Abstract
Formation of polyelectrolyte-surfactant complexes (PESCs) in polymerization process of corresponding amphiphilic monomer is a promising way of pure stoichiometric PESC obtained in salt-free systems. Polymerization of micelle-forming monomer dodecylammonium 2-acrylamido-2-methylpropanesulfonate (DDA-AMPS) in different media was studied by means of 1H-NMR and conductance measurements. Kinetics of polymerization in direct micelles, inverted micelles, and homogeneous solution was considered in terms of classical solution polymerization and microemulsion polymerization. Reaction orders on monomer and initiator were measured in water and dioxane. The rate of polymerization is strongly dependent on monomer micelle existence, being considerably higher in micellar solution than in homogeneous one.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymerization of micelle-forming monomers in micellar state is widely investigated. Most of these works dealt with aqueous solutions of ionic surfactants bearing polymerizable group in the tail or in the head group of the molecule. Much less studies were devoted to micellar systems with polymerizable counterion [1,2,3,4,5,6,7,8,9,10,11] and very few—to inverted micellar polymerization [12,13,14,15,16]. Micellar polymerization usually proceeds with high rate and conversions close to 100%, which is explained by “monomer condensation” effect—increasing of local monomer concentration in the micelle core (up to bulk concentration values) and possible monomer molecule orientation favoring to polymerization [17].
Kinetic studies of polymerization in micellar state are limited to rather few papers [18,19,20,21,22,23,24,25,26] because of quite fast reaction at low concentrations having low volume effects. Most results in these studies established classical kinetics regularities known for free-radical homogeneous polymerization. There are some papers considering mechanistic aspects of micellar polymerization for monomers with high and low cmc [24, 25]. Paper [26] treats micellar polymerization in terms of microemulsion regime described in Morgan and Kaler work [27].
In this paper, we describe micellar precipitation polymerization of micelle-forming monomer dodecylammonium 2-acrylamido-2-methylpropanesulfonate (DDA-AMPS) having hydrophobic cation and hydrophilic polymerizable anion. Polymerization of such monomer leads to formation of polyelectrolyte-surfactant complex (PESC)—a special kind of macromolecular substances, which are usually obtained by mixing together solutions of polyelectrolyte and surfactant. Synthesis of PESC by direct polymerization of micelle-forming monomer [1,2,3,4,5,6,7,8,9, 16] or of the ionic monomer in surfactant solution [28] makes it possible to control molecular weight and dispersity of the resulting polymer [1, 6, 29] and in some cases—morphology of the polymer in the solid state [16]. PESCs obtained by mixing of aqueous solutions of a polyelectrolyte and an oppositely charged surfactant can form different structures in the solid states [30]. Kinetic studies of PESC formation in polymerization process are practically absent in the literature. Most of PESCs are insoluble in water and the polymerization proceeds in precipitation regime that is additional complication for precise kinetic measurements. In this direction, probably the most interesting results were obtained by Walker and Kline [1,2,3,4,5,6] for the polymerization of cetyltrimethylammonium 4-vinylbenzenecarboxylate, which after polymerization formed stable PESC solution in water. The kinetics of structural changes of micellar solutions during polymerization in that works was monitored by SANS [5] but no correlations of these data with monomer conversion were presented.
In our previous papers, we described synthesis of a series of PESC-forming amphiphilic ionic monomers with polymerizable counterion [16, 31], their micelle-forming properties in media of different polarity [32, 33], their polymerization in such media, and the properties of the resulting polymers [34, 35]. The current paper is focused on the polymerization kinetics of a micelle-forming monomer, in which polymerizable group is located in counterion, actually, monomer is AMPS acid anion with about 0.6 degree of binding to the micelles [33]. Polymerization was performed in different states of monomer solution—direct micelles in water and inverted micelles in dioxane and in homogeneous solution in water/dioxane mixture, all under precipitation polymerization conditions. In each case, polymerization resulted in great structural rearrangements of solution [5] originated from the concurrence of hydrophobic interactions of surfactant tails and Coulomb interactions of charged groups to adopt most stable conformation of polymer chain [35]. Direct NMR spectroscopy measurements were used to determine monomer conversion in the course of polymerization.
