Abstract
Real-time thermal analysis is used for the synthesis of silica globules. The rate of the reaction of sol synthesis is determined. The reaction order is found, depending on the concentration of water, and the kinetic and diffusion regions are identified. Dried (not annealed) samples are studied via DSC. The dynamics of ethanol and water evolution from the samples is studied in analyzing the heating and cooling curves. Structural phase transformations of the formation of β-cristobalite and its transition to the α-form are observed. The dependence of the amorphousness of the sample on the amount of water in the solid phase is determined. It is found that when the water–TEOS molar ratio is less than fifty, the process proceeds slowly and hydrolysis is incomplete. An explanation of this pattern is proposed. A reaction equation for the kinetic region is determined for the case when the indicated molar ratio is more than 100. A general chemical formula for the main structure of a globule’s core is proposed, with allowance for the presence of cristobalite fragments in all samples. Based on an analysis of this formula, a condition is found for the case when the hexagonal ring cannot be the basis of the structure, and the amount of chemically retained water can be determined. A stoichiometric formula for the product that forms in the diffusion region is obtained.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Among the numerous applications of silicon dioxide, the ones most promising in the field of medicine are as a precursor of microcapsules for the initiated release of cytotoxic drugs [1], thickeners of dispersed media, sorbents and crystallizing reagents [2], and aerogels and UV filters [3].
The variety of technological parameters for the synthesis of silica (the type and concentration of the system components, the order and rate of their mixing, the type and rate of the reaction system’s mixing, the temperature and thermodynamic mode of synthesis, the presence of electromagnetic fields, and the shape and functionality of the reactor) offers a huge range of options for solving the problem of synthesizing nanoglobules of silica.
The most important problem is therefore real-time control of synthesis, which allows us to reveal details of the multistage process and make initial assessments of the parameters of the internal structure and composition of globules.
EXPERIMENTAL
We used thermal analysis to obtain the kinetic curve of synthesis in the real-time mode by controlling the processes in the liquid phase. The selected process parameters were the standard set of components for preparing silica sol: extra pure Si(OC2H5)4 : C2H5OH : NH4OH : H2О, and distilled water [4, 5]. The speed of mixing was 450 rpm [6]. Stirring was done on a magnetic stirrer with a Teflon-coated magnetic stirring bar. The reactor was a 100 mL glass beaker. The temperature was measured with a platinum–rhodium thermocouple enclosed in a stainless steel casing. The accuracy of measuring temperature was 0.01°C. The series of experiments was performed at a temperature of 24.5 ± 3°C under quasi-adiabatic conditions.
First, two precursor solutions were prepared. The calculated amount of ethanol was halved, and TEOS was added to the first half. An organic precursor solution formed upon stirring. Water and ammonia were added to the second half, and an inorganic precursor solution formed upon stirring. The process was conducted in the inorganic phase, while controlling the temperature at a fixed rotation speed of the magnetic stirrer. This allowed us to homogenize the system at the stage of mixing the components. The molar ratios of the system’s components are presented in Table 1.
In this manner, we gradually replaced the solvent (ethanol) with the reagent (water) and calculated the rate of the process by analyzing the graphs of heat release.
Figure 1 shows a typical thermal analysis (TA) kinetic curve of the onset of synthesis. The solid curve is the temperature controlled in the reactor, and the dotted line is the temperature of the organic phase. The tangent to the graph is drawn at the point of the maximum rate of temperature growth. The time and temperature intervals corresponding to the stage of the exothermic process of polycondensation (explosive nucleation) are noted [7].
A number of assumptions were used in processing the TA plots (Fig. 2) and calculating the rate of nanocluster synthesis.
The multi-stage process of hydrolysis and polycondensation can be formally described by a simple thermochemical equation (gross reaction):
where ΔrH is the thermal effect of the reaction.
Let the depth of the hydrolysis and polycondensation processes be complete; i.e., the product of the reaction will not change when the composition of the system changes from experiment to experiment. To estimate the rate of silica sol synthesis, assuming the composition of the system changes negligibly during synthesis (the solid phase is 1.2 wt %), we can use the formula
where r is the rate of the reaction, mol/(m3 s); R is the gas constant; \({{{v}}_{j}}\) and \({{C}_{p}}_{{,j}}\) are the number of moles and heat capacity of the jth component of the system; T is temperature; and t is time. Processing the experimental data allowed us to relate the growth rate of the solid phase to the concentration of water in the system. The resulting dependence is described by the second-order polynomial
This suggests the complex nature of the processes that occur in the system.
