Abstract
The interaction between calcium hydroxide Ca(OH)2 and amorphous silica SiO2, a product of the polycondensation of silicic acids isolated from serpentines (Mg(Fe))6[Si4O10](OH)8, is studied. It is found that hours-long autoclave treatment can be avoided when sodium hydroxide NaOH is added to an initial aqueous suspension prepared from CaO and SiO2, and amorphous calcium hydro- and hydroxosilicates that transform into β-wollastonite upon annealing in the temperature range of 800–815°C can be obtained with common stirring of a suspension heated to the boiling point under the conditions of atmospheric pressure. The mechanism behind the action of NaOH on the interaction of Ca(OH)2 with this SiO2 is determined, and the optimum parameters of synthesis (the molar ratio of the initial substances and the duration of stirring and annealing) are determined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Due to a number of properties (e.g., a low coefficient of thermal expansion, shrinkage, thermal conductivity, dielectric properties, and strong whiteness), synthetic β-wollastonite (β-CaSiO3) finds wide application in different industrial sectors [1–8].
Common forms of silica SiO2 (e.g., quartz, diatomites, and different industrial silicic wastes) are generally used as the initial raw material for the production of calcium silicates, including β-CaSiO3 [9–12]. To obtain β-CaSiO3 via the interaction between calcium hydroxide Ca(OH)2 and SiO2 in an aqueous medium with subsequent annealing of the resulting compounds, autoclave conditions, hours-long treatment, and thus high energy consumption are required to form intermediate compounds with chain structure (e.g., tobermorite [Ca5Si6O16(OH)2 · 4H2O, Ca5Si6(O,OH)18 · 5H2O], or xonotlite [Ca6Si6O17(OH)2]) from SiO2 [8, 9, 13–20].
Using a new approach to the acid treatment of dehydrated serpentinitesFootnote 1 [21, 22], we managed to isolate silicic acids and obtain from them amorphous silica that (as studies have shown) has higher chemical activity than the conventional forms of SiO2 [23, 24]. This is due to the low energy needed to break the siloxane bonds in the Si–O–Si bridges, which is explained by the presence of unsaturated Si–O(Si) bonds in the structure of the silica [25, 26].
During the thermal treatment of serpentine minerals, two simultaneous processes proceed in the silicate layer: dehydroxylation and the breaking of unsaturated Si–O(Si) bonds, as a result of which the mineral transforms into an amorphous and unstable state. Acid treatment of this mass allows us to easily separate ortho-[SiO4]4−, di-[Si2O7]6−, tri-[Si3O10]8−, and other relatively simple silicate anions with chain structure from the broken silicate layer and transfer them to a solution in the form of silicic acids, along with iron and magnesium compounds [21, 22, 27–29]. Further polycondensation of these acids at 90°C yields the above amorphous silica consisting of different silicate units bound to one another by relatively weak Si–O(Si) bonds [25, 26]. It is logical that the Si–O(Si) bonds formed between silicate anions during polycondensation are weaker than the primary bonds that initially exist inside the silicate units that formed in magma [30, 31].
Considering the above structural characteristic features of this form of amorphous SiO2, it is of interest to study the preparation of various silicate compounds based on it, including calcium silicates.
The aim of this work was to study the interaction between Ca(OH)2 with amorphous SiO2, a product of polycondensation of silicic acids isolated from a serpentine mineral, and the thermal transformation of the synthesized intermediates to β-wollastonite by means of differential thermal (DTA), X-ray diffraction (XRD), and IR spectroscopic (IR) analyses; plus scanning electron microscopy (SEM).
EXPERIMENTAL
Amorphous SiO2, in which the amount of impurities did not exceed 0.2–0.3%, was obtained from silicic acids isolated from a sample of serpentinite taken from the Shorzha deposit (Republic of Armenia) according to the procedure developed in [22].