Experimental section
Dodecylamine (98%, Merck), 2-acrylamido-2-methylpropanesulfonic acid (99%, Merck), potassium persulfate (special purity grade, Vekton, Russia), and azo-bis-cyanovaleric acid (98%, Sigma-Aldrich) were used as received. 1,4-Dioxane (Vekton, Russia) was distilled above potassium hydroxide prior to use. Deionized water with a specific resistance not less than 16 MOh was used for all preparations.
Monomer DDA-AMPS was prepared as described previously [15].
Conductivity measurements were performed using an Expert-002 (Russia) conductometer equipped with Mettler Toledo InLab-710 platinum probe; conductivity values were taken every 10 s. 1H NMR spectra were recorded on a Bruker Avance III 500 spectrometer at 500 MHz.
Polymerizations were carried out in standard 5-mm NMR tubes in non-deuterated solvents. Measuring cell was pre-heated to temperature 60 °C. Shimming and locking were performed using a sample tube with corresponding deuterated pure solvent. Monomer and initiator solution of desired concentration were placed in the NMR tube and bubbled with argon for 15 min prior to heating. After placing the tube into the spectrometer, shimming (without lock) was performed again and 1H NMR spectra were recorded every 18 s (4 scans per spectrum). Monomer conversion was calculated from the decreasing intensity of signals corresponding to the double-bond hydrogens at 6.50, 6.38, and 5.94 ppm (Figs. 1 and 2). Signal of methyl group (from dodecylamine) at 0.9 ppm was used as internal standard for calculation of conversion. Conversion was calculated as p = (1 − I1/I7) × 100%, where I1 and I7 are integral intensities of hydrogen signals of double bond (I1, 3H) and methyl group (I7, 3H). The data was processed with Bruker Topspin Dynamics Centrum software.
Visual effect of polymerization was that polymer precipitated from solution but it was found from NMR and conductance measurements that precipitation started from meta-stable polymer solution when conversion of monomer was quite high (more than 60–70%), so polymerization proceed quicker than it seems from visual observation and it is possible to perform conversion measurements in macroscopically homogeneous solution before precipitation starts.
After polymerization, DDA-PAMPS was isolated by filtration and washed with acetone. To convert it to PAMPS-Na form, the polymer sample was dissolved in methanol containing 20% stoichiometric excess of NaOH and stirred for 24 h then precipitated in diethyl ether, and washed with acetone. Resulting PAMPS-Na was dissolved in water, filtered through a 0.45-μm syringe filter, and freeze-dried. The completeness of ion exchange was checked by NMR by the absence of signals corresponding to the dodecylammonium tail. Chromatographic analysis was performed on Shimadzu LC-20AD apparatus equipped with a refraction detector and TSKgel SuperAW5000 column calibrated according to pullulan standards (Shodex, Mw = 5 × 103–8 × 105). Aqueous 0.5 M NaNO3 with 0.05% NaN3 was used as eluent at 30 °С, flow rate 0.5 mL/min, and pressure 1.8 MPa. Sample concentration was 3 g/L.
Results and discussion
DDA-AMPS is micelle-forming monomer which can form direct and inverted micelles in appropriate media; its aggregation behavior and polymerization in micellar state and in homogeneous solution were studied in detail [16]. In water, DDA-AMPS forms micelles having hydrodynamic radius of 2.4 nm, aggregation number of about 100, and cmc of 1.1 × 10−2 mol/L at 25 °С.
Polymerization of DDA-AMPS proceeds very fast and with high yields in micelles and with considerably lower yields in homogeneous regime. Recently we have published new results [26] for kinetic study of micellar polymerization with semi-empirical model of microemulsion polymerization [27] adopted for the case of pure micelle-forming monomer. Conversion data for polymerization rate calculations in that paper were obtained from UV-spectroscopy measurements. Here we tried to use NMR real-time spectroscopy for investigation of DDA-AMPS polymerization. NMR-spectroscopy allows obtaining directly the data of monomer conversion in the course of polymerization in various media.
Polymerization of DDA-AMPS in water was performed in monomer concentration range 0.013–0.25 mol/L at constant potassium persulfate concentration (3.7 × 10−3 mol/L) or at constant monomer concentration 0.25 mol/L and initiator concentration range 2–11 × 10−3 mol/L. Conversion vs time curves are shown in Fig. 3 a and c.
