Abstract
Subsolidus phase equilibria in the La2O3–(Ni/Со)O–Sb2O5 systems have been studied. A previously unknown compound La4Sb2O11 was found to exist in the La2O3–Sb2O5 system. La4Sb2O11 has been shown to decompose at 1060°C to form La3SbO7 and LaSbO4. Two ternary oxides (LaNi2SbO6 and La2NiSb2O9) have been discovered in the La2O3–NiO–Sb2O5 system. These new compounds are stable and do not undergo polymorphic transformations throughout the range of temperatures studied (25–1350°C). The existence of previously known ternary oxides, namely perovskite La3Ni2SbO9 and rosiaite LaNi1/3Sb5/3O6, has been verified. In the La2O3–CoO–Sb2O5 system, two new compounds (LaCo2SbO6 and La2CoSb2O9) have been found along with previously known perovskite La3Co2SbO9, rosiaite LaCo1/3Sb5/3O6, and rhombohedral pyrochlore La3Co2Sb3O14. These new compounds are isostructural to those found in the nickel oxide system. La2CoSb2O9, unlike its nickel analogue, decomposes at 990°C. For LaCo2SbO6, no thermal events associated with polymorphic transitions or melting have been detected on DSC curves up to 1350°C. An inspection of diffuse reflectance spectra of the newly synthesized LaNi2SbO6, La2NiSb2O9, LaCo2SbO6, and La2CoSb2O9 phases showed that the oxidation state of nickel and cobalt in them is 2+. The 1050°C isothermal sections of La2O3–(Ni/Co)O–Sb2O5 systems have been constructed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Phase equilibria in multicomponent systems are directly related to the search for new functional materials based on complex oxides and the development of optimal methods for their synthesis. Despite an enormous amount of research work carried out to date into phase equilibria in binary and multicomponent oxide systems, a great many of the systems remain poorly studied. Such are systems comprising antinomy oxide, whose high volatility makes investigations difficult. Meanwhile, many promising functional materials, in particular catalysts, have been created based on antimonates. Various M–Sb–O double oxides (where M = Mg, Ca, Sr, Al, Cr, Fe, Co, Ni, Cu, Zn, Y, Ag, In, La, Pb, or Bi) have shown their efficacy in photocatalytic water splitting [1–8]. Transition-metal antimonates MSb2O6, where M = Mn, Fe, Co, and Ni, have been used as low-cost (free of precious metals) catalysts, which are distinguished by their good oxygen binding energy, conductivity, thermal phase stability, and stability in aqueous media. These catalysts can be used in the electrocatalytic reduction of oxygen [3–7] and for the electrochemical release of chlorine [2, 6]. The reason for the catalytic activity of antimonates in redox reactions is the low redox potential of the Sb3+ → Sb5+ transformations [9]. It is due to this feature that iron antimonate FeSbO4 is an efficient oxidation catalyst in various organic syntheses [10–13]. We showed recently [14–20] that pyrochlore ternary oxides (Ln1.8Fe0.2)FeSbO7 (Ln = Pr–Tb, or Bi) and rosiaites LnFe0.5Sb1.5O6 (Ln = La–Sm) and LaNi1/3Sb5/3O6 exhibit high activity and stability in catalytic CO oxidation and complete methane oxidation.
The study of phase equilibria in antimony-containing multicomponent oxide systems will elucidate previously unknown phases and, possibly, expand the range of promising antimonate-based functional materials. In particular, of interest are La2O3–(Ni/Co)O–Sb2O5 systems, which have not been studied but the boundary binary systems of which have been in sufficient detail.
