Abstract
The forming and study of cationic surfactant/polyelectrolyte (PE) polymer colloid systems has been done at different concentrations of an amphiphilic compound and fixed polymer concentration. An imidazolium amphiphilic compound with a tetradecyl radical (IA-14) served as the surfactant; polyacrylic acid (PAA) characterized by a molecular weight of 1800 Da was used as component of the polymer. The aggregation characteristics of the systems have been examined via tensiometry, conductometry and fluorescence spectroscopy. It is shown that introducing of polyelectrolyte initiates the formation of polymer colloid complexes (PCC) at a 50 times lower concentration than the one required for an individual IA-14 system. Dynamic light scattering analysis has shown that the values of the hydrodynamic parameters of PCC lied in the range of 100–120 nm and did not depend on the concentration of the PE or the surfactant. It has been estimated via electrophoretic light scattering that electrostatic interactions make the main contribution to the formation of complexes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Polymer colloid complexes (PCC) formed via the self-assembly of oppositely charged polyelectrolytes and amphiphilic compounds are widely used in many fields of industry (e.g., medicine, oil production, and pharmaceutics) [1–8].
The formation of new composed structures expands the field of application of surfactants and polyelectrolytes, so the search for new mixed compositions based on these components is an important task. Varying the concentration and structure of the components, the pH of solutions, and the temperature and ionic strength are especially important when optimizing the composition and conditions of forming surfactant/polyelectrolyte composite systems. It allows us to alter the properties of PCCs and use them to obtain nanocontainers for hydrophobic compounds [9] and polyelectrolyte capsules [10]. The study of such systems also helps in modeling of interaction between cationic surfactants and natural biopolymers (e.g., oligonucleotides, DNA, and proteins) [11–15].
Mixtures of polyelectrolytes and oppositely charged surfactants are characterized by complicated aggregation behavior in solutions, followed by the formation of a wide diversity of morphological structures [16, 17]. Intermolecular interactions of different nature between polyelectrolytes and surfactants are the reasons for this behavior in solutions. The hydrophobic effect, Van der Waals interactions, and hydrogen bonds can contribute to the formation of complexes, but the strongest contribution comes from electrostatic interaction [18–20].
Tensiometry is one way of studying the aggregation behavior of PCCs in aqueous solutions. The tensiometric dependence of the surface tension of a surfactant/polyelectrolyte system is usually characterized by a more complicated shape than those of the individual amphiphilic systems, due not only to aggregation in the solution’s volume but to adsorption at the interphase boundary as well [21, 22]. Surfactant/PE complexes usually form at surfactant concentrations considerably lower than the critical micelle concentration (CMC) [23]; this is known as the critical aggregation concentration (CAC) in mixed systems [24]. In some cases, however, the value of the CAC is higher than [25, 26] or equal to [27] the CMC of the individual surfactant solution, depending not only on the nature of the components but on the concentration of the polyelectrolyte in the system as well [23].
The above brief analysis of the literary data shows that the formation of complexes based on oppositely charged surfactants and polyelectrolyte is an important line of research. However, the role structural factors play in altering the functional activity of such systems has yet to be determined, despite the available data. In this work, we formed polymer colloid systems based on imidazolium surfactant with tetradecyl radical (IA-14), and synthetic polyelectrolyte (polyacrylic acid (PAA), 1800 Da). The experiments were performed at varied concentrations of the amphiphilic compound and three constant concentrations of polyelectrolyte to optimize the composition of the binary systems and attain better aggregation capability (lower values of aggregation thresholds) [28].
The formulas of our compounds are given below:

EXPERIMENTAL
Imidazolium surfactants IA-14 were synthesized according to the standard experimental procedure in [29, 30]. Polyacrylic acid (Aldrich; 1800 g/mol, 99% purity) was used. The below concentrations of the polymer were calculated against one polymer unit.
The surface tension was measured on a Krüss K06 tensiometer [31] (Germany) using the du Nuy ring technique. An Inolab Cond 720 electrical conductivity meter (Germany) was used to measure the specific conductivity.
The emission spectra of pyrene (1 × 10−6 mol/L) were registered on Cary Eclipse G9800A spectrofluorometer (United States) at 25°С. The exciting wavelength was 335 nm. The emission spectra were registered at 350–500 nm at the scan rate of 120 nm/min. The measurements were made in a 1 cm thick cuvette. The fluorescence of the first (II) and third (IIII) vibrational peaks in the spectrum at 373 and 384 nm, respectively, were determined using spectral data obtained for pyrene.