Analyzing our results, we found that in the initial section of the graph (the range of water concentrations corresponding to 8–10 M), the order of the process with respect to water can be taken equal to 1. At average water concentrations, the order changes smoothly from 1 to 2, and it remains constant and equal to 2 at water concentrations above 22 M. A further increase in the concentration of water has no appreciable effect on the rate of the process.
The formal kinetics equations with respect to water concentration differ, depending on the region of the process. In the diffusion region with respect to water (when \({{C}_{{{{{\text{H}}}_{{\text{2}}}}{\text{O}}}}}\) < 10 M), \(r = k{{C}_{{{{{\text{H}}}_{2}}{\text{O}}}}}\). In the kinetic region (when \({{C}_{{{{{\text{H}}}_{{\text{2}}}}{\text{O}}}}}\) > 22 M), \(r = kC_{{{{{\text{H}}}_{2}}{\text{O}}}}^{2}\). The data on the rate of the reaction with alkaline catalysis of the process (pH ≈12) are in good agreement with the literature data [8].
After the synthesis of the sol was complete (2 h from the moment of mixing the phases), the reaction vessels were placed in an isolated thermostat with forced circulation of the gas phase. A constant temperature of 25 ± 1°C was maintained for 1.5 months, until complete precipitation of the sol and evaporation of the liquid phase.
Non-annealed samples were examined via differential scanning calorimetry (DSC) on a Pegasus DSC 404 F1 device.
Figure 3 shows the DSC heating and cooling curve for sample 1 of the composition.
A sample of the second composition had a similar form of curves. The structure of the first endothermic peak (Fig. 3) separated by another fragment corresponds to the evaporation of (a) adsorbed alcohol, (b) water, and (c) TEOS held in the cavities of the solid phase during synthesis. The boiling points of these components are 78, 100, and 167°C, respectively. The endothermic peak corresponds to the release of chemically bound alcohol in the composition of the unhydrolyzed TEOS. The anomalous temperature dependence of the heat capacity of the samples testifies to their amorphous nature. However, the presence of a high-temperature peak at 1200°C on the heating graph indicates a transition of part of the disordered structure to β-cristobalite. This transition occurs irreversibly, as is confirmed by the cooling curves, on which we observe a low-temperature (210°C) peak corresponding to the β → α transition of cristobalite. On the DSC curve of the third sample (Fig. 4), the endothermic effect of the decomposition of unhydrolyzed TEOS is noticeably reduced (the weak d-peak). Upon cooling, the sample lost its original structure. With repeated heating, it displayed a completely vitrified amorphous composition and no cristobalite structural elements. Figure 5 shows the DSC curves of composition 4. The curves of compositions 5–8 have a similar form. The DSC graphs of compositions 4–8 differ substantially from the curves of the first three compositions by the absence of endothermic effect d, the depth of the anomalous dependence of the heat capacity on temperature (often referred to as the glass transition process in the literature [9]) and the general slope of the 0-line. Common to all of the obtained DSC curves is the presence in the heating curves of a high-temperature phase transition of amorphous silica to β-cristobalite.
Estimating the amount of water in the solid phase from the anomalous behavior of the heat capacity’s temperature dependence [10], we plotted the effect the composition of synthesis had on the content of water in the samples. The data are presented in Fig. 6. The greatest amount of captured water was contained in the sample of the third composition (about 53 wt %), which turned out to be the most amorphous data from DSC analysis.
RESULTS AND DISCUSSION
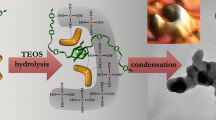
Analysis of gross reaction Eq. (1) and the data in Table 1 shows that a two-fold excess of the concentration of water over that of TEOS is not enough to conduct the process of sol synthesis. DSC data revealed a considerable amount of water in the solid phase. Compositions 1–3 also contained chemically bound alcohol. When n < 50, the process proceeded slowly in the diffusion region with respect to water. Even hydrolysis was incomplete, though the excess of water molecules relative to stoichiometric requirements is substantial. This is explained by the water being bound by Van der Waals forces (hydrogen bonds) to the polar molecules of ammonium hydroxide and alcohol. Like water molecules, ammonia molecules are polar; they can contain up to four water molecules in the first hydration shell, thereby binding the water. TEOS, which has terminal ethyl groups, is solvated by ethanol molecules. It is in this form that it is created in the organic phase, before being added to an inorganic one. This mutual arrangement of the components of the system slows the hydrolysis of TEOS and contributes to its incompleteness. Polycondensation can follow incomplete hydrolysis. These processes are also complicated by the small number of hydroxyl groups in the composition of TEOS molecules. Hydrolysis for the remaining alcohol groups can also proceed in parallel with polycondensation, so the process cannot be described by the formal Eq. (1) alone.
