Abstract
Characteristic features of the gas chromatographic separation of the keto and enol tautomeric forms of ethyl acetoacetate on a capillary column with a BPX-1 standard nonpolar polydimethylsiloxane stationary phase are considered. It is confirmed that the chromatograms of a mixture of tautomers have a specific profile, i.e., a plateau between the peaks of the tautomers, which corresponds to the reversible keto \( \rightleftarrows \) enol transformation during separation. It is shown that the enol and keto forms of ethyl acetoacetate have different coefficients of the temperature dependence of the gas chromatographic retention indices (0.19 ± 0.03 and 0.02 ± 0.02, respectively). It is found there is no dependence of the relative peak areas of the tautomers on the nature of the solvent (polar ethyl alcohol and nonpolar hexane) at different temperatures; i.e., such ratios predominantly reflect the position of the keto \( \rightleftarrows \) enol equilibrium in the vapor phase of the injector of the chromatograph. It is concluded that this results in similar values of the thermodynamic parameters (standard enthalpy and entropy) of the tautomeric equilibrium determined upon the dosing of samples in different solvents. Possible distortions of the results due to the effects of discriminating between the compositions of the samples injected into capillary columns with gas flow splitting are discussed. An impurity in a sample of ethyl acetoacetate after long-term storage is identified as ethyl 2-hydroxy-3-oxobutanoate, the product of oxidation by dissolved atmospheric oxygen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Prototropic tautomerism (a 1,3 shift of a hydrogen atom) is of special interest in chemistry, since it complicates the characterization of the physicochemical and spectral properties of organic compounds as a result of their dependence on the conditions of determination (e.g., temperature, solvent, pH of solutions). β-dicarbonyl compounds are a typical example of the structures for which prototropic tautomerism is typical:

The existence of such a tautomeric equilibrium determines the challenges and uncertainty of the results from the chromatographic separation of analytes capable of tautomerism [1]. In chemical practice, such representatives of β-diketones as 2,4-pentanedione (acetylacetone (I)) and ethyl-3-oxobutanoate (ethyl acetoacetate, ethyl ester of acetoacetic acid (II)) are encountered most often:
According to the literature data, the concentration of the enol form in I under standard conditions (STP) is 80–96% [2]; in II, it is 6.8–8.0 [3] to 9.4–10.5% [4]. The main way of determining the ratio of the keto and enol forms in solutions under STP is 1H NMR spectroscopy, and the estimates of their amounts by different signals of the spectra can differ somewhat [4]. The concentration of less polar enol forms of β-dicarbonyl compounds in solutions grows upon moving from polar to nonpolar solvents [5], and in the vapor phase.
All the above patterns have been observed for individual liquid substances or their solutions. Gas chromatographic separation, however, requires the transition of the components of samples to the vapor phase in the injector of a chromatograph, and the shifting of their zones along the column at elevated temperatures, which changes the ratio of the tautomers. However, all of the original works [6–13] used as sources of information in the NIST database [14] indicate the gas chromatographic retention indices (RIs) of ethyl acetoacetate (II) on standard nonpolar and polar phases without assigning them to any of the tautomers. The same refers to the electron ionization (EI) mass spectrum of ester II [14]. A similar situation is also observed for acetylacetone (I). At the same time, two different mass spectra of the keto and enol forms are presented in the database [14] for methyl ester of 2-oxopentanoic acid isomeric to (II), but the value of the RI is assigned only to the first of them. Meanwhile, as far back as late 1980s, reports on the gas chromatographic separation of the keto and enol forms of 1,3-diketones [15], and, somewhat later, β-ketoesters [16] have emerged, and the differences in their EI mass spectra have been confirmed. The data of [15, 16] allow us to summarize the patterns of the orders of the gas chromatographic elution of the tautomers of β-dicarbonyl compounds, at least on standard nonpolar phases. If there are no substituents at the carbon atom located between the carbonyl groups, the enol form has smaller retention parameters than the keto tautomer. When substituents are present, the order of elution is reversed.
