org/10.1021/cr1003248
H. Zhao and S. V. Malhotra, Aldrichim. Acta 35, 75 (2002). https://doi.org/10.1155/2014/729842
H. Olivier-Bourbigou, L. Magna, and D. Morvan, Appl. Catal., A 373, 1 (2010). https://doi.org/10.1016/j.apcata.2009.10.008
R. Priambodo, T. C. Chen, M. C. Lu, et al., Energy Proc. 75, 84 (2015).https://doi.org/10.1016/j.egypro.2015.07.143
H. J. Kim and Y. Shim, ACS Nano 3, 1693 (2009). https://doi.org/10.1021/nn900195b
M. Watanabe, H. Tokuda, S. Tsuzuki, et al., J. Phys. Chem. B 110, 19593 (2006). https://doi.org/10.1021/jp064159v
V. V. Chaban, I. V. Voroshylova, and O. N. Kalugin, Phys. Chem. Chem. Phys. 13, 7910 (2011). https://doi.org/10.1039/c0cp02778b
E. H. Duan, B. Guo, M. M. Zhang, et al., J. Chem. Eng. Data 55, 4340 (2010). https://doi.org/10.1021/je100361s
A. P. Abbott and K. J. McKenzie, Phys. Chem. Chem. Phys. 8, 4265 (2006).https://doi.org/10.1039/B607329H
R. Kawano, H. Matsui, C. Matsuyama, et al., J. Photochem. Photobiol. A 164, 87 (2004). https://doi.org/10.1016/j.jphotochem.2003.12.019
L. Grande, E. Paillard, G. T. Kim, et al., Int. J. Mol. Sci. 15, 8122 (2014). https://doi.org/10.3390/ijms15058122
H. Sakaebe, H. Matsumoto, and K. Tatsumi, Electrochim. Acta 53, 1048 (2007). https://doi.org/10.1016/j.electacta.2007.02.054
M. B. Foreiter, H. Q. N. Gunaratne, P. Nockemann, et al., Phys. Chem. Chem. Phys. 16, 1208 (2014). https://doi.org/10.1039/c3cp53472c
E. Duan, Y. Guan, B. Guo, et al., J. Mol. Liq. 178, 1 (2013). https://doi.org/10.1016/j.molliq.2012.10.026
Q.-S. Liu, P.-P. Li, U. Welz-Biermann, et al., J. Chem. Thermodyn. 66, 88 (2013). https://doi.org/10.1016/j.jct.2013.06.008
J. Vila, B. Fernández-Castro, E. C. Rilo, et al., Fluid Phase Equilib. 320, 1 (2012). https://doi.org/10.1016/j.fluid.2012.02.006
J. Leys, C. S. P. Tripathi, C. Glorieux, et al., Phys. Chem. Chem. Phys. 16, 10548 (2014). https://doi.org/10.1039/C4CP00259H
O. E. Zhuravlev, L. I. Voronchikhina, and K. P. Gerasimova, Russ. J. Gen. Chem. 86, 2606 (2016).
V. L. Chumak, M. R. Maksimyuk, T. V. Neshta, et al., Vost.-Evrop. Zh. Pered. Tekhnol. 62 (2/5), 59 (2013).
W. H. Lee and R. J. Wheaton, J. Chem. Soc., Faraday Trans. 74, 743 (1978). https://doi.org/10.1039/F29787400743
W. H. Lee and R. J. Wheaton, J. Chem. Soc., Faraday Trans. 74, 1456 (1978). https://doi.org/10.1039/F29787401456
W. H. Lee and R. J. Wheaton, J. Chem. Soc., Faraday Trans. 75, 1128 (1979). https://doi.org/10.1039/f29797501128
A. D. Pethybridge and S. S. Taba, J. Chem. Soc., Faraday Trans. 76, 368 (1980). https://doi.org/10.1039/F19807600368
J. Leys, M. Wubbenhorst, C. P. Menon, et al., J. Chem. Phys. 128, 64509 (2008). https://doi.org/10.1063/1.2827462
J. Leys, R. N. Rajesh, P. C. Menon, et al., J. Chem. Phys. 133, 34503 (2010). https://doi.org/10.1063/1.3455892
Y. Gao, L. Zhang, Y. Wang, et al., J. Phys. Chem. B 114, 2828 (2010). https://doi.org/10.1021/jp910528m
Iu. V. Voroshylova, E. A. Dakhova, V. V. Chaban, et al., Kharkov Univ. Bull. 895 (18), 159 (2010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhuravlev, O.E. Effect of the Structure of Imidazolium Ionic Liquids on the Electrical Conductivity and Processes of Ionic Association in Acetonitrile Solutions. Russ. J. Phys. Chem. 95, 298–302 (2021). https://doi.org/10.1134/S0036024421020308
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421020308