Kinetic data for DDA-AMPS polymerization in water. Conversion curves (a, c) are shifted by 500 s from each other for distinct representation. a Conversion vs time dependences at different monomer concentrations. b Polymerization rate vs monomer concentration dependence. c Conversion vs time dependences at different initiator (PSK) concentrations. d Polymerization rate vs initiator concentration dependence
In all cases, some induction period (1–2 min) was observed after which polymerization started and completed in several minutes. Rates of polymerization were calculated from conversion data (from linear region of conversion curves: 10–70% conversion) and plotted against concentration (Fig. 3b, d). From these plots, reaction orders were calculated: 1.13 ± 0.04 with respect to monomer and 0.46 ± 0.08 with respect to initiator. These values appeared to be in good agreement with classical theory of free-radical polymerization and previously reported data for polymerization of the micelle-forming monomer alkyldimethyl-4-vinylbenzylammonium chloride in water [24, 25].
In dioxane, DDA-AMPS forms inverted micelles with cmc about 3.3 × 10−2 mol/L (value obtained by conductance measurements in dioxane containing 5% water). Polymerization in dioxane was performed in similar way as in water; azo-bis-cyanovaleric acid was used as initiator. Conversion curves (Fig. 4) in dioxane appeared to be very similar to those in water indicating high rate of polymerization retained up to 80% conversion, which is characteristic to micellar or microemulsion type of polymerization [26, 36]. Reaction order with respect to monomer appeared to be 1.32 ± 0.16 that is close to value obtained in water.
Polymerization of DDA-AMPS in dioxane containing 20% of water proceeds considerably different. These conditions correspond to homogeneous monomer solutions; no micelles formed. The rate of polymerization (8.5 × 10−5 mol/L × s) compared with that in water (1.0 × 10−3 mol/L × s) or in pure dioxane (6.1 × 10−4 mol/L × s) is much lower (at the other conditions being equal) and after 1 h of reaction proceeding conversion was not higher than 70% (Fig. 5a). It seems that differences between polymerization in micellar state (pure water, pure dioxane) and homogeneous solution are just quantitative, but there are some observations indicating qualitative distinction between micellar and homogeneous polymerizations.
Conversion data can be treated in the terms of microemulsion polymerization kinetics theory proposed by Morgan and Kaler [27] and used for micellar polymerization characterization. In this approach, dome-shaped dependences of dp/dt on p appeared to be characteristic for microemulsion regime of polymerization having maximum of dp/dt at conversion value of 39%. In homogeneous regime, this dependence extincts to straight line. As it can be seen from Fig. 5b, in pure water and pure dioxane, conversion curves in Morgan-Kaler coordinates have distinct dome shapes, whereas conversion points in water-dioxane mixture drop to linear array.
The main equation for micellar polymerization \( \frac{\partial p}{\partial t}={\left(1-p\right)}^{\alpha}\sqrt{-2{A}^{\ast}\ln \left(1-p\right)} \) having two fitting parameters А* and α were used to fit experimental data of DDA-AMPS conversion (lines on Fig. 5b). In the Morgan-Kaler model, parameter \( A=\frac{2f{k}_{\mathrm{d}}I{k}_{\mathrm{p}}{C}_0}{M_0} \) combines the rate constants of initiator decomposition (kd) and propagation (kp) reaction, initial monomer (M0), and initiator (I) gross concentration and local concentration of monomer in the particles at the moment of the particle formation (С0). In this case, empirical parameter A* = AM0/2 accounting dependence of initiation efficiency f on monomer concentration f ≈ ki[M] ≈ kiM0(1 − p), where ki is relative rate constant of primary radical reaction with monomer. Parameter α corresponds to initiation efficiency and radical transfer from solution to the micelles [19, 21]. Here A* = 0.75 and α = 0.7 in water and A* = 0.25 and α = 1.15 in dioxane. In the case of DDA-AMPS, the polymerization was complicated by polymer precipitation and this might disturb basic regularity, but in general, in all cases of micellar polymerization, we observed dome-shaped curves in Morgan-Kaler plots for NMR-based (or UV-based) conversion data and for conductance changes during polymerization.
