Abstract
The surface composition and surface tension of Ga–Tl binary melts were studied in situ by Auger electron spectroscopy and sessile drop method over the entire range of volume concentrations at temperatures from liquidus temperatures to 773 K. The surface was shown to be enriched with thallium, whose concentration increases with temperature. The differences between the obtained surface tension values and the literature data were revealed, and reasons for them were analyzed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Currently, there are many techniques and instruments for determining the characteristics of metals and alloys in the liquid state such as surface tension, density, work function, etc. Their disadvantage is the inability to control the state of the surface and its chemical composition. In this case, the adsorption and the surface concentrations of components in alloys are calculated, if necessary, using the experimental values of surface tension. However, there are unique methods that allow direct analysis of the surface composition, both in the solid and liquid state. Today the most popular methods are photoelectron spectroscopy and Auger electron spectroscopy. It was interesting to experimentally measure in situ the surface tension and surface composition of metals, combining the capabilities of surface analysis techniques and one of the methods for surface tension determination. The goal of this study was to investigate the surface of Ga and Tl and their binary alloys in the liquid state under the same experimental conditions simultaneously using Auger spectroscopy and the sessile drop method.
EXPERIMENTAL
The experiments were performed on an ultrahigh-vacuum unit (10–8 Pa) of a surface spectrometer, which made it possible to study a liquid metal drop formed according to the rules of the sessile drop method. A detailed description of the unit was given in [1]. The liquid under study was placed on a graphite substrate in the form of a sessile droplet with a maximum diameter. The surface tension was calculated by the sessile drop method [2] using the tables of [3]; the surface concentration was calculated taking into account the matrix effects [4].
The alloys were prepared from metals with 99.99 at % (Tl) and 99.999 at % (Ga) purity in the working chamber of an Auger spectrometer. In addition to pure metals, the surfaces of four gallium–thallium melts with 4.8, 12.2, 83.5, and 92.9 at % Tl at temperatures from liquidus temperatures to 773 K were studied by these methods. After the preparation of the alloys in ultrahigh vacuum, the Auger spectra of their surface contained the lines of the Auger electrons of carbon (KLL, 270 eV), oxygen (KLL, 510 eV), and additionally sulfur for gallium (LMM, 150 eV) in addition to the metal peaks. The surface contaminants were most effectively removed by heating at 573 K with simultaneous ionic bombardment of the surface (Ar+, 600 eV, 1–2 μA) for a few hours.
After the sample was kept at the temperature of experiment for 30–40 min at a heating/cooling rate of 1 K/min, Auger surface analysis for the presence of contaminants was performed; if the latter were absent (or were at a minimum level), the Auger spectrum was recorded, which was used for calculations of surface concentrations. At the same time, a high-resolution photo of the droplet profile was taken.
The time of experiment was a few dozens of minutes. During this time, the surface of the sample was contaminated as a result of adsorption from the residual gas; therefore, periodic flash heating with ion bombardment at 573 K was used to remove the adsorbed layers.
RESULTS AND DISCUSSION
The experiments showed that the Auger spectrum of thallium was characterized by Auger lines of the NOO series at 84 eV, and the lines of pure liquid gallium had a rather intense LMM peak at 1070 eV. For liquid metals, the intensity ratio Tl/Ga of the Auger lines was 1.1. The indicated peaks were used to evaluate the surface concentrations of the binary alloys.
The surface tension of pure gallium and thallium was measured under the given conditions simultaneously with monitoring the state of the surface. Each value was determined with an accuracy of 3%. The results of the surface tension measurements of pure gallium and thallium are well approximated by the equations
where Tm is the melting point of the metal. The results of these studies are given in Table 1 in comparison with the literature data.
According to Table 1, the scatter of values is rather wide for both metals. Many researchers recommended the σ values given in [5]. Several publications suggested higher σ values. For example, according to Wobst [9], the surface tension of pure thallium at the melting temperature is 513 mN/m, whereas the values recommended in [5] are much lower.
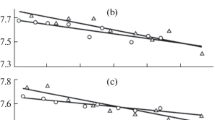
The Ga–Tl system is one of the few systems whose melts were studied by us at different times. Previously, surface tension measurements were performed by the “large” drop method in the “classical” version, which usually suggests using a glass measuring cell placed in a thermostat (unpublished data of 1976). In this case, the surface tension isotherm had clearly pronounced extrema, which were explained by the formation of clusters in the surface layer of the melts (curve 1, Fig. 1).
The surface tension measurements with strict control of the situation on the surface gave the σ values used to construct the isotherm shown in Fig. 1 (curve 2). In addition to pure metals, the surface tension was measured for eight alloys with 4.8, 12.2, 25.7, 37.8, 47.3, 62.3, 83.5, and 92.9 at % Tl. The results of the calculation of the surface composition are shown in Fig. 1 (curve 3). Segregation for this system was studied only for solutions adjacent to the pure components because of the high Tl vapor pressure and the peculiarities of the state diagram of the Ga–Tl system.
