Abstract
The oxidation of a heterocyclic compound—barbituric acid (BBA) by diperiodatoargentate(III) (DPA) was carried out in the absence and presence of ruthenium(III) catalyst in alkaline medium with a constant ionic strength of 0.20 mol dm–3 at 298 K. The reaction was monitored spectrophotometrically. The reaction was of first order with respect to [DPA] and was less than unity order with respect to [BBA] in both catalyzed and uncatalyzed cases. Positive and negative fractional order in [OH–] for uncatalyzed and Ru(III) catalyzed reaction respectively was observed, whereas perioadate has retarding effect in both the cases. A unity order with respect to [Ru(III)] was observed. The uncatalyzed reaction in alkaline medium has been shown to proceed via a DPA–BBA complex, which decomposes in a rate determining step to give the free radicals, which is followed by other fast steps to give the products. Whereas in catalyzed reaction, it has been shown to proceed via a Ru(III)–BBA complex, and similar other steps as in uncatalyzed reaction to give the products. The reaction constants involved in the various steps involved in the mechanisms were calculated for both the reactions. The catalytic constant (kC) was also calculated for catalyzed reaction at four temperatures. The activation parameters with respect to slow step of the mechanism and also the thermodynamic data for all the equilibrium steps were determined and discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Naturally occurring compound uracil is a derivative of pyrimidine [1]. It is one of four nucleobases, and also have other biological functions [2, 3]. Barbituric acid (BBA) or 6-hydroxyuracil, is a crystalline compound, soluble in water [4]. Barbiturate drugs are derivatives of BBA. Even though, barbituric acid shows no pharmacologically activity [5], barbiturates act as allosteric modulator and at higher doses as agonists of GABA receptors [6].
The study of the metals in their highest oxidation state has attracted many researchers in current years. Such oxidation states can be stabilized by chelating with suitable bi- or polydentate ligands. Diperiodatocuprate(III) (DPC) [7], diperiodatoargentate(III) (DPA) [8], and diperiodatonickelate(IV) (DPN) [9] are well known oxidizing agents in a certain buffer medium with a suitable pH value. Diperiodatoargentate(III) (DPA) has the reduction potential 1.74 V and acts as a powerful oxidizing agent in aqueous alkaline medium [10]. Silver(III) is a two electron oxidant. The reactions of silver(III) species have generated further interest mechanistically. The reduction of Ag(III) to Ag(I) may occur either by simultaneous two-electron transfer in one step or in two successive one-electron steps [11]. The structure of this complex is considered as [AgIII(H3IO6)2]– which has a square-planar coordination geometry around the metal center and it is fairly stable in alkaline medium and can be readily prepared [12].
Kinetically, catalytic reactions are particular reactions which involve lower activation energy than the corresponding uncatalyzed reactions resulting in a higher reaction rate at the same temperature and for the same reactant concentrations. Ruthenium(III) is a stable and efficient catalyst active in milder reaction conditions and various media. Many pioneering researcher worked, Oi and Inoue [13] initiated in 2001, Ackermann [14] and Bruneau and Dixneuf [15].
As per the literature survey, there were no reports on the oxidation of BBA by any oxidant from kinetic and mechanistic point of view. Such studies are of much importance in understanding the mechanism of oxidation of BBA and also it is helpful in getting the information of silver metal ions interaction with the biologically active compounds. Hence, the present investigation is aimed to unveil the Ru(III) catalyzed and uncatalyzed oxidation of BBA by DPA and to arrive at plausible mechanism and to understand the reactive species.
EXPERIMENTAL
Chemicals and Solutions
Barbituric acid was purchased from Sigma Aldrich, India. The analytical grade reagents were used in the experiment and Millipore water was used throughout the work. A stock solution was prepared by dissolving a known amount of the BBA in Millipore water. The required concentration of BBA was obtained from its stock solution. A standard stock solution of Ru(III) was prepared by using RuCl3 (S.D. Fine Chem.) in 0.20 mol dm–3 HCl. The concentration was determined [16] by EDTA titration. To maintain ionic strength and alkalinity of the reaction, KNO3 and KOH (BDH) respectively were used. Aqueous solutions of AgNO3 were used to study the product effect, Ag(I). A stock standard solution of \({\text{IO}}_{4}^{ - }\) was prepared by dissolving a known weight of KIO4 (Riedel–de Haen) in hot water; the stock solution used after keeping for 24 h and its concentration was deduced iodometrically [17] at neutral pH maintained using a phosphate buffer. The temperature was maintained constant to within ±0.10 K.