In the Ni–Sb–O system, only two mixed oxides exist: NiSb2O4 and NiSb2O6 [21]. NiSb2O6 has the trirutile structure (space group P42/mnm) [22, 23]; it is stable in air (at least to 1523°C) and can be used in C3H8 and CO sensors [24] and liquefied petroleum gas sensors [25]. NiSb2O6 has a metastable rosiaite-type polymorph (space group P\(\bar {3}\)1m) [26] with the unit cell parameters a = 4.691(7) Å, c = 9.299(3) Å. When heated for more than 1 h at 500°C, however, this compound transforms to a stable phase. NiSb2O4 belongs to the Pb3O4 structure type (space group P42/mbc) [27] and can be prepared only via heating NiO and Sb2O3 mixtures in vacuo or in flowing nitrogen [21].
In the Co–Sb–O system, as in the nickel system, there is a trirutile phase [28]. Also known are spinel Co7/3Sb2/3O4 (space group Fd\(\bar {3}\)m) [29] and pyrochlore Co2Sb2O7 (space group Fd\(\bar {3}\)m) [30, 31]. A Co7/3Sb2/3O4 phase was prepared from cobalt sulfate and antimony oxide (Sb2O3) by annealing at 900°С; Co2Sb2O7 was prepared from cobalt acetate and antimony oxide (Sb2O5) at 450°С, but it is yet unknown whether this phase is stable at high temperatures.
The La–Ni–O system was studied in detail at 1200°C [32]. Under air, the system can form LaNiO3 and Ruddlesden–Popper homologous phases of general formula Lan+1NinO3n+1 (n is the number of octahedral layers) [33]: La2NiO4, La3Ni2O7, and La4Ni3O10. Their crystal lattices are formed by the alternation of perovskite layers and NaCl-type layers. LaNiO3 has the perovskite structure [34]; it is stable below 850°C in air or under an elevated oxygen pressure. Above 850°C, this phase gradually decomposes to Lan+1NinO3n+1 oxides [32, 33, 35]. According to Kitayama [32], an La6Ni5O15 phase exists; it was prepared at 1200°C under air. According to Demina at el. [36], however, a sample of nominal composition La6Ni5O15 has an X-ray diffraction pattern that features reflections from two phases: La4Ni3O10 and NiO. LaNiO2 exists between 300 and 400°C and only under a reductive atmosphere [37].
In the La–Co–O boundary system, double oxides are thermodynamically stable phases: perovskite LaCoO3 and Ruddlesden–Popper phases La2CoO4 and La4Co3O10 [38]. All phases contain cobalt ions in various degrees of oxidation [39]. The stability of phases depends on the partial oxygen pressure and temperature. Cobalt(+3) phases have higher stability at low temperatures and at higher partial oxygen pressures. When synthesis is performed under an air atmosphere (p(O2) = 0.2 atm), LaCoO3 is the only stable compound up to 1600 K.
No systematic inventions have been performed into phase equilibria in the La–Sb–O system in air. There is evidence for the existence of three compounds: La3SbO7, LaSbO4, and LaSb3O9 [40–42]. The orthorhombic LaSb3O9 phase (space group Cmcm) is stable only below 1100°C and melts incongruently above this temperature to yield a LaSbO4 phase [40]. LaSbO4 has a monoclinic structure (space group P21/m [43]) and exists up to 1450°С, at which temperature it decomposes to form orthorhombic La3SbO7 (space group Cmcm) [41]. The antimony in all of the above-listed compounds is antimony(+5).