The above surface tension values are higher than the literature values. The difference can be explained by the following. The literature data on σ were mostly obtained in a vacuum of 10–3–10–5 Pa, where the effect of residual gases on the surface state is rather strong. In some papers [12], the residual pressure in the working chamber was 10–7 Pa, but effective measures to clean the metal surface were not taken, and the σ values for Ga and Pb [12] were obtained at the level of the commonly accepted reference values. At the same time, it is known that even under ultrahigh vacuum conditions, a monolayer coating of surfactant impurities forms within a few seconds on the atomically clean metal surface, which can significantly reduce the surface tension. Later, the effect of these carbon-containing impurities on the surface tension was evaluated in [1] by measuring σBi immediately after the formation of a sessile drop and after obtaining an atomically clean surface, while strictly monitoring the surface condition.
In contrast to the literature data, our σ values were obtained in the absence of oxygen in the residual atmosphere of the working chamber due to the use of ion pumps at the main stage of the ultrahigh-vacuum station. The effect of oxygen on σ was considered using indium as an example in our previous study [13]. After creating a sessile drop, σIn was measured in ultrahigh vacuum while gradually removing the surfactant impurities and during the exposure in an oxygen medium in the range from 0 to 4000 L starting from the atomically clean surface [13].
In all the studies discussed above, the presence of surfactant impurities decreased the σ values; as the carbon-containing compounds were removed from the sample surface, the surface tension increased by a few dozen mJ/m2. The σ values presented in this paper evidently cannot be used as reference data because they were obtained under nonequilibrium conditions. This is confirmed by continuous evacuation during the experiment, the significant volume of the vacuum chamber compared with the sample size, and the presence of a temperature gradient between the design elements of the working chamber of the spectrometer and the sample.
The results may be affected by the incorporation of argon ions during the cleaning of the sample surface, which will change the profile of the drop. This possibility was confirmed by a computer simulation of ion-atomic collisions in SRIM (Stopping and Range of Ions in Matter) [14]. The mean free path of Ar+ ions used in the present work (600 eV) in gallium was calculated, which was 1.7 nm.
We tried to assess the effect of each factor on the results by reducing the power of the evacuation system, leveling the temperature of the design elements of the unit, and changing the energy of argon ions, but the resulting deviations were within the measurement error of 3%.
Thus, the results were a consequence of several effects that cannot be refined under our conditions: the lack of equilibrium with the vapor phase, cleaning the surface to the atomically clean state, and possible dissolution of argon, used for etching the surface, in the surface layers of Ga.
REFERENCES
O. G. Ashkhotov, I. B. Ashkhotova, M. A. Aleroev, and T. T. Magkoev, Russ. J. Phys. Chem. A 91, 386 (2017).
U. I. Naidich and V. K. Eremenko, Fiz. Met. Metalloved., No. 11, 883 (1961).
B. Bashfort and J. Adams, An Attempt to Test the Theories of Capillary Action (Cambridge Univ. Press, Cambridge, 1883).
O. G. Ashkhotov and A. A. Shebzukhov, Poverkhnost, No. 3, 64 (1983).
V. I. Nizhenko and A. I. Floka, Surface Tension of Metals and Alloys (Metallurgiya, Moscow, 1981), p. 206 [in Russian].
Kh. B. Khokonov, S. N. Zadumkin, and B. B. Alchagirov, Dokl. Akad. Nauk SSSR 210, 899 (1973).
B. Kh. Unezhev, S. N. Zadumkin, and M. M. Makhova, in Physical Chemistry of the Liquid Surface (1977), p. 209 [in Russian].
U. V. Arsamikov et al., Adgeziya Raspl. Paika Mater., No. 25, 26 (1991).
M. Wobst and Z. D. Wiss, Wissensch. Zeitschr. Tech. Hochschule Karl-Marx-Stadt 12, 393 (1970).
B. B. Alchagirov, M. A. Kokov, and Kh. B. Khokonov, in Physics of Interphase Phenomena (Nal’chik, 1976), p. 42 [in Russian].
Kh. I. Ibragimov, N. L. Pokrovskii, and V. K. Semenchenko, in Surface Phenomena in Melts (Nal’chik, 1965), p. 269 [in Russian].
C. Serre, P. Wynblatt, and D. Chatain, Surf. Sci. 415, 336 (1998).
O. G. Ashkhotov, M. V. Zdravomyslov, R. V. Plyushchenko, and A. V. Sardlishvili, Russ. J. Phys. Chem. A 71, 121 (1997).
www.srim.org
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Smolina
Rights and permissions
About this article
Cite this article
Ashkhotov, O.G., Ashkhotova, I.B. Surface Tension and Concentration Isotherms of Gallium–Thallium Binary Melts. Russ. J. Phys. Chem. 93, 2137–2139 (2019). https://doi.org/10.1134/S0036024419110037
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419110037