Abstract
Differential scanning calorimetry and high-temperature X-ray diffraction were used to measure the molar heat capacity and lattice thermal expansion of pyrochlore europium hafnate in the temperature range 298–1300 K. Eu2Hf2O7 does not experience structural transformations in this temperature range. The thermal expansivities of europium hafnate were estimated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Europium hafnate Eu2Hf2O7 of pyrochlore structure type (Fd3m) crystallizes with the metal ratio Eu : Zr = 1 : 1 in the region of continuous hafnia-base solid solutions (1 – x)EuO1.5⋅xHfO2 (x ≈ 0.45–1.0), which have disordered fluorite crystal structure (Fm3m). Pyrochlores crystallize at high temperatures (1600–1900 K), where diffusion processes are activated [1]. Europium hafnate is a high-temperature oxide and does not experience structural transformations up to the temperature at which it converts to the disordered fluorite structure (~2500–2700 K) [2–5]. Europium hafnate, as other rare-earth zirconates and hafnates, too, has a good potential for use in the nuclear industry and in power production [6–8]. Of special interest are protective coatings for high-temperature power plants, namely, for gas turbines (thermal barrier coatings, TBCs) and aircraft engines (thermal/environmental barrier coatings, TBC/EBC) [9–11]. The high chemical inertness and corrosion resistance of rare-earth hafnates and zirconates, which should ensure the durability of coatings, needs to be confirmed, especially in high-temperature contact with CMAS (calcium magnesium alumina silicate) oxides. Experimental studies are quite costly and laborious, but methods of chemical thermodynamics modeling chemical equilibria at high temperatures can help to solve this problem [12, 13]. The advantage of this approach is the elimination of kinetic factors that complicate experimental measurements. Model calculations require reliable data on temperature-dependent thermodynamic functions—heat capacity, entropy, enthalpy increment, and Gibbs free energy–over the widest possible range of temperatures. The high-temperature heat capacity of Eu2Hf2O7 was measured by differential scanning calorimetry (DSC) in a helium atmosphere in the temperature range 373–1073 K [14], and was calculated from drop calorimetry measurements of the enthalpy increment in the ranges 774–1679 K [15] and 977–1738 K [16]. The DSC-derived values [14] seem overestimates, and the values derived from drop calorimetry measurements [15] and [16] are not very consistent with each other, especially at temperatures above 1000 K. Thermal expansion parameters are an important characteristic of high-temperature ceramic materials. This work comprised DSC measurements of the Eu2Hf2O7 molar heat capacity in the range 298–1300 K, X-ray diffraction studies of temperature-dependent unit cell parameters, and assessment of thermal expansivities in the range 298–1300 K.
EXPERIMENTAL
Synthesis, measurement and data processing procedures are described in detail elsewhere [17]. The chemicals used in the synthesis were europium sesquioxide (99.99 wt %), hafnia (99.99 wt %) produced by Lanhit, hydrochloric acid (35–38 wt %, specialty grade 20-4), and aqueous ammonia (25–28 wt % NH4OH, specialty grade) produced by Khimmed. The prepared sample was single-phase europium hafnate with the unit cell parameter а = 10.541(4) Å (pyrochlore) as probed by X-ray powder diffraction. It was not nanosized as shown by scanning electron microscopy and its coherent scattering domain sizes estimated from the X-ray diffraction pattern (Figs. 1 and 2). The composition of the sample as probed by chemical analysis was Eu2Hf1.97O6.94; the oxygen index was derived from the metal oxide ratio. As shown previously [18], this deviation from stoichiometry cannot significantly affect the molar heat capacity value. The formula weight calculated as recommended in [19] was 772.9038 g/mol. The heat capacity was measured by DSC (STA 449 F1 Jupiter (Netzsch)). The thermal expansion was measured by high-temperature X-ray powder diffraction (Shimadzu X-ray diffractometer equipped with an HZ-1001 high-temperature attachment) [20].
