Abstract
Boron-doped diamond electrode has been utilized for the study of electrochemical behaviour of an anti-HIV herbal drug wedelolactone in Britton-Robinson buffer (pH-2.5) by square-wave and cyclic voltammetry techniques. The response characteristics of cyclic voltammetry and square wave voltammetry showed a remarkable increase in the anodic peak current and electrochemical impedance spectroscopy revealed a lowering in charge transfer resistance at the boron-doped diamond electrode as compared to the glassy carbon electrode that can be attributed to the higher sensitivity of boron-doped diamond sensor. Cyclic voltammetry at the boron-doped diamond surface revealed the oxidation of wedelolactone with two oxidation peaks (P1 and P2) with Ep1= 0.4V and Ep2=1.00 V with scan rate varying from 10 - 220 mV/s and exhibits diffusion-controlled process. Based on the electrochemical measurements, a probable oxidation mechanism has been deduced and the electrode dynamics parameters have been evaluated. The effect of concentration on the peak currents of wedelolactone was found to have a linear relationship within the concentration range of 50–700 ng/mL. The LOD and LOQ were found to be 43.87 and 132.93 ng/mL respectively. The applicability of the proposed method was further scrutinized by the successful determination of wedelolactone in real plant samples.

Voltammetric method for the determination of an anti-HIV herbal drug Wedelolactone was studied at boron doped diamond sensor. The redox mechanism was proposed and electrode dynamics parameters were explored. The two oxidation peaks were observed (p1 and p2) due to presence of dihydroxy and phenol moiety attached to the ring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Herbs are considered as the storehouse of various secondary metabolites having pharmacological potentials and their chemical partnership leads to favourable synergistic or additive effects with minimum side effects. Their bioactive principles tend to normalize physiological function and provide rational means for the treatment of many obstinate and incurable diseases.[1–3]
Wedelolactone (WDL), 7-Methoxy-5, 11, 12-trihydroxycoumestan, (scheme 1) forms the major constituent of the traditional medicinal plant Eclipta alba Hassk. (Asteracea), which is a perennial herb grown throughout India and Southwestern U.S, in moist and damp land.[4,5] The literature reveals that WDL is known to show anti-HIV activity against HIV-1 integrase (IN), exhibiting high inhibitory effect, and possesses a wide range of biological activities and therapeutic properties.[6–11]It was further found to suppress caspase-11 which plays an important role in regulating apoptosis by inhibiting IKK complex.[12] These reasons fasten the development of an efficient and reliable analytical method for the quantification of WDL.
Different analytical methods have been explored for the estimation and quantification of WDL which includes HPLC,[13–15]HPTLC,[16,17]UV spectrophotometry,[18] ICP-MS analysis, IR, EPR.[19] However, these methods are time consuming and involve derivatization steps prior to the final analysis. Apart from this, the aforementioned procedures involve excess amount of solvents, reagents and sophisticated instrumentation. On the other hand, electrochemical techniques are easy to use, highly sensitive, inexpensive, and provide quick response. Its application includes determination of redox properties of chemical constituents which provides insights into their metabolic fate and in vivo redox processes.[20–30]An extensive literature survey revealed that the electrochemical investigation has so far not been reported for the study of the redox behaviour of WDL.
Boron-doped diamond electrode (BDDE) has been the subject of considerable interest as an electrode material for various electroanalytical applications, as it is more advantageous over the traditionally employed electrodes (e.g., glassy carbon electrode or the platinum electrode).[31–37]This is because of its very wide potential window (upto 3 V) in aqueous solutions, low background currents, resistance to thermal shock, extreme electrochemical stability and corrosion resistant towards aggressive media.[38–41]Moreover boron has low charge carrier activation energy of 0.37 eV,[42] as a consequence of which it is by far the most widely used dopant to produce conducting diamond electrodes. Boron doping leads to a p-type semiconductor.[43]
The present work reports a new and explicit electrochemical method for the study of WDL by square wave voltammetry (SWV) and cyclic voltammetry (CV) at BDDE, using Britton-Robinson buffer (pH 2.5) as a supporting electrolyte. BDDE unveiled the mechanistic aspects of the phytoconstituent in the solution system. Electrochemical impedance spectroscopy (EIS) was employed to scrutinize the charge transfer resistance of the BDDE and GCE detailing higher sensitivity of the boron-doped diamond sensor as compared to the GCE. This method was further successfully employed for the investigation of WDL in plant samples.