Chemically-pure grade (GOST 8677-76) calcium oxide CaO, calcined for 0.5 h at 1000°C, was used for preparing Ca(OH)2.
Four samples of suspensions were prepared from amorphous SiO2, CaO, and H2O. Weighed amounts of silica and calcium oxide, taken in CaO : SiO2 molar ratios (for brevity, referred to as C : S below) of 1 : 1, 1 : 1.2, 1 : 1.4, and 1 : 1.6, were introduced into the reaction vessel and suspended in distilled water at a ratio of the solid and liquid phases (S : L) of 1 : 15. Four more samples of suspensions prepared from the same compounds at the same ratios were mixed with a 0.1 M solution of NaOH. Each suspension was then heated to the boiling point (95°C) and stirred for 1.25 h using a mechanical stirrer. The slurry formed after treating each sample was separated from the solution via filtration through a paper filter and rinsed with distilled water. The precipitate was exposed for 24 h at 60–80°C in a KBC G-100/250 drying oven (Premed, Poland).
Each of the eight dried samples was subjected to DTA from room temperature to 1000°C. They were then cooled to room temperature and studied via XRD. It should be noted that the weight of the investigated samples was 300 mg for DTA and 220–240 mg for XRD.
The optimum ratio of the initial compounds and conditions of synthesis were determined from the experimental data. Six samples of new suspensions were then prepared from the initial reagents taken in the determined optimum C : S ratio and S : L ratio of 1 : 15. The suspensions were treated for 0.25, 0.5, 1, 1.5, 2, and 2.5 h. The synthesized samples of the slurry were then separated from the solution and studied using the above procedure. The optimum duration of stirring of the suspension was determined from the obtained results.
One more sample of the suspension was prepared from the initial reagents taken in the determined optimum C : S ratio (1 : 1.4) and S : L ratio of 1 : 15. The suspension was treated for the determined optimum time (1.5 h). The synthesized sample of the slurry was separated from the solution, dried, and exposed for 0.5 h at the optimum temperature (815°C), determined from the data of the above experiments. The value of the temperature of annealing was set and controlled using a Wise Therm F muffle oven with digital control (China). The synthesized product was studied via IR spectroscopy and SEM.
DTA was performed on a DERIVATOGRAPH Q-1500 D derivatograph (MOM, Hungary) in air at a heating rate of 10°C min−1.
XRD analysis was performed on a DRON-3 diffractometer (Russia) in Ni-filtered CuKα radiation. The images were recorded in air in the angular range 2θ = 8°–80° at 22°C. The counter motion speed was 2 deg min−1. All reflections were interpreted and identified using the 2004 JCPDS–ICDD computer-aided database.
The IR spectrum of the synthesized substance was recorded on a Nicolet/NEXUS Fourier-transform IR spectrometer (United States) in the range of 650–1600 cm−1 with a resolution of 2 cm−1.
The microstructure of the synthesized compound was determined on a Tesla BS-300 electron microscope (Czech Republic).
RESULTS AND DISCUSSION
It should be noted that all of the samples synthesized from CaO and SiO2 taken in different molar ratios and treated at 95°C and atmospheric pressure were in the amorphous state.
Regardless of the C : S molar ratio of the initial substances, a number of endothermic effects differing in intensity and accompanied by weight loss (Fig. 1a, I–IV) were observed in the temperature range of 100–800°C in the DTA curves of the first four samples treated for 1.25 h. The endothermic effects observed in the region of low temperatures (100–210°C) were caused by the removal of adsorbed and crystal water from the synthesized intermediate substances, indirectly indicating the formation of calcium hydrosilicate, while the endothermic effects in the region of 600–800°C were most likely due to the dehydroxylation of the hydroxyl groups OH in the calcium hydroxosilicates, which proceeds with the subsequent formation and removal of constitutional water. The differences between the intensities and values of the minima of the endothermic effects observed in the DTA curves of all our samples can be explained by the different concentrations of water and OH groups in the amorphous calcium hydro- and hydroxosilicates synthesized.