Measuring the ratio of the intensities of the signals of the tautomers of ester II in the 1H NMR spectra at different temperatures is equivalent to determining the keto \( \rightleftarrows \) enol equilibrium constants, on whose basis we can calculate this process’s thermodynamic parameters of activation (ΔH# and ΔS#) [3–5, 17, 18]. The gas chromatographic data were not used for these purposes, since it remained unclear to which phase (system) to attribute the obtained data. There are reports of using such data to estimate the enthalpies of evaporation of tautomers [3].
The aims of this work were:
• to determine the characteristic features of the gas chromatographic separation of two tautomeric forms of ethyl acetoacetate (II), including the temperature dependence of the ratios of the peak areas S(enol)/S(keto) in solvents with different polarities;
• to refine the values of the gas chromatographic retention indices of both tautomers of ethyl acetoacetate, and to characterize their temperature dependence;
• to compare the thermodynamic parameters of activation of the process of establishing the keto \( \rightleftarrows \) enol tautomeric equilibrium for solutions of ethyl acetoacetate in different solvents, in order to clarify the nature of this process under the conditions of gas chromatographic separation;
• to identify the impurity of an earlier uncharacterized compound found in a sample of ethyl acetoacetate after long-term storage.
EXPERIMENTAL
Chemicals Used in Preparing the Samples
The solvents we used were ethyl acetoacetate (reagent grade, Reakhim, Moscow), C6–C12n‑alkanes with an even number of carbon atoms (reagent grade or reagent grade for chromatography, Reakhim, Moscow), and n-hexane (reagent grade, Vekton, St. Petersburg) and ethyl alcohol (Gatchinskii spirtovyi zavod). The samples were prepared by dissolving 150 μL of ethyl acetoacetate in 1.35 mL of ethyl alcohol or n-hexane with the addition of 50 μL each of n-octane and n‑decane (reference hydrocarbons).
Conditions of Gas Separation and Chromatographic–Mass Spectrometric Analysis
The gas chromatographic analysis of the solutions of ethyl acetoacetate in ethyl alcohol and hexane was performed on a Chromatec-Crystal 5000.2 gas chromatograph equipped with a flame ionization detector and a WCOT 10 m long fused quartz column with an internal diameter of 0.53 mm with a BPX-1 standard nonpolar polydimethylsiloxane phase. The film of the stationary phase was 2.65 μm thick. The temperature of the column was 70°C, while the temperature of the injector was varied from 100 to 220°C in increments of 20 K. In the other experiments, isothermal modes from 60 to 100°C were used in increments of 10 K at a constant temperature of the injector of 160°C. The temperature of the detector was 200°C, the carrier gas was nitrogen, the flow rate was 5.2 mL/min (linear velocity, 43.9 cm/s), the split ratio was 1 : 3, and the injection volume was 0.5 μL (MSh-1 microsyringe). The number of parallel determinations for the same samples under the same conditions was two to four. To determine the isothermal retention indices (Kovats), 50 μL each of C8 and C10n-alkanes were added to the samples.
Our chromatographic–mass spectrometric analysis was performed on a Shimadzu QP-2010 SE chromatograph–mass spectrometer with EI (energy of ionization, 70 eV), equipped with an Optima 1 column 25 m long with an internal diameter of 0.32 mm. The film of the stationary phase was 0.35 μm thick. The analysis was performed with temperature programming from 50 to 250°C at a rate of 5 K/min. The temperature of the injector was 180°C, and that of the detector was 200°C. The carrier gas was helium, with a flow rate of 1.82 mL/min (linear velocity of 53.6 cm/s), a split ratio of 1 : 10, and an injection volume of 0.5 μL. The temperatures of the interface and ion source were 200°C. The flow of carrier gas from the chromatographic column to the ion source (solvent delay) was cut off at 1.6 min. A mixture of reference C6–C12n-alkanes with the even number of carbon atoms was added to the samples to determine the linear–logarithmic retention indices.
