Abstract
Complexes [Ln2(H2O)6(μ2-Htba−O,O')4(Htba−O)2]n (Ln = Tb (I), Gd (II), Nd (III); and H2tba is thiobarbituric acid) have been synthesized. According to single-crystal X-ray diffraction, monoclinic crystals of I–III are isostructural. They contain three independent Htba– ions (one terminal and two bridging) and two independent Ln3+ ions. Six Htba– ligands (two terminal and four O,O'-bridging) and two water molecules are coordinated to one Ln3+ ion, and four O,O'-bridging Htba– ions and four water molecules are coordinated to the other Ln3+ ion to form square antiprisms. The antiprisms are bound by Htba– bridging ions into layers. Numerous hydrogen bonds and π–π interactions stabilize the structures of the compounds. Thermal decomposition of complexes I and II performed in air results in mixtures of oxides and oxysulfates, whereas complex III forms Nd2O2SO4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
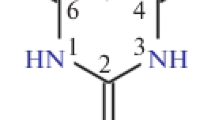
The organometallic coordination polymers of lanthanides(III) possess high monochromaticity of luminescent radiation and are used in clinical diagnostics and biotechnology, organic light-emitting diodes, displays, optical amplifiers, and lasers [1–4]. One of the multifunctional ligands capable of forming coordination polymers of various structures with metal ions is 2-thiobarbituric acid (Fig. 1) [5]; its derivatives—thiobarbiturates—are used as drugs [6]. In the present work, continuing our systematic study of the structure and properties of 2-thiobarbituric and 1,3-diethyl-2-thiobarbituric lanthanide complexes [7–10], three isostructural complexes [Ln2(H2O)6(H2tba)6] (Ln = Tb (I), Gd (II), Nd (III)) have been prepared which possess potentially useful luminescent and magnetic properties [3, 4]. Their thermal decomposition in air has been studied.
EXPERIMENTAL
In this work, TbCl3 ⋅ 6H2O, GdCl3 ⋅ 6H2O, Nd(NO3)3 ⋅ 6H2O, H2tba, and NaOH (chemically pure grade, Russian State Standard) were used without additional purification.
Synthesis of [Ln2(H2O)6(Htba)6]n (Ln = Tb (I), Gd (II), and Nd (III)). The hydrates of the lanthanide salts (0.463 mmol) were dissolved in water (10 mL) followed by the addition of solid H2tba (0.200 g, 1.39 mmol). The mixture was neutralized with 1 M NaOH solution to pH 4. Fine crystalline precipitates I (yellow), II (red), and III (purple) were formed which were filtered off after 8 h. The yield of the compounds was 60–70%.
For C24H30N12Tb2O18S6 anal. calcd. (%): C, 22.4; H, 2.35; N, 13.1; S, 15.0.
Found for I (%): C, 22.1; H, 2.47; N, 12.8; S, 14.8.
For C24H30N12Gd2O18S6 anal. calcd. (%): C, 22.5; H, 2.36; N, 13.1; S, 15.0.
Found for II (%): C, 22.1; H, 2.55; N, 12.9; S, 14.7.
For C24H30N12Gd2O18S6 anal. calcd. (%): C, 23.0; H, 2.41; N, 13.4; S, 15.3.
Found for III (%): C, 22.7; H, 2.66; N, 13.0; S, 15.0.
In the course of slow evaporation of the mother solutions for 2–4 weeks, the corresponding single crystals were formed; the crystals were filtered off and dried in air between sheets of filter paper. The intensities of X-ray reflections from prismatic I–III crystals with sizes of 0.2 × 0.3 × 0.4 mm were measured at 100 K using a D8 VENTURE single crystal diffractometer (Bruker AXS, MoKα radiation). The data were corrected for absorption using the SADABS program [11] by the multiscan method. The structure model was determined by direct methods and refined using the SHELXTL program package [12]. The positions of hydrogen atoms were determined from difference electron density syntheses, which were idealized and refined in the form associated with the main atoms. Table 1 shows the experimental parameters and the results of structure refinement.
The experimental powder X-ray diffraction patterns of fine-crystalline powders of I–III coincide with those theoretically calculated from X-ray diffraction data for the corresponding single crystals, which confirms their phase identity. A comparison of these powder X-ray diffraction patterns is shown in Fig. S1.
Structures of I–III were deposited with the Cambridge Structural Database (CCDC nos. 1576288, 1576438 and 1576287, respectively); the data are available free of charge at deposit@ccdc.cam.ac.uk or http://www.ccdc.cam.ac.uk/data_request/cif.