2 Experimental
2.1 Instrumentation
All electrochemical studies were performed at a PC-controlled AUTOLAB PGSTAT 302N (Eco-Chemie B.V., Utrecht, The Netherlands) potentiostat-galvanostat with IME663 and software NOVA 1.8. EIS was carried out using FRA 2 module. A standard three electrode electrochemical assembly was used in the study that contained BDDE and GCE as the working electrode, platinum wire as counter and Ag/AgCl (3M KCl) as reference electrode that were fitted in one compartment cell, connected with electrochemical workstation through Metrohm 663VA stand. The electrochemical cell was fitted with the nitrogen gas bubbler. All pH measurements were made on a Mettler Toledo pH meter fitted with a glass electrode and a Ag/AgCl electrode as reference which was pre-standardized with buffers of known pH. All measurements were carried out at room temperature.
2.2 Materials and reagents
WDL standard (≥99%) was obtained from the Sigma Aldrich. Ultra-pure water (Milli-Q water with resistivity 18 M Ω.cm) was obtained from ELGA purification system (U.K.). Standard solution of WDL (1 mg/mL) was prepared by dissolving pure compound in methanol and was further diluted with britton-robinson buffer to get the concentration in the working range. To investigate WDL in real samples, plant of Eclipta alba (authenticated by Department of Botany, Dayalbagh Educational Institute, Agra) was purchased from commercial source. Solutions at all the stages of the study were prepared by using analytical grade reagents and were used without further purification.
2.3 Working analytical procedure
BDDE and GCE were polished against the alumina slurry (particle size 0.01 μm–0.3 μm) spread over the Buehler cloth, and copiously rinsed with deionized water followed by gently blowing under the nitrogen stream to remove the residual alumina particles. After this mechanical treatment, electrodes were electrochemically activated against a suitable electrolyte by applying a potential of + 1.3 V for 30 sec.
The stock solution of WDL (1 mg/mL) was prepared in methanol. Working solutions were prepared by further dilution with the supporting electrolyte to get the desired concentration range. Initially, a series of britton-robinson buffer (pH 2.5–12) were prepared in ultrapure water and used as supporting electrolytes. About 10 mL of electrolyte solution containing appropriate amount of standard WDL or sample was added to the electrolytic cell. Prior to all the electrochemical measurements, all the solutions were purged with the pure N2 gas to remove the interference due to O2. Then the electrodes were immersed and the voltammograms were recorded within the potential window of 0 to 1.4 V. Electrochemical impedance measurements were performed in the solution of 5mM K3Fe(CN)6 in 0.1 M phosphate buffer solution (PBS) by applying the AC voltage with 10 mV amplitude in a frequency range from 0.1Hz to 1000Hz. The results were plotted in the form of Nyquist plots. All voltammetric measurements were carried out at ambient temperature.
3 Results and Discussions
3.1 Electrochemical behaviour of WDL at BDDE and GCE
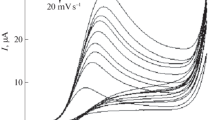
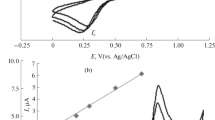
Electrochemical behaviour of WDL at BDDE and at the GCE was investigated by CV and SWV techniques. Figure 1 (A and B) represents the CV and SWV of WDL at the GCE (b) and at BDDE (c). At each of the electrodes, WDL exhibited two oxidation peaks that can be ascribed owing to the presence of functional –OH groups attached to ring structures.
Square wave voltammogram (B, amplitude: 20 mV, frequency: 25 Hz, step potential: 5 mV, scan rate: 125 mV/s) and Cyclic voltammogram (A, scan rate 70 mV/s) comparing the peak potential and current values of WDL obtained at b) GCE and c) BDDE, a) blank in BR buffer pH 2.5 at a concentration of 500 ng/mL and 700 ng/mL respectively.