In the La2O3–NiO–Sb2O3 quasi-ternary system, two ternary oxides are currently known to exist: LaNi1/3Sb5/3O6, and La3Ni2SbO9. LaNi1/3Sb5/3O6 has a rosiaite-type layered structure (space group P\(\bar {3}\)1m) [44, 45]. Layers are formed of six-membered rings of edge-sharing (Ni/Sb)O6 octahedra, in which Ni2+ and Sb5+ ions are arranged randomly. The La3+ ions reside in interlayer spaces, occupying sites on the triple axis in hexagonal-prismatic channels. The unit cell parameters of LaNi1/3Sb5/3O6 are a = 5.26142(4) and с = 5.21945(6) Å. The compound exists up to 1050°С. At higher temperatures, it has not been studied. La3Ni2SbO9 has a monoclinic perovskite-like structure (space group P21/n) with the unit cell parameters a = 5.0675(1), b = 5.6380(1), c = 7.9379(2) Å, β = 89.999(6)° at room temperature [46]. Two crystallographically different octahedral sites are occupied randomly by Ni2+ and Sb5+ ions. There is also evidence for the existence of LaNi1–xSbxO3 (0 ≤ x ≤ 1/3) limited solid solutions in the range 850–950°С. Unlike LaNiO3, which has a rhombohedrally distorted perovskite structure (space group R3c) [47], LaNi1‒xSbxO3 (0.05 ≤ x ≤ 1/3) solid solutions have an orthorhombically distorted perovskite structure (space group Pbmn), in which Ni and Sb ions are distributed randomly over the B lattice sites [48].
Only mixed oxides LaCo1/3Sb5/3O6 [44], La3Co2Sb3O14 [49], and La3Co2SbO9 [50], which occur in the La2O3–CoO–Sb2O5 system, are described in the literature. LaCo1/3Sb5/3O6 is isostructural to the above-mentioned rosiaite LaNi1/3Sb5/3O6. La3Co2Sb3O14 has a rhombohedrally distorted pyrochlore structure (space group R\(\bar {3}\)m, а = 7.52954(2) Å, с = 17.59983(6) Å [49]). In its crystal lattice, Co2+ ions occupy both the La and the Sb sites in accordance with the formula [La3Co′][Sb3Co′′]О14. The La, Sb, and Co ions are distributed orderly. Perovskite La3Co22+Sb5+O9 (space group P21/n) has a monoclinic structure. In La3Co22+Sb5+O9, cobalt and antimony ions occupy the same octahedral sites [50].
Therefore, the available body of data implies that the La2O3–(Ni/Co)О–Sb2O3 systems have been studied to an insufficient extent. Here, we will report the results of our study into subsolidus phase equilibria of the La2O3–(Ni/Co)O–Sb2O5 systems.
EXPERIMENTAL
The synthesis of antimonates is complicated by the high volatility of antimony. Long-term anneals at high temperatures are thereby undesirable. On the other hand, refractory precursors containing lanthanum, nickel, and cobalt react only at high temperatures. Therefore, the citrate method followed by stepwise annealing was chosen to reduce the synthesis temperature. In this synthetic method, the reaction occurs at lower temperatures (compared to those of solid-phase synthesis from oxides), and the differences between the individual behaviors of cations in solution (which lead to the loss of reagents and deviation from the required composition of samples during coprecipitation synthesis) is also leveled out. Other advantages of the citrate method are a high degree of homogenization of the batch and a shorter equilibration time.
The reagents used were La(NO3)3∙6H2O (specialty grade), Ni(NO3)2∙6H2O (pure for analysis grade), Co(NO3)2∙6H2O (pure for analysis grade), and Sb2O3 (99%). Ethylene glycol C2H6O2 (pure grade) and citric acid monohydrate (chemically pure grade) were taken in a twofold excess relative to metal ions. The components were mixed and kept in a water bath at 80°C. Dissolution of all components, thickening of the solution, and foaming were observed. After this, the reaction mixture was dried at 110°C until brittle foam formed and then pounded. The powder was first annealed at 350°C (4 h) and 450°C (4 h) to decompose the nitrates and burn out the organic components. The resulting powder was then annealed at 650 (24 h), 900 (24 h), and 1050°C (48 h) in Pt crucibles under air. The stepped annealing ensured the formation of nonvolatile intermediate products, which prevented the loss of antimony.