Processing of the Results
The components of the reaction mixtures were characterized by their standard retention indices and mass spectra, with subsequent averaging. For solutions in n-hexane, the time required for elution of the peak of the first of the impurities in the solvent, and the time of elution of the solvent for solutions in ethanol, were used as estimates of the hold-up time. The tautomers were characterized by the ratios of the peak areas of the keto and enol forms. With automated recording of the areas in the region of the plateau between the peaks of the tautomers, these values were added to the area of the first peak under the condition tR < [tR(1) + tR(2)]/2, and to the area of the second peak if this condition was not met. The retention indices calculation and statistical data processing was performed using the Excel software (Microsoft Office, 2010), Origin (version 4.2), and QBasic program.
RESULTS AND DISCUSSION
Characteristic Features of the Chromatographic Profiles of the Tautomers of Ethyl Acetoacetate
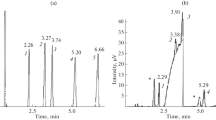
A fragment of the chromatogram of a solution of ethyl acetoacetate (II) in ethyl alcohol in the mode of temperature programming is shown in Fig. 1. Based on the ratio of the intensities of the peaks and in accordance with the literature data on the order of elution of the tautomers [15], the weaker peak with a shorter retention time was attributed to the enol form of the ester. As we expected, the intensity of the signals of the molecular ions of the enol form is higher, due to the presence of a conjugation system. The EI mass spectra of the tautomers are generally similar, but we can notice certain differences between them, e.g., in the intensities of the peaks with m/z= 85 and 69.
No. 1 (enol form), m/z ≥ 39 (Irel, %): 130 (17) M, 115 (5), 102 (12), 88 (16) [M − CH2CO], 87 (16), 86 (3), 85 (31) [M − C2H5O], 84 (15), 70 (3), 69 (24), 61 (4), 60 (8), 58 (4), 56 (2), 55 (2), 45 (5), 44 (3), 43 (100) [CH3CO], 42 (12), 41 (3), 39 (3).
No. 2 (keto tautomer), m/z ≥ 39 (Irel, %): 130 (6) M, 115 (2), 102 (6), 88 (20) [M − CH2CO], 87 (4), 85 (13) [M − C2H5O], 84 (2), 70 (3), 69 (4), 61 (5), 60 (11), 58 (2), 45 (4), 44 (3), 43 (100) [CH3CO], 42 (12), 41 (2).
The only mass spectrum of ethyl acetoacetate presented in the database [14] corresponds approximately to the superpositioning of the two presented spectra. The intensity of the signal with m/z= 69 is 10%.
The specific contour of the chromatographic signal in the region between the peaks of the tautomers, the level of which does not reach the baseline and forms a certain plateau (Fig. 1), is worthy of attention. The recording of the mass spectra at different points of such plateaus observed for some other compounds that reversibly or irreversibly transform into one another during separation in a chromatographic column [19, 20] also shows they are superpositions of the mass spectra of the individual tautomers at different ratios. This shape of the chromatograms clearly testifies to keto \( \rightleftarrows \) enol interconversion as the zones of the analytes move along the column. Similar profiles of the chromatographic signals were observed for four ketoesters [15] and diacetyl on an additionally oxidized RTx-1701 polar stationary phase [21].
Gas chromatographic analysis of the solutions of II under isothermal conditions at different temperatures of the column (60 to 100°C) and a constant temperature of the injector allowed us to characterize the temperature dependence of the retention indices of each of the tautomers and the impurity found in the sample of II, and to determine the character of the dependence of equilibrium constants K, which are equal to the ratios of the peak areas of the tautomers K = Srel = S(enol)/S(keto) on the temperature of the column.
Figure 2 compares fragments of the chromatograms of the tautomers of II under isothermal conditions at column temperatures of (a) 60 and (b) 100°C, which display the notable dependence of the degree of their separation on temperature. The plateau between the peaks reaches its minimum value at 60°C, but its height grows along with temperature. We can draw a conclusion about the incomplete separation of the tautomers already at 100°C, for two reasons. First, the enol and keto forms of ethyl acetoacetate are characterized by different coefficients β of the temperature dependence of retention indices RI(T):
where RI(T) is the value of the retention index at temperature T, RI(T0) is the value at a temperature T0 = 0°C (conditionally), and β = dRI/dT is the coefficient of the temperature dependence of the retention indices. The values of RI(T0) and β were calculated using the least squares method. By processing the data in the 60–100°C range of temperatures, we obtained the results (relative to the value of RIprogr under the conditions of temperature programming) presented in Table 1.