DTA curves of samples obtained (a) by stirring a boiling aqueous suspension consisting of CaO and SiO2 for 1.25 h and (b) upon adding NaOH to the same suspensions. The C : S molar ratios are (I) 1 : 1, (II) 1 : 1.2, (III) 1 : 1.4, and (IV) 1 : 1.6. The conditions are the same for Fig. 2.
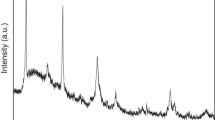
The endothermic effect observed with different intensities in all our DTA curves in the 495–505°C region of temperatures testifies to the decomposition of unreacted Ca(OH)2 (Fig. 1a) [32, 33]. The reflections of CaO, one of the products of this decomposition, were recorded in the X-ray diffraction patterns of all the samples (card no. 82–1690) (Fig. 2a, I–IV). By reacting with the water vapor in air, some unreacted CaO was converted to Ca(OH)2, the reflections of which are also recorded in the X-ray diffraction patterns of the samples with C : S molar ratios of 1 : 1, 1 : 1.2, and 1 : 1.4 (card no. 81–2041) (Fig. 2a, I–III).
X-ray diffraction patterns of samples synthesized (a) via the DTA of compounds preliminarily obtained by stirring a boiling aqueous suspension consisting of CaO and SiO2 for 1.25 h: (1) calcium oxide, (2) calcium hydroxide, (3) β-wollastonite, (4) cristobalite, and (5) quartz and (b) upon adding NaOH to the same suspensions: (1) β-wollastonite, (2) cristobalite, and (3) larnite. See Fig. 1 for the conditions of synthesis.
As for the unreacted amorphous silica, it was detected in the form of reflections characteristic of cristobalite and quartz (card nos. 85–0621 and 86–1630, respectively) (Fig. 2a). The weak reflections caused by the formation of β-CaSiO3 once more suggest incomplete interaction between the initial compounds (Fig. 2a). Due to the identity of the reflections characteristic of polymorphic modifications of β-wollastonite, both monoclinic and triclinic (Card nos. 84–0654 and 84–0655, respectively), the synthesized β-CaSiO3 can be considered either a form of these modifications or a mixture of them [33, 34]. The low-intensity exothermic effect observed in the DTA curves in the temperature range of 800–860°C was likely due to the formation of a negligible amount of β‑CaSiO3 and the crystallization of silica (Fig. 1a).
Based on our results, we may conclude that the conditions generated by this mode of treatment are insufficient for complete interaction between the amorphous silica obtained from silicic acids and Ca(OH)2, and thus cannot promote the formation of large amounts of calcium silicates, particularly β-wollastonite, upon heating to 1000°C.
However, the situation changes radically when a solution of NaOH is added to the initial suspension prior to synthesis.
The endothermic effect observed in the temperature range of 495–505°C due to the decomposition of unreacted Ca(OH)2 (Fig. 1a) is in this case missing entirely from the DTA curves of the samples taken in the same molar ratios (Fig. 1b). Reflections of monoclinic larnite (Ca2SiO4) (card no. 83–0460) are also observed in the X-ray diffraction patterns of the obtained products, in addition to the intense reflections associated with the formation of β‑wollastonite (Fig. 2b).
In contrast to the above DTA curves, two individual medium-intensity exothermic effects are observed in those of the samples taken in C : S molar ratios of 1 : 1 and 1 : 1.6 in the temperature range of 800–900°C (Fig. 1b, I and IV), while three exothermic effects are observed for the sample with the C : S of 1 : 1.2 (Fig. 1b, II). At the same time, a clearly pronounced exothermic peak with the maximum at 810°C is visible in the DTA curve for the sample with the C : S molar ratio of 1 : 1.4 (Fig. 1b, III). Since the most intense reflections of β-wollastonite were recorded in the X‑ray diffraction pattern of this sample (Fig. 2b, III), we may assume the observed exothermic effect was due to the formation of β-CaSiO3 (Fig. 1b, III). The exothermic peaks with maxima at 809, 815, and 812°C, recorded for the samples with C : S molar ratios of 1 : 1, 1 : 1.2, and 1 : 1.6, respectively, were therefore also due to the formation of β-CaSiO3 (Fig. 1b, I, II, and IV). When sodium hydroxide was added, the formation of β‑CaSiO3 was observed at temperatures 25–30°C lower than in the earlier samples (Figs. 1a, 1b).
