Abstract
The electrochemical behavior of ascorbic acid (AA) is studied by means of cyclic voltammetry (CVA) and square-wave voltammetry (SWVA) on a boron doped diamond electrode (BDD). The possibility of using the voltammetric response signal to determine quantitatively the concentration of AA in an aqueous solution is shown. An analytical direct correlation function for SWVA at BDD is obtained. It is shown that the detection limit for AA is 1.87 μM. The applicability of SWVA for determining the content of AA in pharmaceutical preparations is demonstrated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Water-soluble vitamin C (L-ascorbic acid (AA)) plays an important role in the human body. AA is easily oxidized to L-dehydroascorbic acid both chemically and electrochemically. This property is widely employed in developing electrochemical ways of determining the content of AA in different pharmaceutical formulations and foodstuffs [1–3]. To improve the sensitivity and selectivity of electrochemical sensors, their surfaces are often modified in different ways [4–13]. Titration, liquid chromatography, and UV spectroscopy are also used to determine AA. The aim of this work was to assess the possibility of using a boron doped diamond electrode (BDD) in electroanalytical methods such as cyclic voltammetry (CVA) and square wave voltammetry (SWVA).
EXPERIMENTAL
AA manufactured by Sigma was used as the reference substance. Solutions for electrochemical measurements were prepared with bidistilled water. Electrochemical measurements were made in a Britton–Robinson (BR) buffer solution prepared from phosphoric, boric, and glacial acetic acids. The pH was adjusted with NaOH. Electrochemical measurements were performed using a Pro ZAO KRONAS IPC (computer-controlled potentiostat) in a standard three-electrode cell with a silver/silver chloride reference electrode (Ag/AgCl 3 M KCl) and an auxiliary electrode made from a Pt plate with an area of 0.1 cm2. BDD with a niobium substrate that had a working area of 0.1 cm2 (Condias, Germany) was used as the working electrode.
RESULTS AND DISCUSSION
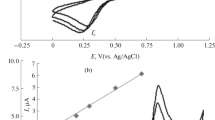
The CVA for AA on BDD in a background solution (0.1 eq/L Na2SO4) with AA concentration of 2.27 mM (0.4 g/L) is shown in Fig. 1. It can be seen that the AA gave a clear response. Dependences and of the peak currents Ip,a and peak potentials Ep,a on potential sweep rate ν are shown in Figs. 2 and 3, respectively. Figure 2 shows that the dependence of the values of Ip,a on ν0.5 is linear for AA on BDD. This means the rate-limiting step in the anodic oxidation of AA is the substrate’s mass transferred to the surface of the anode [14]. This agrees with the literature data, testifying to diffusion control of the reaction [6–9].
Dependence of CVA peak current values (Ip,a) in Fig. 1 on the potential sweep rate.
Dependence of CVA peak potentials (Eр,а) in Fig. 1 on the potential sweep rate.
From Fig. 3, we can see that the dependence of Ер,а on the value of ν is described by the equation [14]

where E0 is the formal potential; F is the Faraday constant; R is the gas constant, equal to 8.135 J/(mol K); T is the absolute temperature of the experiment, 298 K; ks is the reaction rate constant; α is the coefficient of electron transfer; and nα is the number electrons that participate in the limiting stage. In [15], value nα was identified with the total number of electrons participating in the electrode process and was taken to be 2. If we follow this reasoning, the dependence of Ep,a on ln ν (a straight line with a slope of 27.4 mV) the value of α is 0.15. If we nevertheless accept the more realistic approximation that one electron participates in the limiting stage, as is done in most works (i.e., nα = 1), the value of α is 0.3, making the considered process irreversible.
For irreversible anodic processes, Ip,a can be calculated using the Randles–Ševčík equation [16]
where c0 is the concentration of AA in the solution; nα is the number of electrons participating in the limiting stage (1.0); n1 is the total number of electrons participating in the oxidation of the substance per molecule; and D is the coefficient of AA diffusion. For the oxidation of AA [15], n1 = 2, according to the equation