The working surface area of the BDDE and the GCE were obtained by the CV using 1 mM K3Fe(CN)6 as a redox probe at different scan rate. The surface area can be calculated using Randles-Sevcik equation (298°K) as follows[44]
For K3Fe(CN)6, n =1 and D = 7.6 × 10−6 cm2/s. From slope of Ip (μA) vs. ν 1/2 the area were determined to be 0.069 cm2 for the BDDE and 0.028 cm2 for the GCE electrodes. Thus, the current values for BDDE and GCE were observed to be 4.34 μA/cm2 and 3.52 μA/cm2 for CV, 14.49 μ A/cm2 and 12.49 μA/cm2 for SWV.
3.2 Electrochemical Impedance Spectroscopy
The increase in the electron transfer at the BDDE was investigated using electrochemical impedance spectroscopy technique. For this AC amplitude of 10 mV at a frequency of 0.1–100 kHz was applied. The higher electrocatalytic behaviour of BDDE was confirmed by the reduction in the charge transfer resistance (figure 2A). The values observed for the charge transfer resistance, RCT, fitting an appropriate equivalent circuit, were determined and found to be 174 k Ω/cm2 at BDDE and 643 k Ω/cm2 at GCE (figure 2B).
The electrical equivalent circuit compatible with the Nyquist plots comprises of RS the solution resistance, Qdl the double layer capacitance, RCT the charge transfer resistance and W the Warburg impedance. The semi-circle observed in the Nyquist plot reveals the lower charge transfer resistance at BDDE surface as compared to GCE which can be attributed to the higher sensitivity of the electrochemical sensor. A constant phase element was used instead of the pure capacitance because of the electrode surface inhomogeneities.[45]
3.3 Effect of scan rates
To study the effect of scan rate on the oxidation peak of WDL, voltammograms were recorded for different scan rate from 10–220 mV/s at a fixed concentration (700 ng/mL) of WDL at BDDE. The information involving electrochemical mechanism can be obtained from the investigation of the response characteristics of CV on the electrooxidation process of WDL (figure 3). A linear relationship was obtained between the peak current intensity (Ip; P1 and P2 anodic peak currents) and the square root of scan rate (ν)1/2, suggesting the diffusion controlled process at the electrode surface. The linear relationship between Ip and (ν)1/2 for P1 follows Ip(μ) = 0.043(ν)1/2 − 0.111, R2 = 0.991 and for P2, Ip(μA) = 0.025(ν)1/2 − 0.085, R2 = 0.989. The results confirms the electroxidation diffusion controlled process of WDL.
Moreover, from the literature,[46] for an anodic reaction, the relationship between Ep and ν can be described as:
Where E° is the formal redox potential, R and F values are 8.314 J/K.mol and 96480 C/mol respectively, ks is the standard heterogeneous rate constant, α is the charge transfer coefficient and D is the diffusion coefficient.
From the slope of the straight line of Ep against ln ν, αn was calculated to be 0.46 and 0.89 for P1 and P2 respectively. For a diffusion-controlled anodic behaviour, assuming α to be 0.5,[47] number of electrons participating (n) during the oxidation process of WDL was calculated to be one for the anodic peak P1 and two for the anodic peak P2.
3.4 Effect of supporting electrolyte and its pH
To achieve the optimum electrolyte, the influence of various supporting electrolytes such as KCl, phosphate buffer, acetate buffer and Britton-Robinson buffer on the electrooxidation of WDL (700 ng/mL) was investigated. Britton-Robinson buffer was chosen as the optimum buffer with respect to the fairly well defined current responses and prominent peak shape. The effect of pH on the oxidation of WDL was examined over the pH range (2.5–12). The anodic peak currents (P1 and P2) and the peak potential for the oxidation of WDL were found to be sensitive towards the pH of the solution. It was observed that the current values (Ip) for anodic peaks P1 and P2 started to decrease with the increase in pH values and at higher pH (pH >7.9), the peak current almost disappeared (figure 4A). Thereby considering the sensitivity and the peak shape, pH 2.5 was selected as the optimum pH for the entire electrochemical measurement. Moreover there was a sequential shift of the anodic peak potential with the augmentation of solution pH, indicating that the protons had participated in the electrode reaction process of WDL.[48,49]Figure 4B explains the shifting of peak potential with the increase in pH values following the linear equation Ep (mV) = 700.3–88.05pH, R2 = 0.961 (P1) and Ep (mV) = 1092 −45.64pH, R2 = 0.964 (P2). Furthermore, the peak potential and the pH of the solutions can be expressed by the following Nernst equation.[50]
where ‘m’ is the number of protons participating in the electrode reaction. Thus, from the slope of Ep vs. pH and the aforementioned Nernst equation ‘m’ was calculated to be one.