It follows from β = 0.02 ± 0.02 index units/K that the value of the RI of the keto form of ethyl acetoacetate depends weakly on temperature. At the same time, the positive value of β = 0.19 ± 0.03 for the enol form means that the distance between the peaks of the tautomers in the scale of retention indices falls upon an increase in the temperature of the chromatographic column. The reason for the higher value of coefficient β of the enol form of II is the presence of a >C=C–C=O conjugated system in its molecule. The abnormal negative value of the coefficient β for the impurity (−0.10 ± 0.01) should therefore be noted. For most organic compounds, such a value excludes the presence of conjugation systems in the molecule; it is also typical of compounds with functional groups that contain active hydrogen atoms. The rate at which of the keto \( \rightleftarrows \) enol equilibrium is established grows along with temperature, raising the height of the plateau between the peaks of the tautomers. In theory, a subsequent increase should result in the peaks of the tautomers merging (as with the temperature of coalescence in NMR spectroscopy [18]).
Analysis of the solutions of II at different temperature of the gas chromatographic column confirms there was a temperature dependence for the relative areas of the peaks. For example, the values of Srel = S(enol)/S(keto) < 1 fall as the temperature rises, as is illustrated by the data of Table 2. However, it is impossible to linearize the dependence of Srel on the temperature of the chromatographic column according to the Antoine equation
where coefficients a and b are calculated using the least squares method.
The reason for this is the different residence times of the analytes in the heated chromatographic column. Their dependence on temperature is characterized by the equation [22]
As a result, linearization of dependence Srel = f(T) is only possible by combining correlations (2) and (3) into one that contains the exotic function of double logarithm ln ln Srel [21, 23], which is beyond the scope of this work. Instead, it is better to consider the simpler dependence of Srel on the temperature of the injector at a constant temperature of the chromatographic column.
Dependence of the Relative Peak Areas of the Tautomers of Ethyl Acetoacetate on the Injector Temperature
Table 3 presents the average values of relative areas Srel = S(enol)/S(keto) and quantities reciprocal to them at different temperatures of the injector (100 to 220°C) for the solutions of ethyl acetoacetate in ethyl alcohol, while Table 4 presents similar data for the solutions in hexane. The temperature of the column was held constant at 70°C. Tables 3 and 4 also include the ratios of the peak areas of the enol and keto forms of ethyl acetoacetate to the peak area of the inert component of the mixture, n-alkane C8H18 (see below). According to the literature data, the concentration of the enol form in a nonpolar solvent (hexane) is higher than that of the polar solvent (ethanol) (39 and 7.2%, respectively [5]). However, the values of Srel of the tautomers differ slightly for both solvents at all temperatures of the injector. For example, the values of Srel = S(keto)/S(enol) grow by 1.9 times upon raising the temperature from 100 to 200°C for solutions of II in ethanol. For solutions in hexane, they grow by 2.2 times. We may therefore state that the temperature variations of the position of the keto \( \rightleftarrows \) enol tautomeric equilibrium established from the data of gas chromatography are mostly associated with the vapor phase, where the effect the nature of the solvent has on the ratio of the tautomers is minimal, rather than the condensed phase. In addition, the observed differences could be due not to the effect the nature of the solvents has on the position of the tautomeric equilibrium but to those of discriminating between the compositions of the analyzed samples (see below).
Calculating the parameters of linear regression of ln Srel on the reciprocal temperature (as 1000/T with subsequent recalculation) using the least squares approach and the equation
allows us to estimate the values of ΔH# and ΔS# for the tautomeric equilibrium.