RESULTS AND DISCUSSION
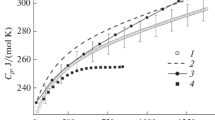
The heat capacity of pyrochlore europium hafnate in the range 298–1300 K measured by DSC (Fig. 3) is well fitted by the Maier–Kelley equation [21]:
The temperature-dependent heat capacity does not feature anomalies due to structural transformations. Figure 3 compares the Eu2Hf2O7 heat capacities in the range 300–1300 K taken from various sources: our measurements (curve 1), calculations from Eu2O3 [22] and HfO2 [23] heat capacities in terms of the Neumann–Kopp rule (curve 2), results borrowed from [15] (curve 3) and from [16] (curve 4). One can see that curves 1 and 2 lie very closely to each other, although the Neumann–Kopp heat capacity estimate at 298.15 K (242.8 J/(mol K))) is 4.5 J/(mol K) higher than our experimental value. The heat capacities calculated from enthalpy increments [15] (curve 3) are also close to our values (curve 1). The heat capacities calculated by the equation found in [16] as
(curve 4) are also close to our values (curve 1) in the range 300–800 K; at higher temperatures, however, they are serious underestimates. Thus, the general trend of the temperature dependences calculated by differentiating enthalpy increments [15, 16] slightly differs from direct heat capacity measurements, and extrapolation of these values to high temperatures can significantly distort actual values. López-Cota at al. [14] measured the high-temperature heat capacity of pyrochlore europium hafnate by DSC. However, their results presented graphically in the paper are significant overestimates (Cp > 380 J/(mol K) for 300–1100 K), most likely, due to a methodological error, namely, the use of helium as an inert gas, with its high thermal conductivity.
The thermal expansion of europium hafnate was studied by high-temperature X-ray diffraction in the range 298–1300 K and by determination of the temperature- dependent unit cell parameter а of the cubic pyrochlore (Fig. 4; Table 1). The thermal expansion of europium hafnate has a positive trend, just as expected, and the general trend confirms the absence of structural transformations in the range of temperatures studied. The temperature-dependent parameter а is satisfactorily fitted by the linear equation
and the relative thermal expansivity remains a constant value to high accuracy:
whereas the current thermal expansivity decreases systematically as temperature rises:
The relative thermal expansion (TE) is described by the relationship
The unit cell parameter versus temperature plots for europium hafnate and gadolinium hafnate [17] are almost parallel (Fig. 4, curves 1 and 4), which is an indication to the closeness of values of derivatives da(T)/dT and relative thermal expansivities: α298 (K–1) = 11.79 × 10–6 and 11.75 × 10–6, respectively. Our temperature-dependent unit cell parameter plot coincides with that in [24] to a 5% error (Fig. 4), but the calculated relative thermal expansion differs from that in [25] almost twofold.
CONCLUSIONS
Pyrochlore europium hafnate has been prepared by reverse precipitation and characterized by scanning electron microscopy, X-ray powder diffraction, and chemical analysis. The molar heat capacity of europium hafnate has been measured by differential scanning calorimetry (at 298–1300 K), and it featured no anomalies due to structural transformations in this range of temperatures. The temperature-dependent unit cell parameter of cubic europium hafnate (pyrochlore) has been determined in high-temperature X-ray diffraction experiments, and thermal expansivities have been estimated. The relative expansivities of europium hafnate and gadolinium hafnate have almost equal values and are invariable in the range 298–1273 K. The results can be used for thermodynamic modeling of processes where Eu2Hf2O7 is involved, for predicting the behavior of a europium hafnate based high-temperature material in corrosive settings, and for developing new europium hafnate production technologies.