The phase behavior of the surfactant/PE systems was studied on a Specord 250 Plus spectrophotometer (Germany). A predetermined solution of polyelectrolyte was added to IA-14 solution of a given concentration, and the mixture was kept for 2 min to reach the equilibrium state. The optical density of the solution was measured at a wavelength of 500 nm.
Our samples were studied via dynamic light scattering on a ZetaSizer Nano ZS analyzer (Malvern, United Kingdom). A He–Ne gas laser (power, 4 mW; wavelength, 633 nm) was the source of laser radiation. The resulting signals were analyzed using the unit’s software, according to the frequency and phase analysis of the scattered light. The measurements were made at a scattering angle of 173°. The size of the particles was determined using the Stokes–Einstein equation for spherical particles: D = kT/6πηR, where k is the Boltzmann constant, Т is the absolute temperature, η is the viscosity of the solvent, and R is the hydrodynamic radius. The electrophoretic mobility in each sample was transformed into the value of the zeta potential using Smoluchowski equation [32]: ζ = μη/ε, where ζ is the zeta potential, η is the dynamic viscosity of the liquid, μ is the electrophoretic mobility of the liquid, and ε is the permittivity.
RESULTS AND DISCUSSION
As noted above, measurements of surface tension are used to study of aggregation in mixed surfactant/PE systems [28, 33, 34]. The concentration of surfactant corresponding to a break in the surface tension–surfactant concentration curve is registered. Study of our IA-14/PAA systems via tensiometry showed there were two breaks on the isotherm of the surface tension despite the content of PE in the system (1, 3, or 5 mM); the values in the breakpoints do not depend on polyelectrolyte concentration (Fig. 1).
Isotherms of surface tension corresponding to (1) individual aqueous solutions of IA-14 [27]; (2–4) correspond to the mixed IA-14/PAA systems at СPAA = 1, 3, 5 mM and 25°С.
We attributed the first breakpoint (CAC1) to the IA-14 concentration that corresponded to the formation of mixed surfactant/PE complexes; the second breakpoint (CAC2) was attributed to the saturation of macromolecules with micelles and the emergence of individual IA-14 aggregates composed of amphiphilic molecules. It should be noted that adding a polymer component produced aggregates at a 50 times lower concentration (0.05 mM) than the one in the individual surfactant system (2.5 mM) [29] in all of the studied systems.
The observed drop in surface tension upon raising the content of the surfactant in the system (at equal concentrations of IA-14) shows that both polyelectrolyte and surfactant molecules participate in adsorption at the water/air interphase boundary. However, the accumulation of polyelectrolyte in the surface layer was limited: the isotherms of surface tension are identical at PAA concentrations in the system of 3 and 5 mM.
The PAA/surfactant systems were studied via conductometry (Fig. 2). The two linear regions on the dependences for specific conductivity on PAA concentration were typical of all the studied systems. (In the system with 3 mM of polyelectrolyte, the values of Pearson correlation coefficient r for two linear regions were 0.980 and 0.997, considerably higher than in the approximation of the dependence using unitary functional dependence (r = 0.953).) The presence of the breakpoint observed in the same narrow range of concentrations (1–2 mM) on the specific conductivity–surfactant concentration dependence is thus typical for all systems. It probably represents the formation of free surfactant micelles, since these concentration were on the level of the CMC of the individual surfactant solution (2 mM) [29].
Fluorescence analysis using pyrene as probe because of its sensitivity to the polarity of the microenvironment has proven to be a reliable way of determining the thresholds of aggregation for amphiphiles in solutions. The quantitative measure of this parameter in the above technique is the ratio between the first and third vibrational peaks of pyrene (I1/I3). A drop in the value of I1/I3 was observed for all of the studied systems (Fig. 3), due to the hydrophobic probe moving from the polar volume medium to the lipophilic microenvironment of aggregates. The last point before the I1/I3 value reaches a plateau is considered the threshold of aggregation in a system of ionic surfactants; with nonionic amphiphiles, the concentration of surfactant that divides the region of a drastic reduction of the I1/I3 value by half is considered the threshold of aggregation [35]. It should be noted that in the IA-14/PAA system, the concentration of surfactant corresponding to the fluorescent ratio of intensities is Δ(I1/I3)/2 equals to 0.05 mM, that is is almost identical to the tensiometric value of the CMC despite the cationic nature of the surfactant.