The most intense reflections of larnite are observed in the X-ray diffraction patterns of the samples with C : S of 1 : 1 and 1 : 1.2 (Fig. 2b, I and II), so the weak exothermic peaks with maxima at 861 and 849°C in the DTA curves of these samples, respectively, could be due to the formation of larnite (Fig. 1b, I and II). As for the weak exothermic effects with the maxima at 876°C for the sample with the C : S ratio of 1 : 1.2 and at 880°C for the sample with the C : S ratio of 1 : 1.6 (Fig. 1b, II and IV), they are most likely due to the phase transformation of excess amorphous silica to cristobalite, as is confirmed by the presence of intense reflections that are characteristic of cristobalite in the X-ray diffraction patterns of these samples (Fig. 2b, II and IV).
The above endothermic effects associated with the removal of water and indirectly indicating the formation of calcium hydro- and hydroxosilicates by stirring a boiling suspension are also observed in the DTA curves of these samples in the same temperature ranges (i.e., 100–210 and 775–790°C, respectively) (Fig. 1b, I–IV).
Analyzing the obtained data, we may conclude that sodium hydroxide not only promotes the full interaction between the initial substances upon stirring but also lowers the temperature of formation of β-wollastonite upon heating. The optimum C : S ratio that corresponds to the highest yield of β-wollastonite is 1 : 1.4.
In the DTA curves of the samples with C : S of 1 : 1.4 and treated for different periods, endothermic effects are also observed below 800°C that are due to the release of adsorbed and constitutional water (Fig. 3, I–IV). The very weak endothermic effect in the region of 450–500°C, which is due to the decomposition of unreacted Ca(OH)2, was only recorded for the samples treated for less than 1.5 h (Fig. 3, I–III). The intensity of the exothermic effect above 800°C, which corresponds to the formation of β-CaSiO3, grew when the duration of treatment was increased (Fig. 3, I–V), and the most intense peak was recorded for the amorphous calcium hydro- and hydroxosilicates obtained after 1.5–2 h of treatment of the initial mixture (Fig. 3, IV and V). The most intense peaks of the reflections characteristic of β-CaSiO3 were also recorded in the X-ray diffraction patterns of samples treated for 1.5 and 2 h (Fig. 4, IV and V). In the DTA curves of samples treated for less than 1.5 h, the exothermic peak in a region above 800°C is distinguished by lower intensity (Fig. 3, I–III), while in the X-ray diffraction patterns of the obtained final products, intense peaks corresponding to the formation of cristobalite and weak reflections of larnite are observed along with the reflections of β-CaSiO3 (Fig. 4, I–III). These data show that the relatively low intensity of the exothermic peak in the DTA curves of the samples treated for less than 1.5 h was due to the low yield of β‑CaSiO3. Lengthening the duration of treatment to 2.5 h has no appreciable effect on the yield of β‑CaSiO3. On the contrary, it drops considerably, as follows from the comparatively weak exothermic effect in the DTA curve (Fig. 3, VI) and reflections of β‑CaSiO3 in the X-ray diffraction pattern of the corresponding sample (Fig. 4, VI).
DTA curves of the samples obtained by stirring a boiling aqueous suspension consisting of CaO and SiO2, taken in the C : S molar ratio of 1 : 1.4, and NaOH. Durations of stirring are (I) 0.25, (II) 0.5, (III) 1, (IV) 1.5, (V) 2, and (VI) 2.5 h. The conditions are the same for Fig. 4.