3.5 Probable oxidation mechanism of WDL
The voltammetric techniques can be utilized to elucidate the electroactivity and possible reaction mechanism of various compounds. Here in the present case, CV and SWV of WDL exhibited two anodic voltammetric peaks (P1 and P2), which can be attributed to the presence of phenol and dihydroxy moieties respectively. According to Corduneanu et al.[51] and Janeiro et al.[52] resorcinol group is found to be less electroactive than phenolic group, suggesting that the oxidative peak P1 appearing at lower potential corresponds to the phenol moiety whereas the peak P2 appearing at higher corresponds to the dihydroxy group. Thus based on all the electrochemical measurements, one electron-proton transfer is observed in each step with a total of two proton-electron coupled reaction and a possible oxidation mechanism of WDL has been proposed as shown in scheme 2.
3.6 Calibration curve and detection limit
Under the optimized condition, response characteristics of SWV exhibited a linear relationship between the peak current values and the concentration of WDL within the range 50 ng/mL to 700 ng/mL (figure 5). The calibration plot of Ip (A) vs. concentration (ng/mL) can be described by the equations Ip (A) = 0.017(ng/mL) + 1.366; R2 = 0.994 (P1) and Ip (A) = 0.003(ng/mL)–0.686; R2 = 0.991 (P2)
Limit of detection (LOD) and limit of quantification (LOQ) (estimated as 3 S/m and 10 S/m, respectively, ‘S’ being the standard deviation and ‘m’ being the slope of the calibration curve) were obtained as 43.87 ng/mL and 132.93 ng/mL. Various statistical parameters for the linear regression equation have been evaluated and reported in table 1.
Further to explore the analytical application and the specificity of the proposed method, the effect of some common excipients (such as glucose, sucrose and lactose) were studied by analyzing sample solution of WDL (100 ng/mL) spiked with varying concentration of each excipients under the optimized conditions. The determination of WDL in the presence of above- mentioned excipients was evaluated and a recovery range from 98.03% to 99.18% was obtained. Thus, the results showed that there was no serious interferences occurred from the classical additives.
3.7 Determination of WDL in plant sample (Eclipta alba)
Isolation of the plant extract was attained by percolation of the methanol (48 h) through the bed of herbaceous material at room temperature to produce an extract having active principles contained in the herbaceous material. Before adding the solvent, plant material was washed, dried at room temperature and grinded to powder form. Then 5g of the dried plant material was weighed and soaked in 100 mL of solvent. Lustrous plant extract was obtained which was highly concentrated and contains a large proportion of the active principles, which can be used in lower unit doses for analytical experiments. The appropriate amount of the extract was taken in the voltammetric cell containing the supporting electrolyte and voltammogram was recorded (figure 6). In order to validate the veracity, WDL standard solution was added into the sample solution for recovery detection. The results are listed in table 2. The assay results indicate that the proposed voltammetric method can be applied for determination of WDL in plant extract effectively. The concentration of the unknown phytoconstituent (WDL) can be determined using the standard addition method by the following equation:[53]
After getting the voltammogram of the supporting electrolyte, known volume (Vu) of unknown concentration (Cu) of the investigated herbal drug is added and the resulting voltammogram is recorded and peak current (Ip1) is measured, then the known volume (Vs) of known concentration (Cs) of standard pure drug is added and peak current (Ip2) is measured. The amount of WDL content was found to contain 0.30% in the plant Eclipta alba.