The value of D, calculated from the experimental data shown in Fig. 1, is 5.6 ×10−7 cm2 s−1, which is close to the one obtained in [15].
The most important factor in developing an electroanalytical method for determining the content of AA is the level of noise on the CVA, which represents non-faradaic currents. In the scenario described above, the level of noise is quite high. This reduces the accuracy of the calculated response currents, which are a measure of the concentration of AA in the solution.
Considerable improvement was achieved using SWVA. It was found that non-faradaic currents make only a slight contribution to the total electrochemical response, and there are well-pronounced peaks of AA oxidation at E = 703 mV on the SWVA curves.
To determine the conditions for electroanalytical SWVA registration at an AA concentration of 200 μM, we optimized the analytical parameters of SWVA (pulse frequency f, pulse amplitude a, and potential increment ΔE). The dependence of Ip,a on frequency f was therefore determined. It was found that the response currents grew along with f, and the current maximum shifted to the anodic region. In the interval f = 20–60 Hz, the magnitude of Ip,a depends linearly on f 0.5. This dependence deviates from linear at high values of f. The dependences of Ip,a on a and ΔE remained linear up to a = 40 mV and ΔE = 3 mV, respectively. The following SWVA parameters were thus chosen for electroanalytical determination: a = 40 mV, f = 60 Hz, and ΔE = 3 mV.
With the selected parameters for BDD, we obtained SWVA for AA solutions with different concentrations (Fig. 4). Using the data in Fig. 4, we constructed an analytical straight line in the 20–200 μM range of AA concentrations (Fig. 5). The regression equation was

The reproducibility of these data was determined by repeating SWVA five times at an AA concentration of 200 μM. It corresponded to a relative standard deviation of 1.7%. The limit of AA detection was found to be 1.87 μM from the triple ratio of the standard deviation of background SWVA to the slope of the analytical line. The possibility of using CVA with a BDD to determine the content of AA in aqueous solutions was thus demonstrated.
This approach was used to determine the content of ascorbic acid in drugs. AA concentrations were calculated using the standard addition technique. It was established that the concentrations of AA determined in this manner were in good agreement with the calibration curve data. The calculated and experimentally obtained concentrations of AA in the tablets are presented in Table 1.
CONCLUSIONS
AA produces strong electrical signals under conditions of the linear or pulsed potential scanning of BDD. It was established that the lowest non-faradaic current is recorded on BDD under conditions of pulsed polarization. Optimizing the conditions of SWVA registration results in a detectable minimum of 40 µM for AA (0.007 g/L) and a relative standard deviation of 1.7% at 12.4 mM (0.2 g/L). It was shown that a combination of these techniques allows us to determine the content of AA in pharmaceutical preparations reliably over a wide range of working solution concentrations.
REFERENCES
R. Thangamuthu, S. M. Senthil Kumar, and K. Chandrasekara Pillai, Sens. Actuators, B 120, 745 (2007).
C. S. Erdurak-Kilic, B. Uslu, B. Dogan, et al., J. Anal. Chem. 61, 1113 (2006).
H. R. Zare and N. Nasirizadeh, Sens. Actuators, B 143, 666 (2010).
Sh.-M. Chen and W.-Y. Chzo, J. Electroanal. Chem. 587, 226 (2006).
S. I. Vijaykumar, M. Algarra, and A. Martins, Electroanalysis 16, 2082 (2004).
S. Thiagarajan, Ts.-H. Tsai, and Sh.-M. Chen, Biosens, Bioelectron. 24, 2712 (2009).
A. Nezamzadeh, M. K. Amini, and H. Faghihian, Int. J. Electrochem. Sci. 2, 583 (2007).
R. R. Protiva, S. S. Madhu, O. Takeyoshi, and O. Takeo, Electrochim. Acta 51, 4447 (2006).
T. Kondo, Y. Niwano, A. Tamura, et al., Electrochim. Acta 54, 2312 (2009).
S.-G. Park, J.-E. Park, E.-I. Cho, J.-H. Hwang, and T. Ohsaka, Res. Chem. Intermed. 32, 595 (2006).
D. A. Tryk, H. Tachibana, H. Inoue, and A. Fujishima, Diamond Rel. Mater. 16, 881 (2007).
S. Shahrokhian and M. Khafajia, Electrochim. Acta 55, 9090 (2010).
S. Shahrokhian and M. Khafajia, Electrochim. Acta 51, 347 (2005).
Z. Galus, Teoretyczne Podstawy Elektroanalizy Chemicznej (Panstw. Wyd. Naukowe, Warszawa, 1971).
M. Motahary, S. M. Ghoreishi, M. Behpour, and M. Golestaneh, J. Appl. Electrochem. 40, 841 (2010).
G. Henze, Polarographie und Voltammetrie: Grundlagen und analytische Praxis (Springer, Berlin, Heidelberg, 2001).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by M. Aladina
Rights and permissions
About this article
Cite this article
Vedenyapina, M.D., Kazakova, M.M. & Skundin, A.M. Voltammetric Determination of Ascorbic Acid in Pharmaceutical Formulations on a Boron Doped Diamond Electrode. Russ. J. Phys. Chem. 93, 1178–1181 (2019). https://doi.org/10.1134/S0036024419060335
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419060335