X-ray diffraction patterns of samples synthesized via DTA of compounds preliminarily obtained by stirring a boiling aqueous suspension consisting of CaO and SiO2, taken at the C : S molar ratio of 1 : 1.4, and NaOH: (1) β-wollastonite, (2) cristobalite, and (3) larnite. See Fig. 3 for the conditions of synthesis.
The formation of β-CaSiO3 is also confirmed by the results from IR spectroscopy. The IR absorption spectrum of the final product, synthesized after holding for 0.5 h at 815°C the intermediate sample obtained after 1.5 h of treating the aqueous suspension prepared from the initial reagents with C : S of 1 : 1.4 and NaOH, is identical to the absorption spectrum of β‑CaSiO3. It displays six main bands of β‑CaSiO3 with wavenumbers 1065.7, 1026.2, 1010.5, 958.1, 931.65, and 891.3 cm−1 corresponding to asymmetric SiOSi and O−SiO− and symmetric O−SiO− stretching vibrations, along with two medium-intensity bands at 682 and 650 cm−1 that belong to the symmetric stretching vibrations of SiOSi bridges (Fig. 5) [35].
The procedure for preparing β-wollastonite is shown schematically in Fig. 6.
The formation of Ca2SiO4 in the samples synthesized with NaOH shows how sodium hydroxide affects the interaction between amorphous silica, a product of the polycondensation of silicic acids, and Ca(OH)2.
Let us recall that the amorphous silica used in this work was a result of the polycondensation of silicic acids formed from ortho-[SiO4]4−, di-[Si2O7]6−, and other silicate anions.
While NaOH was stirred with the initial mixture, sodium cations, by penetrating into the structure of silica, interact with Si–O–H silanol groups and simultaneously break the relatively weak Si–O(Si) siloxane bonds that appeared during polycondensation, thus regenerating ortho- and other silicate anions. As a result, various sodium-containing silicate units are formed. Several processes proceed simultaneously in the boiling suspension. Sodium cations are first replaced with calcium cations, after which the freed Na+ interacts with the remaining amorphous silica. A compound that initiates the formation of Ca2SiO4 upon heating is then synthesized from the reproduced [SiO4]4− anions and Ca(OH)2, while intermediate calcium hydro- and hydroxosilicates that transform into β-CaSiO3 form with the participation of other silicate anions. The formation of β-wollastonite under such mild conditions shows that other silicate anions with chain structure (e.g., [Si3O10]8− and [Si4O13]10−) participate in the formation of silica, in addition to orthosilicate anions.
The results from SEM show that β-wollastonite forms from relatively short chains of silicate anions. We can see from the micrograph of the final product that the synthesized crystals of β-wollastonite are short (Fig. 7).
CONCLUSIONS
The presence of unsaturated (weak) Si–O(Si) bonds and chains of silicate anions in the structure of amorphous silica are the two main factors that allow us to avoid hours-long hydrothermal synthesis under autoclave conditions, and to synthesize β-wollastonite using a simplified technique under normal conditions without excessive consumption of energy by adding NaOH to a reaction mixture consisting of Ca(OH)2, SiO2, and H2O. Penetrating into the structure of amorphous silica, sodium cations break the weak siloxane bonds that emerge during polycondensation and thus restore the primary chains of silicate anions that were initially involved in the process of formation of amorphous silica. In such a state, these freed chains of silicate anions readily react with calcium cations via ordinary stirring under conditions of atmospheric pressure and at 95°C, thus forming intermediate amorphous calcium hydro- and hydroxosilicates that transform into β-wollastonite with small crystals upon thermal treatment in the temperature range of 800–815°C. The highest yields of β-wollastonite are achieved upon annealing amorphous calcium hydro- and hydroxosilicates synthesized by 1.5 h of stirring a suspension prepared from the initial reagents at the C : S molar ratio of 1 : 1.4 and at S : L of 1 : 15. At other C : S ratios, an increase in the formation of undesired side compounds (larnite and cristobalite) is observed. Lengthening the duration of treatment is impractical, while shortening it reduces the yield of β-wollastonite.