Figures 3 and 4 present dependences (4) for the solutions of ethyl acetoacetate in ethyl alcohol and hexane. The parameters of the linear regression equations are specified in the corresponding figure captions. The values of ΔH# and ΔS# calculated with these data are −9.3 ± 0.3 kJ/mol and −32.3 ± 0.7 J K−1 mol−1 for ethanol and −10.9 ± 0.3 kJ/mol and ‒35.8 ± 0.8 J K−1 mol−1 for hexane. The negative signs of the values of ΔH# and ΔS# and the order of their absolute values generally agree with those determined in [21], where they were attributed to the interaction between the tautomers and the polar stationary phase of the chromatographic column, rather than to the equilibrium in the vapor phase.
The negligible value of |ΔH#| ∼ 10 ± 1 kJ/mol explains the readiness of the mutual transformations of the tautomers at relatively low temperatures of gas chromatographic separation to manifest in the specific profiles of the chromatograms.
Effects of Discriminating between the Compositions of the Samples
Any gas chromatographic determinations requiring the dosing the samples into capillary columns with the splitting of the flow at different temperatures of injector or in different solvents must consider the possible distortion of the results due to so-called effects of discriminating between the composition of the samples [23–26]. One of their most typical manifestations is variation in the absolute and relative peak areas, depending on the temperature of the chromatograph’s injector and the nature of the solvent. In the case considered in this work, the characterization of dependence Srel = f(T) for tautomers assumes variations in the temperature of the injector; our comparison of the parameters of this dependence for polar ethanol and nonpolar hexane, the influence of the second factor.
To discuss the effects of discriminating between the compositions of the samples when injecting them into capillary columns with flow splitting, we must go back to considering the data in Table 2. In addition to the relative peak areas of the tautomers of II (a solution in ethyl alcohol) at different temperatures of the chromatographic column, it also gives the ratios of the peak areas of each of the tautomers to those of the inert components, hydrocarbon n-C8H18, S(enol)/S(C8) and S(keto)/S(C8): 0.27 ± 0.01 and 1.10 ± 0.01, respectively. This confirms there is no dependence of the specified ratios on the temperature of the column (no effects of discrimination), so the variations in S(enol)/S(keto) and S(keto)/S(enol) are due exclusively by dependence Srel = f(T) for the tautomers.
In addition to the values of S(enol)/S(C8) and S(keto)/S(C8), Table 3 presents similar S(enol)/S(C8) and S(keto)/S(C8) ratios that show pronounced temperature dependences (descending for the first ratio and ascending for the second ratio). These dependences are illustrated graphically in Fig. 5. The temperature dependence of the sum of these ratios is of special interest, rather than their temperature dependences individually. Table 3 additionally presents the values of [S(enol) + S(keto)]/S(C8), while the curve corresponding to the polynomial approximation of the temperature dependence of the half-sum ([S(enol)/S(C8) + S(keto)/S(C8)]/2) is presented in the graph for illustrative purposes. The keto and enol forms of ethyl acetoacetate are isomeric, which predetermines the flame ionization detector’s similar sensitivity to them, but the data of Table 3 and Fig. 5 reveal a notable increase in the ratios [S(enol) + S(keto)]/S(C8) when the temperature was from 100 to 220°C (1.27 times). This is explained by the effects of discrimination [23–26], since the relative peak areas of the higher-boiling components increase with the increase in the temperature of the injector (Tb = 180.8°C for ethyl acetoacetate and 125.7°C for n‑octane).
(Color online) Graphic representation of the dependences of the relative peak areas: (1) S(enol)/S(С8), (2) S(keto)/S(С8), and (3) S(С10)/S(С8) (for comparison) on the temperature of the injector. The solid line is the result of the polynomial approximation of the ascending dependence of the average values of [S(enol)/S(C8) + S(keto)/S(C8)]/2 on temperature (according to the data for a solution of ethyl acetoacetate in ethyl alcohol).
Figure 5 also presents a set of points corresponding to the relative peak areas of compounds with the same chemical nature, reference hydrocarbons n-C10H22 and n-C8H18, indicating there is no expressed temperature dependence for this pair of components. A similar temperature dependence of the [S(enol) + S(keto)]/S(C8) ratios is also observed for the solutions of ester II in hexane, but the corresponding data were not included in Table 4 to reduce the volume of this section.