A subsequent study of mixed surfactant/PE systems at concentrations higher than CAC1 was performed via dynamic light scattering. This allowed us to analyze the size distribution of particles and make assumptions regarding their morphology. The size distribution of aggregates averaged to the number of particles for IA-14/PAA system (СPAA = 5 mM) is given in Fig. 4 as an example. It was shown that complexes characterized by hydrodynamic diameters DH of 100–150 nm form independently from concentrations of PE and amphiphile in IA-14/PAA systems upon reaching CAC1. The observed effect is the opposite of examples described in the literature that showed compaction of PCCs upon raising of surfactant concentration in the system [34]. This could have been due to the compacting ability of polyelectrolyte macromolecule caused by electrostatic forces being less pronounced as a result of delocalization of the charge in the head group of IA-14. At the same time, specific geometric features of a plain imidazolium ring probably favor stacking intermolecular interactions of a non-covalent nature between imidazolium rings, due to the intermolecular overlapping of the electron clouds of aromatic π-electrons. Such interactions could be the reason for the formation of clusters that consist of several polymer chains bound together by the bridge molecules of IA-14. The formation of such clusters agrees with the sizes of particles registered via dynamic light scattering (DH ≥ 100 nm).
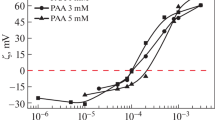
The pH value lies in the narrow range of 4 ± 0.1, which corresponds to a degree of polyelectrolyte ionization of ∼8% [28] in aqueous solutions of PAA at 1, 3, and 5 mM. The value of the zeta potential of the polyelectrolyte is around −20 mV [34], so the electrostatic bonding of the polymer and the IA-14 is expected. Electrophoretic light scattering while raising the concentration of the surfactant in the system was used to examine how efficient the electrostatic mechanism of bonding was in forming of the PCC. The data given in Fig. 5 show that constant titration of the polyelectrolyte with the IA-14 solution was followed by compensating changes in the values of zeta potential, from negative to zero and beyond. Since the concentration of IA-14 that corresponds to the recharge of system grows along with the concentration of PAA (ξ = 0 at СIA-14 = 0.12 ± 0.01 mM for СPAA= 1 mM; СIA-14 = 0.16 ± 0.01 mM for СPAA = 3 mM; and СIA‑14 = 0.36 ± 0.01 mM for СPAA = 5 mM), we may conclude that Coulomb forces play the key role in the formation of IA-14/PAA polymer–colloid complexes.
(1–3) Dependences of the electrokinetic potential of the mixed IA-14/PAA systems on IA-14 concentration at 25°С; see Fig. 2.
In polymer colloid systems composed of oppositely charged components, the formation of stoichiometric complex is often followed by opalescence or sedimentation. The next stage of study was therefore investigating the phase behavior of IA-14/PAA systems. The turbidimetry data presented in Fig. 6 demonstrate the change in the phase state of the mixed IA-14/PAA system as the concentration of surfactant was raised. The data show that the increase of IA-14 concentration led first to the emergence of opalescence properties in the system, and then to their elimination. The fixed bell-shaped dependences are closely related to the change of zeta potential in the system: the most intense opalescence is observed near the isoelectric point, while the solutions are more transparent in the region of the existence of the negatively or positively charged particles. This is explained by the neutrally charged colloid structures being characterized by a high tendency toward aggregation, which results in opalescence. The charged particles are more stable in the solutions due to repulsive forces. The increased value of the maximum of the optical density upon raising the concentration of PAA testifies to the greater number of negatively charged complexes in the system. At the same time, the maxima of the optical density for PAA concentrations of 1, 3, and 5 mM were registered at surfactant concentration equal to 0.2, 0.5, and 0.5 ± 0.01 mM, respectively. The concentrations of IA-14 that correspond to the maximum value of the optical density were thus considerably lower than that of the polyelectrolyte in all cases. This could indicate the formation of non-stoichiometric complexes between the components.
CONCLUSIONS
Mixed systems based on an imidazolium cationic surfactant with tetradecyl radical and polyacrylic acid were studied by varying the concentration of the surfactant and three fixed polyelectrolyte concentrations. Physicochemical measurements showed that polymer–colloid complexes formed between the components at concentrations of the surfactant ∼50 times lower than when there was no polymer. The observed abilities of imidazolium surfactant with respect to neutralization of the polyanion charge and the formation of stable colloid structures characterized by hydrodynamic diameters of 100–150 nm demonstrate the potential of using these systems for condensation with negatively charged polyanions that have practical applications.