The phase composition of the samples was determined by X-ray powder diffraction on a Bruker D8 Advance diffractometer (CuKα radiation, Ni filter, LYNXEYE detector). Phase identification was with reference to the PDF-2 (ICDD) database. The thermal behavior of La4Sb2O11 was studied on a NETZSCH DSC 404 F1 differential scanning calorimeter. Experiments were carried out in platinum crucibles with a lid under dry argon (grade 5.5; 99.9995 vol % Ar) atmosphere at a flow rate of 10 K/min.
A diffuse reflectance spectrum in the range of 300–1100 nm was recorded on an Ocean Optics DH-2000 spectrometer equipped with an integrating sphere (50 mm ISP-50-8-R-GT). An HPX-2000 xenon lamp was used as the radiation source.
RESULTS AND DISCUSSION
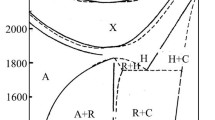
As shown in the Introduction, phase equilibria in the La2O3–Sb2O5 system in air are studied insufficiently, unlike those in its La2O3–МO and МO–Sb2O5 (М = Ni or Co) boundary systems. Therefore, we considered the possibility of the existence of hitherto unknown phases in this system. A number of samples with different La2O3 : Sb2O5 ratios were synthesized, and a new compound La4Sb2O11 was discovered (Fig. 1). The amount of oxygen indicated in the formula of this compound is based on the presence Sb5+ ions. Interplanar distances in La4Sb2O11 are listed in the attachment (Table 1).
The La4Sb2O11 exists up to 1060°С. The DSC heating curve in the range 1000–1500°С features three endotherm effects (Fig. 2a). The first one begins at 1060°C and corresponds to the decomposition of the La4Sb2O11 phase to La3SbO7 and LaSbO4 (Fig. 2b). The effect with the onset at 1320°С is due to incongruent melting of LaSbO4 which results in the formation of a liquid phase. The presence of the liquid creates favorable conditions for the volatilization of antimony oxide; therefore, the composition of the sample upon cooling does not correspond to the original one. This explains the nonappearance of effects on the cooling curve. We have not discovered other phases that would have been previously unknown in the La2O3–Sb2O5 system.
The triangulation of the La2O3–(Ni/Со)O–Sb2O5 system at 1050°С was carried out by the cross sections methods based on XRD data.
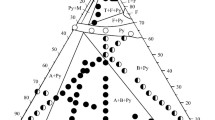
We were the first to discover new ternary oxides in the La2O3–NiO–Sb2O5 quasi-ternary system: LaNi2SbO6 and La2NiSb2O9 (Fig. 3; Tables 2 and 3). These compounds were stable under heating throughout the range of temperatures studied (25–1350°С). The existence of two previously known phases, namely, perovskite La3Ni2SbO9 and rosiaite LaNi1/3Sb5/3O6 [44–46], was verified.
The 1050°С isothermal section of the La2O3–NiO–Sb2O5 system (Fig. 4) features four ternary oxides: LaNi1/3Sb5/3O6, La3Ni2SbO9, LaNi2SbO6, and La2NiSb2O9. Since the stable antimony oxide species at 1050°С in air is Sb2O4, the area between LaSb3O9, NiSb2O6, and antimony oxide is the projection of a more complex system: LaSb3O9–NiSb2O6–Sb2O3–Sb2O5. We have not studied the La2O3–NiOx–La3SbO7 area in detail, as it should be regarded the projection of a quaternary system because of a mixed oxidation state of nickel in this area. We have not found new ternary oxides in the areas belonging to quaternary systems. The La3SbO7–NiO–NiSb2O6–LaSb3O9 section may be represented by 11 triangles of coexisting phases: La3SbO7–La3Ni2SbO9–La4Sb2O11, La4Sb2O11–La3Ni2SbO9–LaSbO4, La3Ni2SbO9–LaSbO4–La2NiSb2O9, La3Ni2SbO9–LaNi2SbO6–La2NiSb2O9, La3Ni2SbO9–LaNi2SbO6–NiO, LaNi2SbO6–NiO–NiSb2O6, LaNi2SbO6–LaNi1/3Sb5/3O6–NiSb2O6, LaNi2SbO6–LaNi1/3Sb5/3O6–La2NiSb2O9, LaSbO4–LaNi1/3Sb5/3O6–La2NiSb2O9, LaSbO4–LaNi1/3Sb5/3O6–LaSb3O9, and LaNi1/3Sb5/3O6–NiSb2O6–LaSb3O9.