Diperiodatoargentate(III) was prepared by oxidising Ag(I) in presence of KIO4 as described elsewhere [8]. The complex was characterized by its UV–Vis spectrum which exhibited three peaks at 216, 255, and 362 nm [11]. The magnetic moment study revealed that the complex is diamagnetic. The compound prepared was analyzed [18] for silver and periodate by acidifying a solution with HCl, recovering and weighing the AgCl for Ag, and titrating the iodine liberated when excess KI was added to the filtrate for \({\text{IO}}_{4}^{ - }\). The stock solution of DPA was used for the required [DPA] in the reaction mixture.
Instrumentation
The kinetic measurements were carried out on Varian CARY 50 Bio UV–Vis spectrophotometer (Varian, Victoria-3170, Australia) with a Peltier accessory for temperature control. For pH measurement an Elico pH meter model LI 120 was used. ESI–MS data were obtained on a 17A Shimadzu gas chromatograph with a QP-5050A Shimadzu mass spectrometer using the EI ionization technique.
Kinetic Measurements
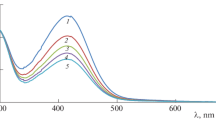
In all the kinetic measurements, a fixed concentration, 1.0 × 10–5 mol dm–3 of KIO4 was used throughout the study unless otherwise stated. Thus, the possibility of oxidation of BBA by periodate was tested. We found that there was no significant interference due to KIO4 under our experimental conditions. Precaution was also taken to avoid the dissolution of O2 and CO2 in the solution by maintaining an inert atmosphere with N2 throughout the study. The kinetics was followed under pseudo-first-order conditions where [BBA] > [DPA] at 298 K, unless specified. The reaction was initiated by mixing the DPA to BBA solution which also contained required concentrations of Ru(III) or KNO3, KOH, and KIO4; the progress of the reaction was monitored spectrophotometrically at 362 nm from the decrease in absorbance due to DPA with the molar absorbency index ε at 13 900 ± 100 dm3 mol–1 cm–1. It was confirmed that there is a negligible effect from other species present in the reaction mixture at this wavelength. The spectral changes during the chemical reaction in the standard conditions at 298 K are shown in Fig. 1 (for uncatalyzed reaction). It is evident from the figure that the concentration of DPA decreases at 362 nm. The pseudo-first order rate constants kT were determined from the log(absorbance) versus time plots (Fig. 2). The plots were linear up to 85% completion of reaction.
RESULTS
Stoichiometry and Product Analysis
Different sets of reaction mixtures containing varying ratios of DPA to barbituric acid in the presence of constant amounts of KOH and KNO3 in uncatalyzed and constant amount of Ru(III) in catalyzed reaction were kept for 6 h in closed vessels under nitrogen atmosphere. The remaining concentration of DPA was estimated spectrophotometrically at 362 nm. The results indicated 3 : 3 stoichiometry (BBA : DPA) for both the reactions as given in following equations:


The main reaction product for both the uncatalyzed and Ru(III) catalyzed reactions was identified as trimer of barbituric acid which was confirmed by ESI–mass spectral analysis. The mass spectrum showed a molecular ion peak at 338 amu confirming the presence of cyclic acyl ion which is a fragmented molecular ion of trimer of barbituric acid (Fig. 3). Similar oxidation product of BBA was obtained by Kato et al. [19]. The formation of free Ag+ in solution was detected by adding KCl solution to the reaction mixture, which produced white turbidity due to the formation of AgCl.
Reaction Orders
As the diperiodatoargentate(III) oxidation of barbituric acid in alkaline medium proceeds with a measurable rate in the absence of ruthenium(III), the catalyzed reaction is understood to occur in parallel paths with contributions from both the catalyzed and uncatalyzed paths.
Thus, the total rate constant (kT) is equal to the sum of the rate constants of the catalyzed (kC) and uncatalyzed (kU) reactions, so kC = kT – kU. Hence the reaction orders have been determined from the slopes of log kC vs. log(concentration) plots by varying the concentrations of barbituric acid, Ru(III) and alkali in turn while keeping the others constant. The uncatalyzed reaction was followed under the conditions [BBA] = 5.0 × 10–4; [DPA] = 5.0 × 10–5; [OH–] = 0.10; [I] = 0.20 mol dm–3. The rate constant of uncatalyzed reaction (kU) was obtained by the plot of log A (A is absorbance) versus time by following the progress of the reaction spectrophotometrically at 362 nm.
Effect of [Diperiodatoargentate(III)]
The DPA concentration was varied in the range of 1.0 × 10–5 to 5.0 × 10–5 mol dm–3 for uncatalyzed and Ru(III) catalyzed cases. The linearity of the plots of log absorbance versus time up to 85% completion of the reaction indicates a reaction order of unity in [DPA]. This was also confirmed by varying [DPA], which did not result in any change in the pseudo-first-order rate constants, kC (Table 1).