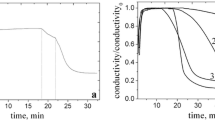
Polymerization of DDA-AMPS and some related monomers was studied by means of conductance measurements. Conductance changes during polymerization (Fig. 6a) retrace conversion curves when plotted in appropriate coordinates (normalized conductance S vs time, \( S=1-\frac{\chi -{\chi}_{\mathrm{f}}}{\chi_0-{\chi}_{\mathrm{f}}} \), where starting value of conductance χ0 taken as a datum and final value of conductance χf taken as unity) [26]. Real-time conductance measurements are quite simple and substantially cheaper than NMR. In our case, the data obtained by means of conductometry appeared to be in good agreement with NMR observations and the kinetics model (Fig. 6b) at the early stages of polymerization (up to 40–50% conversion, until large amount of polymer was formed, affecting conductance due to increasing viscosity or precipitation).
Molecular characteristic of the polymers
Since DDA-PAMPS is PESC, it is relatively complicated object for chromatographic characterization due to its high adsorption. Some DDA-PAMPS polymers obtained in micellar regime (direct or inverted micelles) and in homogeneous solution were converted to polyelectrolyte form (PAMPS-Na) and analyzed by means of SEC. Results are shown in Table 1. One can see that molecular weights of the polymers obtained in micellar regime appeared to be considerably higher than those of the polymers obtained in homogeneous solution, whereas dispersity of the polymers had comparable values of 1.9–3.3. Such values of dispersities are common for free-radical polymerization, but a little bit higher than those (1.2–1.7) obtained for polymers of ω-N-acryloylaminoalkanoates in micellar regime [37]. Probably, in this case, some dispersity in molecular weights may be caused by coexistence of two possible mechanisms of polymerization and PESC formation—the first (and the main) is polymerization of AMPS anion bound to dodecylammonium micelles (degree of binding about 60% [33]) and the second is polymerization of “free” AMPS anion with subsequent binding with micelles. Precipitation of the polymer during polymerization also may contribute to polymer dispersity.
Conclusion
Micellar polymerization and its kinetics are of great interest because of their academic and applied significance. PESC formation via polymerization of tailored amphiphilic monomers in appropriate media allows obtaining pure stoichiometric products with controllable molecular weights. Thus, it becomes clear from the results of NMR experiments presented in this paper that polymerization of DDA-AMPS (micelle-forming alkylammonium salt of ionic monomer AMPS) proceeds in micellar regime in both direct micelles (in water) and inverted micelles (in dioxane) and can be treated in terms of microemulsion polymerization theory. Molecular weights of the polymers obtained in micellar regime are higher than those of polymers obtained in homogeneous solution. No considerable differences in dispersity of “micellar” or “homogeneous” polymers were observed for DDA-AMPS.
References
Gerber MJ, Walker LM (2006) Controlling dimensions of polymerized micelles: micelle template versus reaction conditions. Langmuir. 22:941–948. https://doi.org/10.1021/la052297q
Gerber MJ, Kline SR, Walker LM (2004) Characterization of rodlike aggregates generated from a cationic surfactant and a polymerizable counterion. Langmuir. 20:8510–8516. https://doi.org/10.1021/la048929a
Kuntz DM, Walker LM (2007) Solution behavior of rod-like polyelectrolyte-surfactant aggregates polymerized from wormlike micelles. J Phys Chem B 111:6417–6424. https://doi.org/10.1021/jp0688308
Walker LM, Kuntz DM (2007) Wormlike micelles as a template for polymerization. Curr Opin Colloid Interface Sci 12:101–105. https://doi.org/10.1016/j.cocis.2007.07.004
Kline S (2000) Structural evolution during micelle polymerization. J Appl Crystallogr 33:618–622. https://doi.org/10.1107/S0021889899012753
Kline SR (1999) Polymerization of rodlike micelles. Langmuir. 15:2726–2732. https://doi.org/10.1021/la981451o