Under real conditions, the chemistry is a complex combination of several chemical and physical processes: hydrolysis, homogeneous and heterogeneous polycondensation, aggregation during sol coagulation, and recondensation at high pH. Reaction (1) can be written in the following form, allowing for the water that is part of the solid phase:
where 2 < m < 4, and 2n < m < 4n.
Equation (4) can be used for samples obtained under kinetic mode synthesis conditions when n > 100. Ethanol must also be included in the sediment formula when considering processes at the ratio n < 50 of the concentration of water to TEOS.
Since all samples contain cristobalite fragments that are based on two hexagonal rings displaced relative to each other by one radius and linked by two siloxane bonds [11], we may assume this structure is the basis of that of globule cores with the common chemical formula
where n = 1, 2, 3 is the number of rings in the silica cluster; m and l are the numbers of molecules of chemisorbed water and alcohol in the cluster; and \(m = 4n + 2 - l\).
A hexagonal ring cannot be the basis of the structure for a sample of composition 3, since a ring cannot form when 53% of the composition is chemically bound water. The maximum allowable amount of water when n = 1 and l = 0 is 30 wt %. All other samples may well have a hexagonal structure, especially since the amount of chemically retained water falls upon raising the ratio of the concentrations of water to TEOS and stabilizes at a level of 20 wt %.
Since the mass fraction of chemisorbed alcohol for compositions 1 and 2 is (1.5–2)% according to DSC data, the formula of the product obtained in the diffusion region can be written as \({{({\text{Si}}{{{\text{O}}}_{2}})}_{6}}{{({{{\text{H}}}_{2}}{\text{O}})}_{{9.75}}}{{({{{\text{C}}}_{2}}{{{\text{H}}}_{5}}{\text{OH}})}_{{0.25}}}\). In other words, every fourth to sixth ring contains an unhydrolyzed alcohol molecule, so 96% of all silicon bonds were hydrolyzed. Starting from the fourth, the compositions undergo complete hydrolysis and are described by the formula \({{({\text{Si}}{{{\text{O}}}_{2}})}_{{6n}}}{{({{{\text{H}}}_{2}}{\text{O}})}_{m}}\). The compositions are approximately the same when n = 2 (two combined hexagonal rings), and their chemical formula is \({{({\text{Si}}{{{\text{O}}}_{2}})}_{{12}}}{{({{{\text{H}}}_{2}}{\text{O}})}_{{10}}}\). Siloxane bonds thus form in around 58% of all possible cases.
Our findings validate the assumptions made in processing the data from thermal analysis, and the possibility of using formula (2) to calculate the rate of the synthesis process.
A general chemical formula for the basic structure of the globule core was proposed. It was found that neither the hexagonal ring nor the amount of chemically retained water can be the basis of the structure. A stoichiometric formula was obtained for the products that form in the diffusion and kinetic regions of the process.
REFERENCES
M. Ali, S. P. Meaney, M. J. Abedin, et al., J. Colloid Interface Sci. 552, 528 (2019).
Y. Li, L. Zhang, and C. Li, J. Colloid Interface Sci. 559, 273 (2020).
G. Zaccariello, M. Back, A. Benedetti, et al., J. Colloid Interface Sci. 549, 1 (2019).
G. Kolbe, PhD Thesis (Jena, 1956).
W. Stöber, A. Fink, and E. Bohn, J. Colloid Interface Sci. 26, 62 (1968).
M. N. Galinurova and A. I. Rykova, Vestn. Kurgan. Univ., No. 4, 86 (2005).
V. K. LaMer and R. H. Dinegar, J. Am. Chem. Soc. 72, 4847 (1950).
N. A. Shabanova, V. V. Popov, and Yu. G. Frolov, Available from VINITI No. 125-81 (Moscow, 1981).
V. A. Bernshtein and V. M. Egorov, Differential Scanning Calorimetry in Polymer Physicochemistry (Khimiya, Leningrad, 1990) [in Russian].
N. I. Yurasov, I. I. Yurasova, A. I. Plokhikh, et al., J. Phys.: Conf. Ser. 1348, 012082 (2019).
P. J. Heaney, Rev. Mineral. 29, 1 (1994).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yurasova, I.I., Yurasov, N.I., Plokhikh, A.I. et al. Growth Rate of SiO2 Nanoclusters at Different Water : TEOS Molar Ratios in the Presence of an Alkaline Catalyst. Russ. J. Phys. Chem. 95, 1207–1211 (2021). https://doi.org/10.1134/S0036024421060297
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421060297