Effect of [Barbituric Acid]
The barbituric acid concentration was varied in the range 1.0 × 10–4–1.0 × 10–3 mol dm–3 at 25°C while keeping other reactant concentrations and conditions constant in the absence and in the presence of catalyst. The kU and kC values were increased with the increase in concentration of barbituric acid, indicating an apparent less than unit order dependence on [BBA]. This was also confirmed by the plots of kU vs. [BBA]0.69 which is linear rather than the direct plot of kU vs. [BBA] (Fig. 4) (r = 0.9925, S < 0.003 for uncatalyzed, r = 0.9809, S < 0.062 for catalyzed) (Table 1).
Plots of (a) kU vs. [BBA]0.69 and [BBA], (b) kC vs. [BBA]0.73 and [BBA] (conditions as in Table 1).
Effect of [Ru(III)]
The ruthenium(III) concentration was varied from 0.2 × 10–6 to 2.0 × 10–6 mol dm–3 range, at constant concentrations of diperiodatoargentate(III), barbituric acid, alkali, and at constant ionic strength. The order in [Ru(III)] was found to be unity from the linearity of the plots of kC vs. [Ru(III)] (r = 0.9958, S ≤ 0.021) (Table 1).
Effect of [Alkali] and [Periodate]
The effect of alkali on the reaction was studied at constant concentrations of barbituric acid and DPA and at a constant ionic strength of 0.2 mol dm–3 for both the cases at 298 K. Surprisingly alkali has different effect on the rate of the reaction in absence and presence of catalyst. The rate constant increased and decreased with increase in [alkali] in the absence and presence of catalyst less than unit order dependence on [alkali] as given in Table 1. The effect of [\({\text{IO}}_{4}^{ - }\)] was observed by varying the concentration from 0.2 × 10‒5 to 2.0 × 10–5 mol dm–3 while keeping all other reactants concentrations constant. It was observed that the rate constants decreased by increasing [\({\text{IO}}_{4}^{ - }\)] in both the cases (Table 1).
Effect of Dielectric Constant of the Medium (D)
The dielectric constant of the medium, D, was varied by varying the t-butyl alcohol-water percentage. The dielectric constants of the reaction medium at various composition of t-butyl alcohol and water (v/v) were calculated from the equation, D = V1D1 + V2D2, where D1 and D2 are dielectric constants of pure water and t-butyl alcohol, i.e., 78.5 and 10.9 at 25°C, respectively, and, V1 and V2 are the volume fractions of the components, water and t-butyl alcohol respectively, in the total volume of mixture. It was found that the rate constants increased with decrease in the dielectric constant of the medium for both uncatalyzed and Ru(III) catalyzed reactions. The plots of log kU vs. 1/D and log kC vs. 1/D were linear with negative slopes (r > 0.9876, S ≤ 0.0131; r > 0.9679, S ≤ 0.0321) as shown in Fig. 5.
Effect of Ionic Strength (I)
The addition of KNO3 at constant [DPA], [Ru(III)], [BBA], [OH–], and [\({\text{IO}}_{4}^{ - }\)] was found that increasing ionic strength had no significant effect on the rate of the reaction (for both the cases).
Effect of Initially Added Products
In both the cases initially added products, Ag(I) and trimer of barbituric acid did not have any significant effect on the rate of reaction.
Test for Free Radicals
The involvement of free radicals in the reaction was examined as follows. A known quantity of acrylonitrile monomer was initially added to the reaction mixture and was kept for 2 h in an inert atmosphere. A white precipitate was formed on diluting the reaction mixture with methanol, indicating the involvement of free radicals in the reaction [20]. The blank experiments of either DPA or barbituric acid alone with acrylonitrile did not induce any polymerization under the same conditions.
Effect of Temperature
The influence of temperature on the rate of reaction was studied for both uncatalyzed and catalyzed reaction at four different temperatures (288, 298, 308, and 318 K) under varying concentrations of barbituric acid and alkali keeping other conditions constant. The rate constant increased with increase in temperature. The rate constant (k1) of the slow step of the uncatalyzed reaction mechanism was obtained from the slopes and intercepts of plots of 1/kU vs. 1/[BBA], 1/kU vs. [OH–], and 1/kU vs. [\({\text{IO}}_{4}^{ - }\)] plots at four different temperatures and were used to calculate the activation parameters. The energy of activation corresponding to these constants was evaluated from the Arrhenius plot of log k1 vs. 1/T (r = 0.9921, S ≤ 0.014) and other activation parameters obtained are tabulated in Tables 2, 3.
Similarly the rate constant (k2) of the slow step of catalyzed reaction mechanism was obtained from the slopes and the intercept of the plots of [Ru(III)]/kC vs. 1/[BBA], [Ru(III)]/kC vs. [OH–], and [Ru(III)]/kC vs. [\({\text{IO}}_{4}^{ - }\)] at four different temperatures. The values are given in Tables 4–6. The activation energy for the rate determining step was obtained by the least square method of plot of log k2 vs. 1/T (r = 0.9956, S ≤ 0.017) and other activation parameters calculated for the reaction are presented in Tables 4–6.