Summers M, Eastoe J, Davis S, Du Z, Richardson RM, Heenan RK, Steytler D, Grillo I (2001) Polymerization of cationic surfactant phases. Langmuir. 17:5388–5397. https://doi.org/10.1021/la010541h
Lerebours B, Perly B, Pileni MP (1989) Polymerization of cetyltrimethylammonium methacrylate direct micelles, Trends Colloid Interface Sci III. Prog Colloid Polym Sci 79:239–243. https://doi.org/10.1007/BFb0116215
Lerebours B, Perly B, Pileni MP (1988) Polymerization of cetyltrimethylammonium methacrylate micellar solution. Chem Phys Lett 147:503–508. https://doi.org/10.1016/0009-2614(88)85016-4
Hartmann PC, Dieudonné P, Sanderson RD (2005) Self-assembly and influence of the organic counterion in the ternary systems dodecylamine/acrylic acid/water and dodecylamine/methacrylic acid/water. J Colloid Interface Sci 284:289–297. https://doi.org/10.1016/j.jcis.2004.10.009
Samakande A, Hartmann PC, Sanderson RD (2006) Synthesis and characterization of new cationic quaternary ammonium polymerizable surfactants. J Colloid Interface Sci 296:316–323. https://doi.org/10.1016/j.jcis.2005.09.005
Nagai K, Ohishi Y (1987) Polymerization of surface-active monomers. II. Polymerization of quarternary alkyl salts of dimethylaminoethyl methacrylate with a different alkyl chain length. J Polym Sci Polym Chem 25:1–14. https://doi.org/10.1002/pola.1987.080250101
Nagai K, Ohishi Y, Inaba H, Kudo S (1985) Polymerization of surface-active monomers. I. Micellization and polymerization of higher alkyl salts of dimethylaminoethyl methacrylate. J Polym Sci Polym Chem Ed 23:1221–1230. https://doi.org/10.1002/pol.1985.170230425
Bezzaoucha F, Lochon P, Jonquières A, Fischer A, Brembilla A, Aïnad-Tabet D (2007) New amphiphilic polyacrylamides: synthesis and characterisation of pseudo-micellar organisation in aqueous media. Eur Polym J 43:4440–4452. https://doi.org/10.1016/j.eurpolymj.2007.07.005
Bilibin AY, Sukhanova TM, Matuschkin NI, Mel’nikov AB, Zorin IM (2012) Polymerization of dodecylammonium 2-acrylamido-2-methylpropane sulfonate in solvents with different dielectric constants and study of the resulting ionic complexes. Macromol Symp 317–318:160–168. https://doi.org/10.1002/masy.201100112
Bilibin AY, Shcherbinina TM, Kondratenko YA, Zorina NA, Zorin IM (2015) Micellar polymerization of alkylammonium 2-acrylamido-2-methylpropane sulfonates in the solvents of different polarities and properties of resulting polyelectrolyte-surfactant complexes. Colloid Polym Sci 293:1215–1225. https://doi.org/10.1007/s00396-015-3497-8
Tajima K, Aida T (2000) Controlled polymerizations with constrained geometries. Chem Commun:2399–2412. https://doi.org/10.1039/b007618j
Yegorov VV, Batrakova YV, Zubov VP (1988) Influence of the nature of the initiator on the kinetics of radical polymerization of N,N-dimethyl-N-acetoxydecyl-methacryloylethyl ammonium bromide in water. Polym Sci USSR 30:1972–1975. https://doi.org/10.1016/0032-3950(88)90047-0
Yegorov VV, Zaitsev SY, Zubov VP (1991) Radical polymerization of monomers capable of association in water. Review. Polym. Sci. U.S.S.R. 33:1475–1496. https://doi.org/10.1016/0032-3950(91)90031-K
Yegorov VV, Batrakova YV, Zubov VP (1990) Radical polymerization in spherical micelles of unsaturated alkylammonium halides in water. Polym. Sci. U.S.S.R. 32:861–866. https://doi.org/10.1016/0032-3950(90)90216-S
Egorov VV, Zubov VP (1987) Radical polymerisation in the associated species of ionogenic surface-active monomers in water. Russ Chem Rev 56:1153–1165. https://doi.org/10.1070/RC1987v056n12ABEH003328
Egorov V (1990) Radical polymerization in micelles of surface-active monomers. Makromol Chemie Macromol Symp 31:157–161. https://doi.org/10.1002/masy.19900310113
Egorov VV (1995) Radical polymerization of micelle-forming monomers in water. J. Polym. Sci. A Polym. Chem. 33:1727–1733. https://doi.org/10.1002/pola.1995.080331020
Cochin D, Zana R, Candau FF (1993) Photopolymerization of micelle-forming monomers. 2. Kinetic study and mechanism. Macromolecules. 26:5765–5771. https://doi.org/10.1021/ma00073a034