Studies in this direction are of great practical interest. On the one hand, they create the prerequisites for the further development of a new, simplified way of preparing β-wollastonite. On the other hand, they expand the areas of application of this silica, a byproduct of treating serpentinized ultrabasic rocks, thus increasing the profitability of their use.
Notes
Serpentinite is a rock consisting mainly of the serpentine mineral (Mg(Fe))6[Si4O10](OH)8.
REFERENCES
Q. Ding, Z. Zhang, C. Wang, et al., J. Therm. Anal. Calorim. 115, 675 (2014). https://doi.org/10.1007/s10973-013-3171-7
R. Morsy, R. Abuelkhair, and T. Elnimr, Silicon, 1 (2014). https://doi.org/10.1007/s12633-014-9243-x
Sreekanth R. P. Chakradhar, B. M. Nagabhushana, G. T. Chandrappa, et al., Mater. Chem. Phys. 95, 169 (2006). https://doi.org/10.1016/j.matchemphys.2005.06.002
Y. H. Yun, C. H. Yoon, Y. H. Kim, et al., Ceram. Int. 28, 503 (2002). https://doi.org/10.1016/S0272-8842(02)00002-0
N. S. Negmatov and Z. Z. Abdullaev, Glass Ceram. 58, 396 (2001). https://doi.org/10.1023/a:1014914526841
T. Kokubo, Biomaterials 12, 155 (1991). https://doi.org/10.1016/0142-9612(91)90194-F
L. Jingjiang, W. Xiufen, and G. Qipeng, J. Appl. Polym. Sci. 41, 2829 (1990). https://doi.org/10.1002/app.1990.070411125
V. D. Gladun, A. I. Khol’kin, and L. V. Akat’eva, Theor. Found. Chem. Eng. 41, 606 (2007). https://doi.org/10.1134/s0040579507050259
K. G. Grigoryan, G. A. Arutunyan, L. G. Baginova, and G. O. Grigoryan, Theor. Found. Chem. Eng. 42, 583 (2008). https://doi.org/10.1134/s0040579508050163
A. I. Khol’kin, V. D. Gladun, and L. V. Akat’eva, Theor. Found. Chem. Eng. 46, 515 (2012). https://doi.org/10.1134/s0040579512050041
L. V. Akat’eva, A. E. Baranchikov, V. K. Ivanov, and A. I. Khol’kin, Theor. Found. Chem. Eng. 49, 736 (2015). https://doi.org/10.1134/s0040579515050036
L. V. Akat’eva, V. K. Ivanov, S. A. Kozyukhin, et al., Theor. Found. Chem. Eng. 50, 490 (2016). https://doi.org/10.1134/s0040579516040023
H. Wu, J. Yang, H. W. Ma, and M. W. Wang, Integr. Ferroelectr. 146, 144 (2013). https://doi.org/10.1080/10584587.2013.789777
A. Yazdani, H. R. Rezaie, H. Ghassai, and M. Mahmoudian, J. Ceram. Process. Res. 14, 12 (2013).
K. Lin, J. Chang, X. Liu, and C. Ning, Int. J. Appl. Ceram. Technol. 7, 178 (2010). https://doi.org/10.1111/j.1744-7402.2009.02474.x
J. Wu, Y. J. Zhu, G. F. Cheng, and Y. H. Huang, Mater. Res. Bull. 45, 509 (2010). https://doi.org/10.1016/j.materresbull.2009.10.006