Catalytic Activity
It has been pointed out by Moelwyn–Hughes [21] that, in the presence of the catalyst, the uncatalyzed and catalyzed reactions proceed simultaneously, so that
Here kT is the observed pseudo-first-order rate constant in the presence of Ru(III) catalyst, kU the pseudo-first-order rate constant for the uncatalysed reaction, KC the catalytic constant, and x is the order of the reaction with respect to [Ru(III)]. In the present investigations, x values for the standard run were found to be 1.0 for Ru(III). Then the value of KC can be calculated using the equation:
where kT – kU = kC.
The values of KC were evaluated for the catalyst at different temperatures and found to vary at different temperatures. The values of KC and the corresponding activation parameters with respect to Ru(III) catalyst are presented in Table 7.
DISCUSSION
The kinetics of oxidation of various organic and inorganic substrates have been studied by Ag(III) species, this strategy may be due to its strong and versatile nature as a two-electron oxidant. Among the various species of Ag(III), Ag(OH)\(_{4}^{ - }\), DPA, and ethylenebis(-biguanide) silver(III) (EBS) species are of maximum interest to the researchers due to their relative stability [22]. The stability of Ag(OH)\(_{4}^{ - }\) is very sensitive towards traces of dissolved oxygen and other impurities in the reaction medium; so it had not drawn much attention. However, the other two forms of Ag(III) [23] are quite stable; the DPA is used in highly alkaline medium and EBS is used in highly acidic medium. A literature survey [11] reveals that the water-soluble DPA has a formula [Ag(IO6)2]7– with dsp2 configuration of square planar structure, similar to diperiodatocopper(III) complex with two bidentate ligands, periodate, to form a planar molecule. When the same molecule is present in alkaline medium, it is unlikely to exist as [Ag(IO6)2]7‒, though a periodate is known to be in various protonated forms [24] depending on pH of the solution as given in the following multiple equilibria:
Periodic acid exists as H5IO6 in acid medium and as H4IO\(_{6}^{ - }\) near pH 7. Hence, under alkaline conditions as employed in this study, the main species are expected to be \({{{\text{H}}}_{{\text{3}}}}{\text{IO}}_{6}^{{2 - }}\) and \({{{\text{H}}}_{{\text{2}}}}{\text{IO}}_{6}^{{3 - }}\). At higher concentrations, periodate also tends to dimerize [10]. However, formation of this species is negligible under conditions employed for kinetic study. On contrary, the authors [25] in their recent studies have proposed the DPA species as [Ag(HL)2]x– in which L is a periodate with uncertain number of protons and HL is a protonated periodate of uncertain number of protons. This can be ruled out by considering the alternative form [24] of \({\text{IO}}_{4}^{ - }\) at pH >7 which is in the form \({{{\text{H}}}_{{\text{3}}}}{\text{IO}}_{6}^{{2 - }}\) or \({{{\text{H}}}_{{\text{2}}}}{\text{IO}}_{6}^{{3 - }}\). Hence, DPA could be as [Ag(H3IO6)2]– or [Ag(H2IO6)2]3– in alkaline medium. Therefore, under the present condition, diperiodatoargentate(III), may be depicted as [Ag(H3IO6)2]–. The similar speciation of periodate in alkali was proposed [26] for diperiodatonickelate(IV). It is known that barbituric acid exists as negative ion (deprotonated form) in aqueous alkaline medium [27]. In highly acidic medium it exists in the protonated form.
Mechanism for Uncatalyzed Reaction
Since, the effect of [OH–] was enhanced reaction rate, added periodate has the retarded the rate, the effect of [DPA] and [BBA] showed first and fractional order reaction rate respectively. The [OH–] deprotonates the DPA to give a deprotonated Diperiodatoargentate(III) (DPA) before equilibrium step 1, displacement of a ligand takes place in the second step, periodate dissociates to give free periodate which is evidenced by decrease in the rate with increase in free [periodate] (Table 1). It may be expected that lower Ag(III) periodate species such as monoperiodatoargentate(III) (MPA) is more important in the reaction than the DPA. The inverse fractional order in [\({{{\text{H}}}_{{\text{3}}}}{\text{IO}}_{6}^{{2 - }}\)] might also be due to this reason. In the pre-rate-determining stage, this monoperiodatoargentate (MPA), combines with a molecule of the of BBA to give an intermediate complex which decomposes in a slow step to give the intermediate free radical species of barbituric acid, Ag(II) and species by a one equivalent change of Ag(III) in a single step, as intervention of free radical has been observed. This intermediate free radical species reacts with another molecule of free radical to give dimer of barbituric acid. Further, this dimer reacts with one more molecule of MPA species in a fast step to yield carbocation of dimer compound. The active intermediate carbocation dimer combines with another molecule of BBA to form a trimer compound of barbituric acid. Trimer compound gives final product upon reacting with MPA which was confirmed by ESI-mass spectrum. The plot of 1/kU vs. 1/[BBA] proved the complex formation between oxidant and reductant, which explains less than unit order in [BBA].