The effects of discrimination do affect the characteristics of the temperature dependence of the relative peak areas of the tautomers, and thus the reliability of determining the thermodynamic parameters of the keto \( \rightleftarrows \) enol tautomeric equilibrium. Note that the S(keto)/S(C8) ratios for the solutions of II in ethanol (Table 3) grow by 2.2 times upon an increase in temperature, while the estimate allowing for the effects of discrimination corresponds to an increase of approximately 1.3 times. More precise consideration of their effect requires special consideration and improved ways of processing the experimental data.
Identifying the Unknown Impurity in a Sample of Ethyl Acetoacetate
In addition to two tautomers with RI = 880 and 909 (the values for the mode of temperature programming), an impurity with RI = 957 with the following EI mass spectrum was detected in a sample of ethyl acetoacetate after long-term storage: m/z ≥ 39 (Irel ≥ 2%): 146 (1), 119 (1), 75 (17), 74 (74), 73 (24), 59 (8), 57 (17), 56 (17), 47 (8), 46 (7), 45 (100), 44 (18), 43 (40), 42 (6), 41 (11).
A characteristic feature of this mass spectrum is two intense signals with m/z = 45 and 74 that belong to homologous groups y = 3 and 4. Such combinations of mass numbers are rarely encountered in the mass spectra of organic compounds; a library search for these two peaks using the NIST database [14] resulted in only ten alternative responses with coincidence factors of less than 0.5, so none of them can be accepted for further consideration. Identification according to the numbers of the homologous groups of the main signals and the mass spectra of ion series [27] also yielded no positive results. Attempts to determine the nature of this component from the scheme of ethyl acetoacetate synthesis and a hypothesis about the possible formation of the ethyl ether in enol form were fruitless [28]. It should be noted that any derivatives of enol form II must be excluded from consideration, based on the negative value of the coefficient of the retention indices’ temperature dependence (β = ‒0.10 ± 0.01). The peak with RI = 957 thus belongs to unidentified component X.
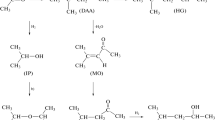
We can still, however, determine its structure through joint interpretation of the EI mass spectrum and retention index on a standard nonpolar stationary phase. The weak signal in the mass spectrum with a maximum value of m/z = 146 most likely belongs to molecular ions. This is confirmed by the presence of one more weak signal with m/z = 119 in the mass spectrum, which corresponds to the [M − C2H3]+ fragment ions typical of ethyl esters of carboxylic acids (bond cleavage with double hydrogen rearrangement [29]). The formation of a compound with M = 146 from ethyl acetoacetate C6H10O3 with M = 130 thus results from its oxidation, which agrees with the long-term storage of the sample in contact with air. Oxidation proceeds with air dissolved in ester II via a free-radical mechanism.
The hydroperoxides and polymer peroxides formed in this case are unstable when heated, and thus cannot be used in gas chromatographic analysis. Observed component X in this case likely has the structure of ethyl-2-hydroxy-3-oxobutanoate C6H10O4:

This compound is mentioned in literature sources, as can be seen from its CAS no. 15863-59-9. However, we could not find any of its physicochemical characteristics or spectral data for it. We can only assume that the intense signal in the mass spectrum with m/z = 74 corresponds to ions that form as a result of C–C bonds cleavage with the migration of hydrogen atoms; e.g., M+ (m/z = 146) → [M − CH3COCHO]+ ([M − 72] = 74). In determining the structure of this compound, the main source of information should therefore be the coincidence between the experimental value of its gas chromatographic retention index (957 in the mode of temperature programming) and the value calculated theoretically, based on its structure.