Unlike in the nickel-containing system, three compounds have been hitherto known in the La2O3–СоO–Sb2O5 system: rosiaite LaСо1/3Sb5/3O6, rhombohedrally distorted pyrochlore La3Со2Sb3O14, and perovskite La3Со2SbO9 [44, 49, 50]. In addition to these compounds, we showed the existence of two more compounds, namely La2СоSb2O9 and LaСо2SbO6, which are isostructural to those found in the nickel system (Fig. 5; Tables 4 and 5). LaСо2SbO6 is stable over the entire range of investigation up to 1350°С. La2СоSb2O9, unlike its isostructural nickel analogue, decomposes at 1003°С. In the La2O3–СоOx–La3SbO7 area, no ternary oxides have been found. Therefore, the 1050°С isothermal section of the La2O3–CoO–Sb2O5 system in the La3SbO7–CoO–LaSb3O9–CoSb2O6 area may be represented by 14 triangles of coexisting phases (Fig. 6): La3SbO7–La3Co2SbO9–La4Sb2O11, La4Sb2O11–La3Co2SbO9–La3Co2Sb3O14, La3Co2Sb3O14–La4Sb2O11–La2CoSb2O9, La4Sb2O11–La2CoSb2O9–LaSbO4, La3Co2SbO9–La3Co2Sb3O14–LaCo2SbO6, La3Co2SbO9–LaCo2SbO6–CoO, LaCo2SbO6–CoO–Co7Sb2O12, LaCo2SbO6–Co7Sb2O12–La3Co2Sb3O14, Co7Sb2O12–La3Co2Sb3O14–CoSb2O6, La3Co2Sb3O14–CoSb2O6–LaCo1/3Sb5/3O6, La3Co2Sb3O14–La2CoSb2O9–LaCo1/3Sb5/3O6, La2CoSb2O9–LaSbO4–LaCo1/3Sb5/3O6, LaSbO4–LaCo1/3Sb5/3O6–LaSb3O9, and LaCo1/3Sb5/3O6–LaSb3O9–CoSb2O6.
The diffuse reflectance optical spectra of the new phases (Fig. 7) confirmed that nickel and cobalt are in the +2 oxidation state. The spectrum of octahedrally coordinated Ni2+ is known to feature three allowed d–d transitions from the 3A2g ground level to 3T2g, 3T1g, and 3T1g(P) [51]. In oxides, bands corresponding to these transitions appear in the ranges 7000–10 000, 12 000–16 000, and 22 000–27 000 cm–1. Bands associated with 3A2g → 1Eg and 3A2g → 1T2g spin-forbidden transitions can also be observable. The 1Eg and 3T1g states are close in energy to each other. As a result of spin–orbit coupling, the 3A2g → 1Eg forbidden transition grows in intensity due to the allowed transition, so a doublet appears in Ni2+ spectra in the range 12 000–16 000 cm–1. The 3A2g → 1T2g spin-forbidden transition usually appears as a weak band at the long-wavelength edge of the 3A2g → 3T1g transition. All the above-listed features appear in the LaNi2SbO6, La2NiSb2O9, and LaNi1/3Sb5/3O6 spectra (Fig. 7). LaNi1/3Sb5/3O6 is shown as a reference. This indicates that nickel ions in these compounds are in the +2 oxidation state.