Spectroscopic evidence for the complex formation between oxidant and substrate was obtained from UV–Vis spectra of BBA (5.0 × 10–4), DPA (5.0 × 10–5), [OH–] (0.1 mol dm–3), and mixture of both. A bathochromic shift of about 5 nm from 362 to 367 nm in the spectra of DPA was observed. However, the Michaelis–Menten plot proved the complex formation between DPA and BBA, which explains the less than unity order dependence on [BBA]. Such complex between an oxidant and substrate has also been observed in other studies [28]. Scheme 1 leads to the rate law:
This explains all the observed kinetic orders of different species. The rate law (9) can be rearranged in to the following form, which is suitable for verification:
According to Eq. (10), other conditions being constant, plots of 1/kU vs. [\({{{\text{H}}}_{{\text{3}}}}{\text{IO}}_{6}^{{2 - }}\)] (r = 0.9827, S ≤ 0.014), 1/kU vs. 1/[OH–] (r = 0.9649, S ≤ 0.034), and 1/kU vs. 1/[BBA] (r = 0.9982, S ≤ 0.019) should be linear and are found to be linear (Fig. 6). The slopes and intercepts of such plots lead to the values of K1, K2, K3, and k1 as (0.64 ± 0.009) dm3 mol–1, (0.25 ± 0.03) × 10–4 mol dm–3, (1.1 ± 0.10) × 104 dm3 mol–1, and (8.5 ± 0.10) × 10–3 s–1, respectively. The value of K1 and K2 is in good agreement with earlier literature [28]. These constants were used to calculate the rate constants and compared with the experimental kU values and found to be in reasonable agreement with each other, which fortifies Scheme 1.
Verification of rate law (9) for the oxidation of BBA by diperiodatoargentate(III). Plots of (a) 1/kU vs. 1/[BBA], (b) 1/kU vs. 1/[OH–], (c) 1/kU vs. [\({{{\text{H}}}_{{\text{3}}}}{\text{IO}}_{6}^{{2 - }}\)], at four different temperatures (conditions as in Table 1).
The thermodynamic quantities for the first, second and third equilibrium steps of Scheme 1 can be evaluated as follows. [BBA], [OH–], and [\({{{\text{H}}}_{{\text{3}}}}{\text{IO}}_{6}^{{2 - }}\)] (as in Table 1) were varied at four different temperatures. From the slopes and intercepts, the values of K1, K2, and K3 were calculated at different temperatures and these values are given in Tables 2, 3. The vant Hoff’s plots were made for variation of K1, K2, and K3 with temperature (log K1 vs. 1/T (r = 0.9742, S ≤ 0.007), log K2 vs. 1/T (r = 0.9712, S ≤ 0.01), and log K3 vs. 1/T (r = 0.99921, S ≤ 0.02) the values of enthalpy of reaction ΔH, entropy of reaction ΔS, and free energy of reaction ΔG, were calculated for all the equilibrium steps. These values are given in Tables 2, 3. A comparison of the thermodynamic quantities of first step of (ΔH = 20.77 kJ mol–1). Scheme 1 with those obtained for the slow step (ΔH# = 18.58 kJ mol–1) of the reaction shows that these values mainly refer to the rate limiting step, supporting the fact that the reaction before rate determining step is fairly fast and has low activation energy [29]. A negative value of ΔS# (‒48.86 J K–1 mol–1) suggests that intermediate complex is more ordered than the reactants [30]. The observed modest enthalpy of activation and a higher rate constant for the slow step indicates that the oxidation presumably occurs via an inner-sphere mechanism which was supported by earlier works [31].