3.8 Comparison of the sensitivity of the present work with other reported methods
Detection limit of the proposed method is compared with the other reported methods.[54,55]Table 3 reveals that the present method is more sensitive and the detection limit of WDL is lower than check the reported methods.
4 Conclusions
A new method for the selective determination of WDL in Britton-Robinson buffer (pH 2.5) at BDDE has been presented in this paper. BDDE offered high sensitivity towards WDL and found to be appropriate and effective for the selective determination of the solution system. The sensitivity of the electrodes can be explained on the basis of electrochemical impedance spectra against the ferricyanide solution with lower charge transfer resistance at BDDE as compared to GCE. The voltammetric study of WDL at different scan rates revealed diffusion controlled reaction process. Electrode process dynamics parameters have been evaluated and based on this a plausible reaction mechanism has been deduced. The calibration plot reveals linearity within the range of 50–700 ng/mL with a correlation coefficient of 0.994 for P1 and 0.991 for P2. The lower limit of detection (43.87 ng/mL) proved the sensitivity of the proposed method and the method was successfully employed for the determination of WDL in a real plant sample.
References
Murray M T and Pizzorno J E 1999 In Textbook of Natural Medicine (China: Churchill Living)
Matsuse I T, Lim Y A, Hattori M, Correa M and Gupta M P 1999 J. Ethnopharmacol. 64 15
Rajabhandari M, Wegner U, Julich M, Schopke T and Mental R 2001 J. Ethnopharmacol. 74 251
Uniyal R C, Sandhu S and Chandok J K 1998 In Herbology: The Ayurvedic Encyclopedia (India: Sri Sadguru Publications) pp. 77–88
Kirtikar K R and Basu B D 1998 In Indian Medicinal Plants 2nd ed., (India: International Book Distributors) pp. 1360-1361
Tewtrakul S, Subhadhirasakul S, Cheenpracha S and Karalai C 2007 Phytother. Res. 21 1092
Saxena A K, Singh B and Anand K K 1993 J. Ethnopharmacol. 40 155
Singh B, Saxena A K, Chandan B K, Agarwal S G, Bhatia M S and Anand K K 1993 Phytother. Res. 7 154
Singh B, Saxena A K, Chandan B K, Agarwal S G and Anand K K 2001 Indian J. Pharmacol. 45 435
Simonsen H T, Nordskjold J B, Smitt U W, Nyman U, Palpu P, Joshi P and Varughese G 2001 J. Ethnopharmacol. 74 195
Thakur V D and Mengi S A 2005 J. Ethnopharmacol. 102 23
Kobori M, Yang Z, Gong D, Heissmeyer V, Zhu H, Jung Y K, Angelica M, Gakidis M, Rao A, Sekine T, Kegami F I, Yuan C and Yuan J 2004 Cell Death Differ. 11 123
Hamrapurkar P, Chachad P and Phale M 2009 Natural Products: An Indian Journal 5 57
Wagner H, Geyer B, Kiso Y, Hikino H and Rao G S 1986 Planta Med. 52 370
Murali B, Amit A, Anand M S and Samiulla D S 2002 J. Nat. Remedies. 2 99
Thorat R M, Jhadhav V M, Kadam V J, Kamble S S and Salaskar K P 2009 Int. J. Chem. Tech. Res. 1 1079
Patel M B, Kadakia V M and Mishra S H 2008 Indian J. Pharm. Sci. 70 689
Das N, Bhavsar G C and Chauhan M G 1990 Indian Drugs 28 100
Reddy S L, Fayazuddin M D, Ahmad A and Reddy G S 2008 Spectrochim. Acta. 71 31
Tsopelas F, Petropoulou M O, Zikos N, Spyropoulou E, Andreadou I and Kakoulidou A T 2011 J. Solid State Electrochem. 15 1099