REFERENCES
V. V. Popov and A. P. Menushenkov, Ya. V. Zubavichus, et al., Russ. J. Inorg. Chem. 60, 602 (2015). https://doi.org/10.1134/S0036023615050162
E. R. Andrievskaya, J. Eur. Ceram. Soc. 28, 2363 (2008). https://doi.org/10.1016/jeurceramsoc.2008.01.009
R. W. Scheidecker, D. R. Wilder, and H. Moeller, J. Am. Ceram. Soc. 60, 501 (1977). https://doi.org/10.1111/j.1151-2916.1977.tb14092.x
C. R. Stanek and R. W. Grimes, J. Am. Ceram. Soc. 85, 2139 (2002). https://doi.org/10.1111/j.1151-2916.2002.tb00423.x
M. J. D. Rushton, R. W. Grimes, C. R. Stanek, and S. Owens, J. Mater. Res. 19, 1603 (2004).https://doi.org/10.1557/JMR.2004.0231
R. C. Ewing, W. J. Weber, and J. Lian, J. Appl. Phys. 95, 5949 (2004). https://doi.org/10.1063/1.1707213
H. Yamamura, Solid State Ionics 158, 359 (2003).https://doi.org/10.1016/s0167-2738(02)00874-3
A. V. Shlyakhtina and L. G. Shcherbakova, Solid State Ionics 192, 200 (2011). https://doi.org/10.1016/j.ssi.2010.07.013
X. Q. Cao, R. Vassen, and D. Stoever, J. Eur. Ceram. Soc. 24, 1 (2004). https://doi.org/10.1016/S0955-2219(03)00129-8
G. Mehboob, M.-J. Liu, T. Xu, et al., Ceram. Int. 46, 8497 (2020). https://doi.org/10.1016/j.ceramint.2019.12.200
N. P. Padture, Science 296, 280 (2002).https://doi.org/10.1126/science.1068609
G. Costa, B. J. Harder, V. L. Wiesner, et al., J. Am. Ceram. Soc. 102, 2948 (2019). https://doi.org/10.1111/jace.16113
O. Fabrichnaya and H. J. Seifert, J. Phase Equilib. Diffus. 32, 2 (2010). https://doi.org/10.1007/s11669-010-9815-4
F. A. López-Cota, N. M. Cepeda-Sánchez, J. A. Díaz-Guillén, et al., J. Am. Ceram. Soc. 100, 1994 (2017). https://doi.org/10.1111/jace.14712
R. Kandan, B. P. Reddy, G. Panneerselvan, and U. K. Mudali, J. Therm. Anal. Calorim. 131, 2687 (2018). https://doi.org/10.1007/s10973-017-6802-6
R. Babu and K. Nagarajan, J. Alloys Compd. 265, 137 (1998). https://doi.org/10.1016/s0925-8388(97)00430-1
V. N. Guskov, A. V. Tyurin, A. V. Guskov, et al., Ceram. Int. 46, 12822 (2020). https://doi.org/10.1016/j.ceramint.2020.02.052
P. G. Gagarin, A. V. Guskov, V. N. Guskov, et al., Ceram. Int. 47, 2892 (2021). https://doi.org/10.1016/j.ceramint.2020.09072
M. E. Wieser, Pure Appl. Chem. 78, 2051 (2006). https://doi.org/10.1351/pac200678112051
T. Yu. Kolomiets, G. B. Tel’nova, A. A. Ashmarin, et al., Inorg. Mater. 53, 874 (2017). https://doi.org/10.7868/S0002337X17080152
C. G. Maier and K. K. Kelley, J. Am. Chem. Soc. 54, 3243 (1932). https://doi.org/10.1021/ja01347a029
R. J. M. Konings, O. Beneš, O. A. Kovács, et al., J. Phys. Chem. Ref. Data 43, 013101 (2014). https://doi.org/10.1063/1.4825256
L. B. Pankratz, Bull. U. S., Bur. Mines 672, 188 (1982).
K. V. G. Kutty, S. Rajagopalan, C. K. Mathews, et al., Mater. Res. Bull. 29, 759 (1994). https://doi.org/10.1016/0025-5408(94)90201-1
K. V. G. Kutty, S. Rajagolapan, and R. Asuvathraman, Thermochim. Acta 168, 205 (1990). https://doi.org/10.1016/0040-6031(90)80639-G
ACKNOWLEDGMENTS
This work was carried out using the equipment of the Center for Collective Use of the Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences. The assistance of PhD A.A. Ashmarin in HTXRD studies is kindly appreciated.
Funding
This work was supported by the Russian Science Foundation project no. 18-13-00025: https://rscf.ru/project/18-13-00025/
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Translated by O. Fedorova
Rights and permissions
About this article
Cite this article
Guskov, A.V., Gagarin, P.G., Guskov, V.N. et al. Thermal Expansion and Thermodynamic Functions of Europium Hafnate at 298–1300 K. Russ. J. Inorg. Chem. 66, 1710–1713 (2021). https://doi.org/10.1134/S0036023621110085
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023621110085