Mechanism for Ru(III) Catalyzed Reaction
Ruthenium(III) chloride acts as an efficient catalyst in many redox reactions, particularly in an alkaline medium [20]. It is interesting to identify the active ruthenium(III) chloride species in alkaline media. In the present study it is quite probable that the [Ru(H2O)5OH]2+ species might assume the general form [Ru(III)(OH)x]3 – x. The x value would always be less than six because there are no definite reports of any hexahydroxy ruthenium species. The remainder of the coordination sphere would be filled by water molecules. At higher pH, the electronic spectra studies have confirmed [32] that the ruthenium(III) chloride exists in the hydrated form as [Ru(H2O)6]3+. Metal ions of the form [Ru(H2O)6]3+ are also known to exist as [Ru(H2O)5OH]2+ in an alkaline medium and are most likely mononuclear species. Hence, under the conditions employed, e.g., [OH–] ≫ [Ru(III)], ruthenium(III) is mostly present as the hydroxylated species, [Ru(H2O)5OH]2+. Similar species have been reported between Ru(III) catalyzed oxidation of several other substrates with various oxidants in alkaline medium [33]. In earlier reports of Ru(III) catalyzed oxidation [34], it has been observed that, if there is a fractional order dependence with respect to [substrate] and [Ru(III)] and unit order with respect to [oxidant], Ru(III) forms a complex with the substrate. It gets oxidized by the oxidant to form Ru(IV)–substrate complex followed by the rapid redox decomposition to regenerate Ru(III). In another case [35], if the process shows a zeroth order dependence with respect to [oxidant], first order with respect to [Ru(III)] and a fractional order with respect to [substrate], there involves the formation of a Ru(III)–substrate complex. It undergoes further cleavage in a concerted manner giving rise to a Ru(I) species, which is rapidly oxidized by the oxidant to regenerate the catalyst. In some other reports [36], it is observed that Ru(III) forms a complex with substrate and is oxidized by the oxidant with the regeneration of the catalyst. Hence, the study of behavior of Ru(III) in catalyzed reaction becomes significant.
Stoichiometry was same as in the case uncatalyzed reaction but the equilibrium steps 1 and 2 were different from uncatalyzed reaction. In the Ru(III) catalyzed reaction, [DPA] an Ru(III) were first order dependence, an apparent order of less than unit order in [BBA], a negative fractional order dependence on [alkali] and [\({\text{IO}}_{4}^{ - }\)] and order with respect to Ru(III) was found to be unity. No effect of added products was observed. Based on the experimental results, a mechanism is proposed for which all the observed orders in each constituent such as [DPA], [BBA], [Ru(III)], [OH–], and [\({\text{IO}}_{4}^{ - }\)] may be well accommodated.
It is well known that in both uncatalyzed and catalyzed reactions DPA oxidation reactions the increasing and decreasing effect on the rate of the reactions was observed with respect to [OH–] and [\({\text{IO}}_{4}^{ - }\)], respectively. But in the present study different finding was obtained. As usual in the uncatalyzed reaction [OH–] has positive effect. However, a negative fractional order with respect to [OH–] in case of Ru(III) catalyzed reaction. Also in some earlier works [37] [\({\text{IO}}_{4}^{ - }\)] has no influence on the rate of reaction when [OH–] has negative effect. Totally dissimilar observation was found in Ru(III) catalyzed oxidation of BBA. The decrease in rate of reaction with increase in alkalinity (Table 1) can be explained in terms of prevailing first equilibrium step of formation of [Ag(OH)2(H3IO6)2]3– from [Ag(OH)2(H3IO6)(H2IO6)]4– hydrolysis as given in the following equation:
The protonated form of DPA i.e., [Ag(OH)2(H3IO6)2]3– can decompose into [Ag(OH)2(H3IO6)]– and a periodate ion, which reveals the negative effect of [\({\text{IO}}_{4}^{ - }\)] as shown in equation:
In the prior equilibrium steps (1) and (2) in view of the relatively less than unit order in OH– concentration, the main oxidant species is likely to be [Ag(OH)2(H3IO6)]–, and its formation equilibrium is important in the reaction. The less than unit order in [BBA] presumably results from formation of a complex (C2) between the Ru(III) species and Barbituric acid. This complex (C2) reacts with one mole of DPA in a slow step to give the free radical species of BBA as intervention of free radical has been observed and Ag(II) species by a one equivalent change of Ag(III) with the regeneration of catalyst, Ru(III) in a single step. In further fast steps the reaction leads to the final product similar to uncatalyzed reaction. The detailed Ru(III) catalyzed oxidation mechanism was sketched in Scheme 2. The plot of 1/kC vs. 1/[BBA] proved the complex formation between oxidant and reductant, which explains less than unit order in [BBA].