Saxena S, Shrivastava R and Satsangee S P 2012 Maced. J. Chem. Chem. Eng. 31 195
Yan J, Wang W, Chen L and Chen S 2008 Colloid Surf. B. 67 205
Teradal N L, Kalanur S S, Prashanth S N and Seetharamappa J 2012 J. Appl. Electrochem. 42 917
Jain R, Radhapyari K and Jadon N 2007 J. Colloid Interface Sci. 314 572
Jain R, Gupta V K, Jadon N and Radhapyari K 2010 Anal. Biochem. 407 79
Jain R, Mishra R and Diwedi A 2009 J. Sci. Ind. Res. 68 540
Jain R and Yadav R K 2011 Drug Test Anal. 3 171
Jain R and Yadav V 2011 Colloid Surf., B. 88 729
Raj K R, Sharma S, Saxena S, Shrivastava M M and Satsangee S P 2010 E-J. Chem. 7 169
Jain R, Dwivedi A and Mishra R 2009 J. Hazard. Mater. 169 667
Peckova K, Musilova J, Barek J and Zima J 2008 In Progress on drinking water research M H Lefebvre and M M Roux (Eds.) (New York: Nova Sci. Pub) p. 103
Souza C D, Braga O C, Vieira I C and Spinelli A 2008 Sens. Actuators, B. 135 66
Gumustas M and Ozkan S A 2010 Anal. Bioanal. Chem. 397 189
Deimkova H, Scampicchio M, Zima J, Barek J and Mannino S 2009 Electroanalysis 21 1014
Peckova K, Musilova J and Barek J 2009 Crit. Rev. Ana. Chem. 39 148
Santos K D, Braga O C, Vieira I C and Spinelli A 2010 Talanta 80 1999
Sartori E R, Medeiros R A, Rocha-Filho R C and Fatibello-Filho O 2010 Talanta 81 1418
Bozal B and Uslu B 2010 Comb. Chem. High Throughput Screening. 13 599
Peleskov Y V 2002 Russ. J. Electrochem. 38 1275
Panizza M and Cerisola G 2005 Electrochim. Acta. 51 191
Alfaro M A Q, Ferro S, Martínez-Huitle C A and Vong Y M 2006 J. Braz. Chem. Soc. 17 227
Haenni W, Rychen P, Fryda M and Comninellis C 2004 In Thin-film diamond Part B Ch. Nebel (Ed.) Semiconductors and Semimetals series (Academic Press, Elsevier) p. 149
Kraft A 2007 Int. J. Electrochem. Sci 2 355
Liu L Q, Xiao F, Li J W, Wu W B, Zhao F Q and Zheng B Z 2008 Electroanalysis 20 1194
Tabeshnia M, Heli H, Jabbari A and Movahedi A A M 2010 Turk. J. Chem. 34 35
Laviron E 1974 J. Electroanal. Chem. 52 355
Xing T L, Wang F, Mao Y Y, Wang L P and Ye B X 2009 J. Chin. Chem. Soci. 56 303
Yang S, Yang R, Li G, Li J and Qu L 2010 J. Chem. Sci. 122 919
Dar R A, Brahman P K, Tiwari S and Pitre K S 2011 J. App. Electrochem. 41 1311
Erk N 2004 Anal. Bioanal. Chem. 378 1351
Corduneanu O, Janeiro P and Brett A M O 2006 Electroanalysis 18 757
Janeiro P and Brett A M O 2004 Anal. Chim. Acta. 518 109
Ewing G W 1995 In Instrumental methods of chemical analysis 5th edition (Philadelphia: Lippincott- Raven) p. 465
Kumar S and Dhanani T 2013 Braz. J. Pharm. Sci. 49 57
Patil A A, Sachin B S, Wakte P S and Shinde D B J. Adv. Res. 4 479
Acknowledgements
The authors acknowledge the Ministry of Human Resource Development project, Dayalbagh Educational Institute, Dayalbagh, Agra, India, for providing financial assistance under an NMEICT project. They are also thankful to UGC for providing fellowship to two of the authors (Sachin Saxena and Ratnanjali Shrivastava).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
SAXENA, S., SHRIVASTAVA, R. & SATSANGEE, S.P. Voltammetric determination of wedelolactone, an anti-HIV herbal drug, at boron-doped diamond electrode. J Chem Sci 127, 959–966 (2015). https://doi.org/10.1007/s12039-015-0853-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0853-7