The thermal decomposition of the compounds was studied on an SDT-Q600 thermal analyzer combined with a Nicolet380 FT-IR spectrometer for the qualitative determination of gases emitted (air flow rate, 50 mL/min; heating rate, 10 K/min; temperature range, 24–850°C).
RESULTS AND DISCUSSION
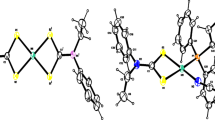
Complexes I–III, together with [Eu2(H2O)6(Htba)6]n [7] and [Sm2(H2O)6(Htba)6]n [10], form a series of isostructural compounds [Ln2(H2O)6(Htba)6]n. The independent part of their unit cells contains two Ln3+ ions in special positions, three Htba– ions, and three water molecules in general positions. The Htba– ions are coordinated to the Ln3+ cations only through O atoms. The two independent Ln3+ ions have different coordination environments (Fig. 2). The polyhedra Ln(1)O8 and Ln(2)O8 are square antiprisms connected to each other by bridging Htba– to form an infinite layer in the plane perpendicular to the direction a + c (Fig. 3). As a result of the bridging O,O'-coordination of Htba– ions to Ln3+, 24-membered cycles are formed. The structure of the complexes under consideration corresponds to the general formula [Ln2(H2O)6(Htba−O,O')4(Htba−O)2]n.
Main geometric characteristics of compounds I–III are given in the Appendix (Table S1). The Ln–O bond lengths fall in the range 2.3–2.5 Å and have usual values [13]. Like the unit cell volumes V (Table 1), the Ln–O bond lengths regularly increase when passing from compound I to III, which is explained by a monotonic increase in the crystallographic radius (r) of the Ln3+ ion [14]. The [Sm2(H2O)6(Htba)6]n [10] and [Eu2(H2O)6(Htba)6]n [7] structures were determined at different temperatures (296 and 300 K, respectively); therefore, the dependence of V on r for complexes [Ln2(H2O)6(Htba)6]n (Ln = Tb, Gd, Eu, Sm, Nd) was found to be nonmonotonic.
The geometric parameters of independent Htba– ions (one terminal and two bridging ones) in all three compounds coincide almost completely (Table S1), for example, the lengths of the C−O (1.257(2)–1.273(2) Å), C(4)–C(5), and C(5)–C(6) bonds (1.389(3)–1.398(3) Å) as well as C–S bonds (1.678(2)–1.684(2) Å) and the bond angle C(6A)C(5A)C(4A) (119.8°–119.9°).
In I–III, twelve hydrogen bonds N−H⋅⋅⋅O, N−H⋅⋅⋅S, O−H⋅⋅⋅O, and O−H⋅⋅⋅S are formed (Table S2), in which all Htba– ions and all water molecules are involved. Hydrogen bonds form a three-dimensional framework in which supramolecular motifs \({\text{R}}_{{\text{2}}}^{{\text{2}}}\)(8), S(6), \({\text{R}}_{{\text{2}}}^{{\text{2}}}\)(28), and \({\text{R}}_{{\text{4}}}^{{\text{4}}}\)(26) can be distinguished [15]. Using the PLATON program [16], the parameters of the π–π interaction between Htba– ions (Table S3) were determined to belong to the head-to-tail type.
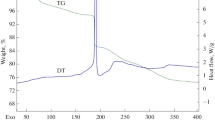
The thermal decomposition of compounds I–III proceeds in several stages (Fig. 4). At the first stage, the complexes are dehydrated to form crystalline phases that were not identified by powder X-ray diffraction. The experimental mass loss of substances (Δmexp) was close to that calculated (Δmcalcd) assuming the removal of all six H2O molecules (for Imexp = 8.71%, mcalcd = 8.41%; for IImexp = 9.03%, mcalcd = 8.50%; for III, mexp = 8.60%, mcalcd = 8.61%). Dehydration is accompanied by endotherms on DSC curves (255°, 311° for I; 247° for II, and 246° for III). The oxidation of the organic ligand in I–III begins at ~300°C. In the range of 400–800°C, this process is accompanied by two or three exotherms and the evolution of H2O, CO2, COS, NO, and SO2 gases. According to powder X-ray diffraction data, the final product of the decomposition of III at 800°C, as in the thermolysis of [Nd(H2O)2(Htba)2(CH3COO)] ⋅ 2H2O [8], is pure Nd2O2SO4 (Δmexp = 33.0%, Δmcalcd = 33.2%). Oxysulfates Ln2O2SO4 have a large capacity for oxygen atoms [17] and are promising as reaction catalysts for hydrogen production from CO and H2O [18] resistant to sulfur poisoning. The thermal decomposition of III can be considered as an alternative method to prepare Nd(III) oxysulfate, which, in turn, can serve as a precursor for the preparation of Nd2O2S being a promising laser material [19]. The final products of decomposition of I and II are mixtures of the corresponding oxides and oxysulfates. The composition of the intermediate decomposition products of the compounds was not studied.