A hypsochromic shift of about 6 nm from 276 to 270 nm of Ru(III) to Ru(III)–BBA complex was observed. Attempts to separate and isolate the complex were not successful. The Michaelis–Menten plot proved the complex formation between catalyst and substrate, which explains less than unit order in [BBA]. Such a complex between a catalyst and substrate has also been observed in other studies [37]. The rate law (13) for Scheme 2 could be derived as,
This explains all the observed kinetic orders of different species. The rate law (14) can be rearranged in to the following form, which is suitable for verification:
The thermodynamic quantities for the different equilibrium steps, in Scheme 2 can be evaluated as follows. The Barbituric acid, hydroxide ion and periodate concentrations (Table 1) were varied at different temperatures. The plots of [Ru(III)]/kC vs. 1/[BBA] (r = 0.9966, S ≤ 0.0023), [Ru(III)]/kC vs. [OH–] (r = 0.9931, S ≤ 0.0015), and [Ru(III)]/kC vs. [\({{{\text{H}}}_{{\text{3}}}}{\text{IO}}_{6}^{{2 - }}\)] (r = 0.9932, S ≤ 0.026) should be linear as shown in Fig. 7. From the intercepts and slopes of such plots, the reaction constants K4, K5, K6, and k2 were calculated as (3.69 ± 0.2) × 10–3 mol dm–3, (0.298 ± 0.1) × 10–4 dm3 mol–1, (10.5 ± 0.4) × 103 dm3 mol–1, (6.2 ± 0.3) × 104 s–1, respectively as shown in Tables 4–6. These constants used to calculate the rate constants and compared with the experimental kC values and found to be in reasonable agreement with each other, which fortifies Scheme 2. A vant Hoff’s plot was made for the variation of K4, K5, K6 with temperature [(log K4 vs. 1/T (r = 0.9891, S ≤ 0.011), log K5 vs. 1/T (r = 0.9956, S ≤ 0.09), and log K6 vs. 1/T (r = 0.9517, S ≤ 0.1)] and the values of the enthalpy of reaction ΔH, entropy of reaction ΔS and free energy of reaction ΔG, were calculated. These values are also given in Tables 4–6. A comparison of the ΔH value of second step (31.8 kJ mol–1) of Scheme 2 with that of ΔH# (16.61 kJ mol–1) obtained for the slow step of the reaction shows that these values mainly refer to the rate limiting step, supporting the fact that the reaction before rate determining step is fairly slow and involves high activation energy. The values of ΔH# and ΔS# were both favorable for electron transfer processes. The favorable enthalpy was due to release of energy on solutions changes in the transition state.
Verification of rate law (14) for the oxidation of BBA by diperiodatoargentate(III); (a) [Ru(III)]/kC vs. 1/[BBA], (b) [Ru(III)]/kC vs. [OH–], (c) [Ru(III)]/kC vs. [\({{{\text{H}}}_{{\text{3}}}}{\text{IO}}_{6}^{{2 - }}\)], at four different temperatures (conditions as in Table 1).
The negative value of ΔS# suggests that the intermediate complex is more ordered than the reactants [30]. The observed modest enthalpy of activation and a higher rate constant for the slow step indicates that the oxidation presumably occurs via an inner-sphere mechanism which was supported by earlier works [31]. The activation parameters evaluated for the catalyzed and uncatalysed reaction explain the catalytic effect on the reaction. The catalyst Ru(III) forms the complex (C2) with the substrate, which enhances the reducing property of the substrate than that without the Ru(III) catalyst. Further, the Ru(III) catalyst modifies the reaction path by lowering the energy of activation. Further, plots of log KC vs. 1/T were linear and the values of energy of activation and other activation parameters with reference to catalyst were computed. These results are summarized in Table 7. The value of KC at 298 K is 1.85 × 104.
The negligible effect of ionic strength on rate of reaction reveals the involvement of neutral species in reaction as seen in (Schemes 1 and 2). The effect of solvent on the reaction rate is described in detail in the literature [38]. For the limiting case of a zero angle approach between two dipoles or anion dipole system, Amis [39] has shown that log kobs vs. 1/D gives a straight line, with a negative slope for a reaction between negative ion and a dipole or between two dipoles, while a positive slope is obtained for positive ion–dipole reactions. In the present investigations, plots of log kU vs. 1/D and log kC vs. 1/D (Fig. 5) for uncatalyzed and Ru(III) catalyzed reactions were linear with negative slopes, which supports the involvement of negative ions as in Scheme 1.
CONCLUSIONS
The comparative study of uncatalyzed and ruthenium(III) catalyzed oxidation of barbituric acid by diperiodatoargentate(III) was performed. Oxidation products were identified. Among the various species of Ag(III) in alkaline medium, [Ag(H2IO6)(H2O)2] and [Ag(OH)2(H3IO6)]– were considered as active species for uncatalyzed and Ru(III) catalyzed reactions respectively. Active species of Ru(III) is found to be [Ru(H2O)5OH]2+. Based on experimental results, the probable mechanisms were proposed for both the reactions. Thermodynamic quantities and activation parameters of individual steps in the mechanisms were evaluated for uncatalyzed and Ru(III) catalyzed reactions at different temperatures, respectively. The catalytic constants and the activation parameters with reference to catalyst were also computed. The descriptions of the mechanisms are consistent with all the experimental evidences including kinetic, spectral and product studies.
REFERENCES
R. H. Garrett and C. M. Grisham, Principals of Biochemistry with a Human Focus (Brooks/Cole Thomson Learning, U. S., 1997).