The spectra of octahedrally coordinated Со2+ usually feature two strong bands in the ranges 8000–10 000 and 16 000–20 000 cm–1 referring to 4T1g(P) → 4T2g and 4T1g(P) → 4T1g allowed transitions, respectively [51]. The 4T1g(P) → 4T1g band, as a rule, is a multiplet due to an admixture of spin-forbidden transitions. In the range 11 000–14 000 cm–1, a less strong 4T1g(P) → 4A2g transition is observed; it frequently appears as a shoulder at the low-energy side of the 4T1g(P) → 4T1g transition band. The LaСо2SbO6 and La3Со2SbO9 spectra, just as the La3Со2Sb3O14 spectrum given as a reference, have all the listed features, indicating the presence of Co2+ ions in an octahedral surrounding in these compounds. The different degrees of splitting of the observed transitions are explained by the structural specifics of the compounds.
Therefore, the fact that the nickel and cobalt are Ni2+ and Co2+ in all the prepared ternary oxides, implies that, indeed, the above-considered La3SbO7–NiO–NiSb2O6–LaSb3O9 and La3SbO7–CoO–LaSb3O9–CoSb2O6 areas belong to the La2O3–NiO–Sb2O5 and La2O3–СоO–Sb2O5 systems, respectively.
CONCLUSIONS
Altogether, the La2O3–(Ni/Со)O–Sb2O5 systems are not completely alike. They implement four pairs of isostructural compounds: La(Ni/Co)1/3Sb5/3O6, La3(Ni/Со)2SbO9, La(Ni/Со)2SbO6, and La2(Ni/Со)Sb2O9; in the cobalt oxide system, however, one more compound exists: La3Со2SbO9. The above-listed compounds differ from one another in (Ni/Со)2+/Sb5+ cation ratios and have different structures. The known ternary oxides were characterized previously. Some of them were shown to have functional properties that allowed them to be considered promising for use. For example, the rosiaite compound LaNi1/3Sb5/3O6 exhibited high catalytic activity in СО oxidation [20]. Therefore, a further study of the new phases first synthesized in this work would be of undoubted interest.
REFERENCES
J. Sato, N. Saito, H. Nishiyama, et al., J. Photochem. Photobiol., A 148, 85 (2002). https://doi.org/10.1016/S1010-6030(02)00076-X
I. A. Moreno-Hernandez, B. S. Brunschwig, and N. S. Lewis, Energy Environ. Sci. 12, 1241 (2019). https://doi.org/10.1039/C8EE03676D
G. K. K. Gunasooriya, M. E. Kreider, Y. Liu, et al., ACS Nano 16, 6334 (2022). https://doi.org/10.1021/acsnano.2c00420
I. A. Moreno-Hernandez, C. A. MacFarland, C. G. Read, et al., Energy Environ. Sci. 10, 2103 (2017). https://doi.org/10.1039/C7EE01486D
L. Zhou, A. Shinde, J. H. Montoya, et al., ACS Catal. 8, 10938 (2018). https://doi.org/10.1021/acscatal.8b02689
T. A. Evans and K.-S. Choi, ACS Appl. Energy Mater. 3, 5563 (2020). https://doi.org/10.1021/acsaem.0c00526