Cochin D, Zana R, Candau F (1993) Polymerization of micelle-forming monomers: mechanistic study and characterization of the systems before and after polymerization. Polym Int 30:491–498. https://doi.org/10.1002/pi.4990300412
Zorin IM, Podolskaya EP, Bilibin AY (2019) On the kinetics of micellar polymerization. Acryloylaminoalkanoates case study. Eur Polym J 110:355–363. https://doi.org/10.1016/j.eurpolymj.2018.11.045
Morgan JD, Lusvardi KM, Kaler EW (1997) Kinetics and mechanism of microemulsion polymerization of hexyl methacrylate. Macromolecules. 30:1897–1905. https://doi.org/10.1021/ma9613704
Shulevich YV, Petzold G, Navrotskii AV, Novakov IA (2012) Properties of polyelectrolyte–surfactant complexes obtained by polymerization of an ionic monomer in a solution of an oppositely charged surfactant. Colloids Surfaces A Physicochem Eng Asp 415:148–152. https://doi.org/10.1016/j.colsurfa.2012.10.013
Shulevich Y, Dukhanina E, Navrotskii A, Novakov I (2018) Polymerization of trimethylmethacryloyloxyethylammonium methyl sulfate in surfactant micellar solution of sodium alkyl sulfates and properties of the resultant polyelectrolytes. Colloid Polym Sci 296:871–881. https://doi.org/10.1007/s00396-018-4302-2
Koetz J, Kosmella S, Beitz T (2001) Self-assembled polyelectrolyte systems. Prog Polym Sci 26:1199–1232. https://doi.org/10.1016/S0079-6700(01)00016-8
Bilibin AY, Sukhanova TM, Kondratenko YA, Zorin IM (2013) n-Alkyl ammonium 2-acrylamido-2-methylpropanesulfonates: synthesis, properties, and polymerization. Polym Sci Ser B 55:22–30. https://doi.org/10.1134/S1560090412100028
Zorin IM, Shcherbinina TM, Mel’nikov AB, Molchanov VS, Bilibin AY (2014) A study of n-dodecylammonium acrylamido-2-methylpropanesulfonate association in aqueous solutions. Colloid J 76:314–318. https://doi.org/10.1134/S1061933X14030168
Bilibin AY, Shcherbinina TM, Girbasova NV, Lebedev VT, Kulvelis YV, Molchanov VS, Zorin IM (2016) Colloidal properties of polymerizable counterion surfmers solutions based on alkylamino 2-acrylamido-2-methylpropanesulfonates in different solvents. Des Monomers Polym 19:369–380. https://doi.org/10.1080/15685551.2016.1169371
Tsvetkov NV, Fetin PA, Lezov AA, Gubarev AS, Achmadeeva LI, Lezova AA, Zorin IM, Bilibin AY (2015) Colloid solution of surfactant monomers and polyelectrolyte: polymerization and properties of the resulting interpolyelectrolyte complexes. J Mol Liq 211:239–246. https://doi.org/10.1016/j.molliq.2015.07.003
Andreeva LN, Shcherbinina TM, Zorin IM, Bezrukova MA, Bushin SV, Bilibin AY (2013) Molecular, conformational, and optical characteristics of poly(dodecylammonium-2-acrylamido-2-methylpropanesulfonate) in organic solvents. Polym Sci Ser A 55:289–294. https://doi.org/10.1134/S0965545X13050015
Yo P, Chow LMG (2005) Microemulsion polymerizations and reactions. Adv Polym Sci 175:257–298. https://doi.org/10.1007/b100117
Tsvetkov NV, Andreeva LN, Zorin IM, Bushin SV, Lebedeva EV, Strelina IA, Bezrukova MA, Lezov AA, Makarov IA, Bilibin AYu (2011) Synthesis, hydrodynamic, and conformational properties of poly(N-acryloyl-11-aminoundecanoic acid) in solutions. Polym Sci Ser A 53:355–363. https://doi.org/10.1134/S0965545X11050087
Acknowledgments
The work was performed on the equipment of the Research park of St. Petersburg State University: Center of Magnetic resonance and the Chemistry educational center.
Funding
The work was supported by RFBR (grant #18-33-00618-mol_a) in the part of PESC synthesis and SEC studies performed by P.A. Fetin.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zorin, I.M., Shcherbinina, T.M., Demidov, E.I. et al. Polyelectrolyte-colloid complex formation via polymerization: reaction kinetics in direct micelles, inverted micelles, and homogeneous solution studied by NMR and conductometry. Colloid Polym Sci 297, 1169–1176 (2019). https://doi.org/10.1007/s00396-019-04531-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-019-04531-4