Of all known ways of predicting gas chromatographic retention indices, an algorithm based on the hypothetical assembly of the required molecular structure from those of simpler analogs, followed by arithmetic operations (adding and subtraction) using known values of the RIs of such analogs, would appear to be the one most informative [30–32]. This is in fact a version of an additive scheme. The solution to the problem is in this case complicated by the presence of an intramolecular hydrogen bond. As a result, its contribution to the additive estimate of the RI must be determined. We therefore compare, e.g., the estimate of the RI of 2-hydroxy-3-pentanone with a similar hydrogen bond to the experimental value of the RI of this compound. Data from database [14] are used as the RI reference values:

The increment of the intramolecular hydrogen bond not considered in this additive scheme is 862 − (787 ± 10) = 75 ± 10 index units. In a similar manner, we then assemble the structure of target ethyl-2-hydroxy-3-oxobutanoate while allowing for the obtained increment:

We eventually obtain 1027 − 75 = 952.
The estimate of the standard deviation of the obtained result is a square root of the sum of the squares of the standard deviations of all the data used in the calculations, i.e., (42 + 52 + 102 + 22 + 22)1/2 ≈ 12. The calculated value of RI = 952 ± 12 thus coincides with the experimental value (957), which can in this case be considered conclusive evidence of the correctness of our proposed structure of component X: ethyl-2-hydroxy-3-oxobutanoate.
CONCLUSIONS
Characteristic features of the gas chromatographic separation of the tautomers of ethyl acetoacetate II were considered in this work.
• The absence of a dependence of the relative peak areas of the tautomers on the nature of the solvent at different temperatures shows that the results from gas chromatographic analysis predominantly reflect the position of the keto \( \rightleftarrows \) enol equilibrium in the vapor phase of the injector of the chromatograph.
• This results in the similar values of the thermodynamic parameters (standard enthalpy and entropy of activation) of the tautomeric equilibrium determined using solutions of ethyl acetoacetate in different solvents.
• The temperature variations of the relative peak areas of the tautomers notably exceed their temperature variations due to the effects of discriminating between the compositions of the samples dosed into capillary columns with splitting of the flow. We may nevertheless assume that discrimination effects particularly introduce certain errors into the results from determination.
• The low absolute values of ΔH# result in the ease of the interconversions of the tautomers during gas chromatographic separation. Their separation is apparently impossible at column temperatures of 100°C and above.
• It was found that the keto and enol tautomers of ethyl acetoacetate are characterized by substantial differences in the temperature coefficients of the gas chromatographic retention indices on standard nonpolar phases.
• Using a modified version of the additive schemes for estimating gas chromatographic retention indices allowed us to identify an impurity in a sample of ethyl acetoacetate as the product of its oxidation by dissolved atmospheric oxygen: ethyl-2-hydroxy-3-oxobutanoate.
REFERENCES
V. I. Minkin, L. P. Olekhnovich, and Yu. A. Zhdanov, Molecular Design of Tautomeric Compounds (Kluwer, Dordrecht, Boston, Tokio, 1958). https://doi.org/10.1007/978-94-009-1429-2