K. Ham, S. Hong, S. Kang, et al., ACS Energy Lett. 6, 364 (2021). https://doi.org/10.1021/acsenergylett.0c02359
L. Zhou, Y. Wang, K. Kan, et al., ACS Sustainable Chem. Eng. 10, 15898 (2022). https://doi.org/10.1021/acssuschemeng.2c05239
M. M. Gadgil and S. K. Kulshreshtha, J. Mol. Catal. A: Chem. 95, 211 (1995). https://doi.org/10.1016/1381-1169(94)00027-1
M. Karimi, S. Dariush, A. Kobra, et al., Tetrahedron Lett. 56, 2674 (2015). https://doi.org/10.1016/j.tetlet.2015.03.114
R. K. Grasselli, J. Chem. Educ. 63, 216 (1986). https://doi.org/10.1021/ed063p216
N. Burriesci, F. Garbassi, M. Petrera, et al., J. Chem. Soc., Faraday Trans. 78, 817 (1982). https://doi.org/10.1039/F19827800817
R. G. Teller, J. F. Brazdil, R. K. Grasselli, et al., J. Chem. Soc., Faraday Trans. 81, 1693 (1985). https://doi.org/10.1039/F19858101693
A. V. Egorysheva, O. G. Ellert, and E. Yu. Liberman, J. Alloys Compd. 777, 655 (2019). https://doi.org/10.1016/j.jallcom.2018.11.008
O. G. Ellert, A. V. Egorysheva, and E. Yu. Liberman, Inorg. Mater. 55, 1257 (2019). https://doi.org/10.1134/S0020168519120033
E. Yu. Liberman, O. G. Ellert, A. V. Naumkin, et al., Russ. J. Inorg. Chem. 65, 592 (2020). https://doi.org/10.1134/S0036023620040117
O. G. Ellert, A. V. Egorysheva, E. Yu. Liberman, et al., Ceram. Int. 46, 27725 (2020). https://doi.org/10.1016/j.ceramint.2020.07.271
A. V. Egorysheva, O. G. Ellert, E. Yu. Liberman, et al., Russ. J. Inorg. Chem. 67, 2127 (2022). https://doi.org/10.1134/S0036023622601349
A. V. Egorysheva, K. R. Plukchi, S. V. Golodukhina, et al., Mendeleev Commun. 33, 608 (2023). https://doi.org/10.1016/j.mencom.2023.09.005
A. V. Egorysheva, S. V. Golodukhina, K. R. Plukchi, et al., Russ. J. Inorg. Chem 68, 1725 (2023). https://doi.org/10.1134/S0036023623602106
K. Swaminathan and O. M. Sreedharan, J. Alloys Compd. 292, 100 (1999). https://doi.org/10.1016/S0925-8388(99)00283-2
H. Haeuseler, Spectrochim. Acta, Part A 37, 487 (1981). https://doi.org/10.1016/0584-8539(81)80036-0
H. Ehrenberg, G. Wltschek, J. Rodriguez-Carvajal, et al., J. Magn. Magn. Mater. 184, 111 (1998). https://doi.org/10.1016/S0304-8853(97)01122-0
V. M. Rodríguez-Betancourtt, H. G. Bonilla, M. F. Martínez, et al., J. Nanomater 2017, 8792567 (2017). https://doi.org/10.1155/2017/8792567
A. Singh, A. Singh, S. Singh, et al., Chem. Phys. Lett. 646, 41 (2016). https://doi.org/10.1016/j.cplett.2016.01.005
A. Y. Nikulin, E. A. Zvereva, V. B. Nalbandyan, et al., Dalton Trans. 46, 6059 (2017). https://doi.org/10.1039/C6DT04859E
J. R. Gavarri, R. Chater, and J. Ziolkowski, J. Solid State Chem. 73, 305 (1988). https://doi.org/10.1016/0022-4596(88)90114-4