A. Yazdani, H. R. Rezaie, and H. Ghassai, J. Ceram. Process. Res. 11, 348 (2010).
K. Lin, J. Chang, G. Chen, et al., J. Cryst. Growth 300, 267 (2007). https://doi.org/10.1016/j.jcrysgro.2006.11.215.
K. Lin, J. Chang, and J. Lu, Mater. Lett. 60, 3007 (2006). https://doi.org/10.1016/j.matlet.2006.02.034
G. Matekonis, R. Šiaučiunas, and D. de Vaičiukynienė, Mater. Sci. Medziagotyra 16, 242 (2010).
N. O. Zulumyan, A. R. Isaakyan, and Z. G. Oganesyan, Russ. J. Appl. Chem. 80, 1020 (2007). https://doi.org/10.1134/s1070427207060353
RF Patent No. 2407704 C2 (2010).
A. Torosyan, N. Zulumyan, Z. Hovhannisyan, and S. Ghazaryan, in Proceedings of the EPD Congress 2005 and 2005 TMS Annual Meeting, 2005, p. 779.
N. Zulumyan, A. Isahakyan, Z. Hovhannisyan, and A. Torosyan, Magnesium Technol. 2006, 351 (2006).
N. O. Zulumyan, A. R. Isaakyan, P. A. Pirumyan, and A. A. Beglaryan, Russ. J. Phys. Chem. A 84, 700 (2010). https://doi.org/10.1134/S003602441004031X
A. R. Isahakyan, H. A. Beglaryan, P. A. Pirumyan, L. R. Papakhchyan, and N. H. Zulumyan, Russ. J. Phys. Chem. A 85, 72 (2011). https://doi.org/10.1134/S0036024410121015
N. Zulumyan, A. Mirgorodski, A. Isahakyan, and H. Beglaryan, J. Therm. Anal. Calorim. 115, 1003 (2014). https://doi.org/10.1007/s10973-013-3483-7
N. H. Zulumyan, L. R. Papakhchyan, A. M. Terzyan, H. A. Beglaryan, and A. R. Isahakyan, Theor. Found. Chem. Eng. 47, 185 (2013). https://doi.org/10.1134/S0040579512040215
N. H. Zulumyan, L. R. Papakhchyan, A. R. Isahakyan, et al., Geochem. Int. 49, 937 (2011). https://doi.org/10.1134/S0016702911090084
N. H. Zulumyan, L. R. Papakhchyan, A. R. Isahakyan, H. A. Beglaryan, and S. G. Aloyan, Russ. J. Phys. Chem. A 86, 1008 (2012). https://doi.org/10.1134/S0036024412060350
N. H. Zulumyan, L. R. Papakhchyan, A. R. Isahakyan, H. A. Beglaryan, and S. G. Aloyan, Russ. J. Phys. Chem. A 86, 1887 (2012). https://doi.org/10.1134/S003602441212028X
Mené. aendez, L. Vega, and C. Andrade, J. Therm. Anal. Calorim. 110, 203 (2012). https://doi.org/10.1007/s10973-011-2159-4
J. Zelić, D. Rušić, and R. Krstulović, J. Therm. Anal. Calorim. 67, 613 (2002). https://doi.org/10.1023/a:1014348603686
J. Tolliday, Nature (London, U.K.). 182, 1012 (1958). https://doi.org/10.1038/1821012a0
A. N. Lazarev, Vibrational Spectra and Structure of Silicates, 1st ed. (Springer US, 1995).
ACKNOWLEDGMENTS
This work was supported by the State Committee of Science of the Ministry of Education and Science of Armenia, in the frames of the research project no. 16YR-1D025.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by E. Boltukhina
Rights and permissions
About this article
Cite this article
Beglaryan, H.A., Zulumyan, N.H., Isahakyan, A.R. et al. Study of the Interaction between Calcium Hydroxide and Amorphous Silica Obtained from Serpentinites. Russ. J. Phys. Chem. 93, 924–931 (2019). https://doi.org/10.1134/S0036024419050042
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419050042