REFERENCES
E. Guzmán, S. Llamas, A. Maestro, et al., Adv. Colloid Interface Sci. 233, 38 (2016).
Z. H. Asadov, S. M. Nasibova, G. A. Ahmadova, et al., Colloids Surf., A 527, 95 (2017).
K. Bodnár, E. Fegyver, M. Nagy, et al., Langmuir 32, 1259 (2016).
S. Hocine and M.-H. Li, Soft Matter 9, 5839 (2013).
J. Ramos, J. Forcada, and R. Hidalgo, Chem. Rev. 114, 367 (2014).
B. J. Lele and R. D. Tilton, Langmuir 35, 15937 (2019).
M. Gradzielski and I. Hoffmann, Curr. Opin. Colloid Interface Sci. 35, 124 (2018).
E. Piccinini, J. S. Tuninetti, J. Irigoyen Otamendi, et al., Phys. Chem. Chem. Phys. 20, 9298 (2018).
M. Klučáková, S. Jarábková, T. Velcer, et al., Colloids Surf., A 573, 73 (2019).
G. A. Gaynanova, A. M. Bekmukhametova, R. R. Kashapov, et al., Surf. Innov. 8, 38 (2020).
Y. Zhang, H. F. Chan, and K. W. Leong, Adv. Drug Deliv. Rev. 65, 104 (2013).
E. A. Vasilieva, D. A. Samarkina, G. A. Gaynanova, et al., J. Mol. Liq. 272, 892 (2018).
D. A. Kuznetsova, D. R. Gabdrakhmanov, S. S. Lukashenko, et al., Chem. Phys. Lipids 223, 104791 (2019).
D. A. Samarkina, D. R. Gabdrakhmanov, S. S. Lukashenko, et al., J. Mol. Liq. 275, 232 (2019).
D. A. Kuznetsova, D. R. Gabdrakhmanov, S. S. Lukashenko, et al., J. Mol. Liq. 307, 113001 (2020).
H. S. Nalwa, Handbook of Polyelectrolytes and Their Applications (Am. Sci., Stevenson Ranch, 2002), Vol. 2.
D. Ray and B. Das, J. Solution Chem. 48, 1576 (2019).
P. Yan, C. Jin, and C. Wang, J. Colloid Interface Sci. 282, 188 (2005).
D. Langevin, Adv. Colloid Interface Sci. 147, 170 (2009).
C. Wang and K. C. Tam, J. Phys. Chem. B 108, 8976 (2004).
K. Szczepanowicz, U. Bazylinska, J. Pietkiewicz, et al., Adv. Colloid. Interface Sci. 222, 678 (2015).
A. Pal and S. Yadav, J. Mol. Liq. 229, 309 (2017).
M. Koolivand-Salookia, A. Javadia, A. Bahramiana, et al., Colloids Surf., A 562, 345 (2019).
N. Jain, S. Trabelsi, S. Guillot, et al., Langmuir 20, 8496 (2004).
D. J. F. Taylor, R. K. Thomas, and P. X. Li, Langmuir 19, 3712 (2003).
D. A. Kuznetsova, D. R. Gabdrakhmanov, E. A. Vasil’eva, S. S. Lukashenko, L. R. Ahtamyanova, I. Sh. Siraev, and L. Ya. Zakharova, Russ. J. Org. Chem. 55, 11 (2019).
A. Akanno, E. Guzmán, L. Fernández-Peña, et al., Molecules 24, 3442 (2019).
E. A. Vasilieva, S. S. Lukashenko, L. A. Vasileva, et al., Russ. Chem. Bull. 68, 341 (2019).
D. A. Samarkina, D. R. Gabdrakhmanov, S. S. Lukashenko, et al., Colloids Surf., A 529, 990 (2017).
D. A. Kuznetsova, D. R. Gabdrakhmanov, S. S. Lukashenko, et al., J. Mol. Liq. 289, 111058 (2019).
A. Pal and S. Yadav, Fluid Phase Equilib. 412, 71 (2016).
L. Ya. Zakharova, G. I. Kaupova, D. R. Gabdrakhmanov, et al., Phys. Chem. Chem. Phys. 21, 16706 (2019).
D. R. Gabdrakhmanov, D. A. Kuznetsova, E. A. Vasil’eva, et al., Zhidk. Krist. Prakt. Ispol’z. 18 (4), 16 (2018).
E. Vasilieva, A. Ibragimova, S. Lukashenko, et al., Fluid Phase Equilib. 376, 172 (2014).
J. Aguiar, P. Carpena, J. A. Molina-Bolívar, et al., J. Colloid Interface Sci. 258, 116 (2003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors state they have no conflicts of interest to declare.
Additional information
Translated by P. Vlasov
Rights and permissions
About this article
Cite this article
Kuznetsova, D.A., Gabdrakhmanov, D.R., Kuznetsov, D.M. et al. Polymer Colloid Complexes Based on an Imidazolium Surfactant and Polyacrylic Acid. Russ. J. Phys. Chem. 94, 2337–2341 (2020). https://doi.org/10.1134/S0036024420110199
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420110199