D. J. Brown, Heterocyclic Compounds: The Pyrimidines (Interscience, New York, 1994), Vol. 52.
E. G. Brown, Ring Nitrogen and Key Biomolecules: The Biochemistry of N-Heterocycles (Kluwer Academic, Boston, 1998).
H. C. Box and E. E. Budzinski, J. Chem. Phys. 59, 1588 (1973).
E. R. Garrett, J. T. Bojarski and G. J. Yakatan, J. Pharm. Sci. 60, 1145 (1971).
W. Löscher and M. A. Rogawski, Epilepsia 53, 12 (2012).
S. B. Konnur and S. T. Nandibewoor, Russ. J. Phys. Chem. A. 93, 1686 (2019)
J. H. Shan, H. Y. Wang, C. Y. Song, and F. Wang, Bull. Chem. Soc. Ethiop. 23, 297 (2009).
R. S. Shettar and S. T. Nandibewoor, J. Mol. Catal. A: Chem. 234, 137 (2005).
B. Sethuram, Some Aspects of Electron-Transfer Reactions Involving Organic Molecules (Allied, New Delhi, 2003), p. 151.
A. Kumar and P. Kumar, J. Phys. Org. Chem. 12, 79 (1999).
C. Yang, Z. Zhang, and J. Wang, Luminiscence 25, 36 (2010).
S. Oi, Y. Ogino, S. Fukita, and Y. Inoue, Org. Lett. 4, 1783 (2002).
L. Ackermann, S. I. Kozhushkov, and D. S. Yufit, Chem. Eur. 18, 12068 (2012).
Z. F. Ke and T. R. Cundari, Organometallics 29, 821 (2010).
C. S. Reddy and T. Vijaykumar, Indian J. Chem. A 34, 615 (1995).
P. A. Magdum, A. M. Bagoji, and S. T. Nandibewoor, J. Phys. Org. Chem. 28, 743 (2015).
G. H. Jeffery, J. Bassett, J. Mendham, and R. C. Denney, Vogel’s Textbook of Quantitative Chemical Analysis, 5th ed. (Longmans Singapore, Singapore, 1996), pp. 467, 391.
S. Kato and G. Dryhurst, J. Electroanal. Chem. 80, 181 (1977).
R. V. Jagdeesh and Puttaswamy, J. Phys. Org. Chem. 21, 844 (2008).
E. A. Moelwyn-Hughes, Kinetics of Reactions in Solutions (Oxford Univ. Press, London, 1947), p. 297.
L. J. Krishenbaum and L. Mrozowski, Inorg. Chem. 17, 3718 (1978).
R. Banerjee, R. Das, and S. Mukhopadhyay, J. Chem. Soc., Dalton Trans. 28, 1317 (1992).
C. E. Crouthamel, A. M. Hayes, and D. S. Martin, J. Am. Chem. Soc. 73, 82 (1951).
P. Jayaprakash Rao, B. Sethuram, and T. Navneeth Rao, React. Kinet. Catal. Lett. 29, 289 (1985).
S. Bhattacharya, B. Saha, A. Datta, and P. Banerjee, Coord. Chem. Rev. 170, 47 (1998).
R. Chang, Physical Chemistry with Applications to Biological Systems (McMillan, New York, 1981), p. 326.
T. S. Kiran and S. T. Nandibewoor, J. Chem. Res. 6, 431 (2006).
K. S. Rangappa, M. P. Raghavendra, D. S. Mahadevappa, and D. Channegouda, J. Org. Chem. 63, 531 (1998).
A. Weissberger and E. S. Lewis, Investigation of Rates and Mechanism of Reactions in Techniques of Chemistry (Wiley Interscience, New York, 1974), Vol. 4, pp. 421.
T. S. Kiran, C. V. Hiremath, and S. T. Nandibewoor, Appl. Catal. A 305, 79 (2006).
R. E. Connick and D. A. Fine, J. Am. Chem. Soc. 82, 4187 (1960).
F. A. Cotton, G. Wilkinson, C. A. Murillo, and M. Bochmann, Advanced Inorganic Chemistry, 6th ed. (Wiley, New York, 1999).
S. Sandu, B. Sethuram, and T. N. Rao, J. Indian Chem. Soc. 60, 198 (1983).
H. P. Panda and B. D. Sahu, Indian J. Chem. 28A, 323 (1989).
V. Tegginamath, C. V. Hiremath, and S. T. Nandibewoor, J. Phys. Org. Chem. 20, 55 (2007).
S. J. Malode, J. C. Abbar, and S. T. Nandibewoor, Inorg. Chim. Acta 363, 2430 (2010).
E. A. Moelwyn-Hughes, Physical Chemistry (Pergamon, New York, 1961).
E. S. Amis, Solvents Effect on Reaction Rates and Mechanisms (Academic, New York, 1966).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Bagoji, A.M., Konnur, S.B., Gokavi, N.M. et al. Silver(III) Periodate Complex—An Oxidant for Free Radical Induced Uncatalyzed and Ruthenium(III) Catalyzed Oxidation of Barbituric Acid. Russ. J. Phys. Chem. 94, 2010–2023 (2020). https://doi.org/10.1134/S0036024420100052
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420100052