J. N. Spencer, E. S. Holmboe, M. R. Kirshembaum, D. W. Firth, and P. B. Pinto, Can. J. Chem. 68, 1178 (1982).
P. Umnahanant and J. S. Chickos, J. Chem. Eng. Data 50, 1720 (2005). https://doi.org/10.1021/je050179z
D. Antic, Thermo Scientific Application Note No. AN52327 (2017).
M. T. Rogers and J. L. Burdett, Can. J. Chem. 43, 1516 (1965).
D. Tiess and Z. Wiess, Willhelm-Pieck-Univ. Rostock Math. Naturwiss. Reiche 33, 6 (1984).
T. L. Peppard, J. Agric. Food Chem. 40, 257 (1992). https://doi.org/10.1021/jf00014a018
E. Tudor, J. Chromatogr., A 779, 287 (1997). https://doi.org/10.1016/S0021-9673(97)00453-6
E. Tudor, D. Moldovan, and N. Zarna, Rev. Roum. Chim. 44, 665 (1999).
M. J. Jordan, K. L. Goodner, and P. E. Shau, J. Agric. Food Chem. 50, 1523 (2002). https://doi.org/10.1021/jf011077p
M. Adamova, A. Orinak, and L. Halas, J. Chromatogr., A 1087, 131 (2005). https://doi.org/10.1016/j.chroma.2005.01.003
J. A. Pino, J. Mesa, Y. Munos, M. P. Marti, and R. Marbot, J. Agric. Food Chem. 53, 2213 (2005).https://doi.org/10.1021/jf0402633
F. Bianchi, M. Careri, A. Mangia, and M. Musci, J. Sep. Sci. 39, 563 (2007).
The NIST 17 Mass Spectral Library (NIST17/2017/EPA/NIH), Software/Data Version (NIST17), NIST Standard Reference Database, Number 69 (Natl. Inst. Standards Technol., Gaithersburg, MD, 2017). http://webbook.nist.gov. Accessed October 2019.
M. Masur, H. F. Grutzmascher, H. Munster, and H. Budzikieicz, Org. Mass Spectrom. 22, 493 (1987).
P. E. Allegretti, M. M. Schiavoni, H. E. di Loreto, and J. J. P. Furlong, and C. O. Della Vedova, J. Mol. Struct. 560, 327 (2001).
S. J. Ruggiero and V. M. Luaces, J. Chem. Educ. 65, 629 (1988). https://doi.org/10.1021/ed065p629
V. Krishman, Inventions 4, 15 (2019). https://doi.org/10.3390/inventions4010013
T. A. Kornilova, A. I. Ukolov, R. R. Kostikov, and I. G. Zenkevich, Rapid Commun. Mass Spectrom. 27, 461 (2013). https://doi.org/10.1002/rcm.6457
I. G. Zenkevich and N. E. Podol’skii, Anal. Kontrol’ 21 (2), 125 (2017). https://doi.org/10.15825/analitika.2017.21.2.002
P. J. Skrdla, V. Antomucci, and C. Lindemann, J. Chromatogr. Sci. 39, 431 (2001).
Guide on Gas Chromatography, Ed. by E. Leibnitz and H. G. Struppe (Akad. Verlag, Leipzig, 1966; Mir, Moscow, 1988).
K. Grob and H. P. Neukom, J. Chromatogr., A 236, 297 (1982). https://doi.org/10.1016/S0021-9673(00)84878-5
I. G. Zenkevich and D. A. Olisov, Labor. Pr-vo, No. 2, 92 (2018).
I. G. Zenkevich and E. Leleev, Anal. Kontrol’ 23, 110 (2019). https://doi.org/10.15826/analitika.2019.23.1.012
I. G. Zenkevich and D. A. Olisov, J. Anal. Chem. 74 (Suppl. 1), S32 (2019). https://doi.org/10.1134/S1061934819070190
I. G. Zenkevich and V. V. Ioffe, The Interpretation of Mass-Spectra of Organic Compounds (Khimiya, Leningrad, 1986) [in Russian].
I. G. Zenkevich and V. M. Lukina, Anal. Kontrol’ 23, 410 (2019). https://doi.org/10.15826/analitika.2019.23.3.009
M. C. Hamming and N. G. Foster, Interpretation of Mass Spectra of Organic Compounds (Academic, New York, 1979).
I. G. Zenkevich, M. Moeder, G. Koeller, and S. Schrader, J. Chromatogr., A 1025, 227 (2004). https://doi.org/10.1016/j.chroma.2003.10.106
I. G. Zenkevich and A. I. Ukolov, Russ. J. Gen. Chem. 81, 1818 (2011). https://doi.org/10.1134/1070363211090143
I. G. Zenkevich and A. I. Ukolov, Mass-Spektrom. 8, 264 (2011). https://doi.org/10.1134/S1061934812130114
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Boltukhina
Rights and permissions
About this article
Cite this article
Zenkevich, I.G., Lukina, V.M. Characteristic Features of the Gas Chromatographic Separation of Tautomers of Ethyl Acetoacetate. Russ. J. Phys. Chem. 94, 1214–1223 (2020). https://doi.org/10.1134/S0036024420060357
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420060357