J. P. Turbil and J. C. Bernier, C. R. Acad. Sci., Ser. C 227, 1347 (1973).
P. Odier, Y. Nigara, J. Coutures, et al., J. Solid State Chem. 56, 32 (1985). https://doi.org/10.1016/0022-4596(85)90249-X
M. S. L. Brito, M. T. Escote, C. O. P. Santos, et al., Mater. Chem. Phys. 88, 404 (2004). https://doi.org/10.1016/j.matchemphys.2004.08.008
H. D. Zhou, C. R. Wiebe, J. A. Janik, et al., J. Solid State Chem. 183, 890 (2010). https://doi.org/10.1016/j.jssc.2010.01.025
K. Kitayama, J. Solid State Chem. 87, 165 (1990). https://doi.org/10.1016/0022-4596(90)90078-C
R. A. M. Ram, L. Ganapathi, P. Ganguly, et al., J. Solid State Chem. 63, 139 (1986). https://doi.org/10.1016/0022-4596(86)90163-5
A. Wold, B. Post, and E. Banks, J. Am. Chem. Soc. 79, 4911 (1957). https://doi.org/10.1021/ja01575a022
M. Zinkevich and F. Aldinger, J. Alloys Compd. 375, 147 (2004). https://doi.org/10.1016/j.jallcom.2003.11.138
A. N. Demina, V. A. Cherepanov, A. N. Petrov, et al., Inorg. Mater. 41, 736 (2005). https://doi.org/10.1007/s10789-005-0201-2
M. A. Hayward, M. A. Green, M. J. Rosseinsky, et al., J. Am. Chem. Soc. 121, 8843 (1999). https://doi.org/10.1021/ja991573i
W.-W. Zhang, E. Povoden-Karadeniz, H. Xu, et al., J. Phase Equilib. Diff. 40, 219 (2019). https://doi.org/10.1007/s11669-019-00717-z
Y. Adachi, N. Hatada, and T. Uda, J. Electrochem. Soc. 163, F1084 (2016). https://doi.org/10.1149/2.0811609je
K. M. Ok, A. Gittens, L. Zhang, et al., J. Mater. Chem. 14, 116 (2004). https://doi.org/10.1039/B307496J
K. P. F. Siqueira, R. M. Borges, E. Granado, et al., J. Solid State Chem. 203, 326 (2013). https://doi.org/10.1016/j.jssc.2013.05.001
M. B. Varfolomeev, T. A. Toporenskaya, and V. V. Burlyaev, Russ. J. Inorg. Chem. 26, 171 (1981).
K. P. F. Siqueira, R. M. Borges, J. C. Soares, et al., Mater. Chem. Phys. 140, 255 (2013). https://doi.org/10.1016/j.matchemphys.2013.03.031
G. Blasse and A. D. M. De Pauw, J. Inorg. Nucl. Chem. 32, 2533 (1970). https://doi.org/10.1016/0022-1902(70)80298-6
O. G. Ellert, A. V. Egorysheva, S. V. Golodukhina, et al., Russ. Chem. Bull. 70, 2397 (2021). https://doi.org/10.1007/s11172-021-3359-0
P. D. Battle, S. I. Evers, E. C. Hunter, et al., Inorg. Chem. 52, 6648 (2013). https://doi.org/10.1021/ic400675r
I. Alvarez, M. L. Veiga, and C. Pico, Solid State Ionics 91, 265 (1996). https://doi.org/10.1016/S0167-2738(96)83028-1
I. Alvarez, M. L. Veiga, and C. Pico, J. Alloys Compd. 255, 74 (1997). https://doi.org/10.1016/S0925-8388(96)02870-8
K. Li, Y. Hu, Y. Wang, et al., J. Solid State Chem. 217, 80 (2014). https://doi.org/10.1016/j.jssc.2014.05.003
D. G. Franco, V. C. Fuertes, M. C. Blanco, et al., J. Solid State Chem. 194, 385 (2012). https://doi.org/10.1016/j.jssc.2012.05.045
A. B. P. Lever, Inorganic Electronic Spectroscopy, vol. 2 (Elsevier, 1984).
ACKNOWLEDGMENTS
Facilities of the Shared Facilities Center of the Kurnakov Institute were used to fulfill the work.
Funding
This work was supported by the Russian Science Foundation (project No. 23-23-00113).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work described in this article.
Additional information
Translated by O. Fedorova
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Egorysheva, A.V., Golodukhina, S.V., Plukchi, K.R. et al. Subsolidus Phase Equilibria in the La2O3–(Ni/Со)O–Sb2O5 Systems. Russ. J. Inorg. Chem. (2024). https://doi.org/10.1134/S0036023624601107
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S0036023624601107