CONCLUSIONS
At 800°C, some thiobarbituric complexes are converted to Ln2O2SO4 oxysulfates (Ln = Nd (complex III), Sm [10], and Eu [7]), while others (I and II) transform to mixtures containing mainly oxides TbO2, Tb2O3, and Gd2O3, respectively, and relatively small amounts of Ln2O2SO4 (Ln = Gd, Tb). The different composition of the products of thermal decomposition of [Ln2(H2O)6(Htba)6]n can be explained by a decrease in the enthalpies of formation of Ln2O3 oxides with an increase in the lanthanide atomic number [20].
REFERENCES
M. C. Heffern, L. M. Matosziuk, and T. J. Meade, Chem. Rev. 114, 4496 (2014). https://doi.org/10.1021/cr400477t
Y. Yang, Q. Zhao, W. Feng, and F. Li, Chem. Rev. 113, 192 (2013). https://doi.org/10.1021/cr2004103
S. Cotton, Lanthanide and Actinide Chemistry (Wiley, Uppingham, Rutland, 2006).
K. Binnemans, Chem. Rev. 109, 4283 (2009). https://doi.org/10.1021/cr8003983
N. N. Golovnev and M. S. Molokeev, 2-Thiobarbituric Acid and Its Complexes with Metals: Synthesis, Structure, and Properties (Sib. Feder. Univ, Krasnoyarsk, 2014) [in Russian].
M. D. Mashkovskii, Medicines: A Manual for Doctors (Novaya volna, Moscow, 2008) [in Russian].
N. N. Golovnev, and M. S. Molokeev, Russ. J. Coord. Chem. 40, 648 (2014). https://doi.org/10.1134/S1070328414090036
N. N. Golovnev, M. S. Molokeev, S. N. Vereshchagin, and V. V. Atuchin, J. Coord. Chem. 68, 1865 (2015). https://doi.org/10.1080/00958972.2015.1031119
N. N. Golovnev, M. S. Molokeev, and S. N. Vereshchagin, J. Struct. Chem. 57, 167 (2016). https://doi.org/10.1134/S0022476616010200
N. N. Golovnev, M. S. Molokeev, I. V. Sterkhova, et al., J. Struct. Chem. 58, 539 (2017). https://doi.org/10.1134/S0022476617030155
G. M. Sheldrick, SADABS, Version 2.01 (Bruker, Madison (WI), 2004).
G. M. Sheldrick, SHELXTL, Version 6.10 (Bruker, Madison (WI), 2004).
Cambridge Structural Database, Version 5.37 (Univ. of Cambridge, Univ. of Cambridge, Cambridge (UK), 2015).
R. D. Shannon and C. T. Prewitt, Acta Crystallogr., Sect. B 25, 925 (1969). https://doi.org/10.1107/S0567740869003220
J. W. Steed and J. L. Atwood, Supramolecular Chemistry, 1st ed. (CRC, 2004; Akademkniga, Moscow, 2007).
PLATON—A Multipurpose Crystallographic Tool (Utrecht Univ., Utrecht, The Netherlands, 2008).
M. Machida, K. Kawamura, K. Ito, and K. Ikeue, Chem. Mater. 17, 1487 (2005). https://doi.org/10.1021/cm0479640
I. Valsamakis and M. Flytzani-Stephanopoulos, Appl. Catal. 106, 255 (2011). https://doi.org/10.1016/j.apcatb.2011.05.037
J. D. Lessard, I. Valsamakis, and M. Flytzani-Stephanopoulos, Chem. Commun. 48, 4857 (2012). https://doi.org/10.1039/C2CC31105D
P. O. Andreev, E. I. Sal’nikova, and A. A. Kislitsyn, Russ. J. Phys. Chem. A 87, 1482 (2013). https://doi.org/10.1134/S0036024413080050
F. A. Khamidov, I. U. Mirsaidov, and A. Badalov, Dokl. Akad. Nauk Resp. Tadzh. 57, 676 (2014).
Funding
The work was performed as part of the State Assignment of the Ministry of Education and Science of the Russian Federation to the Siberian Federal University in 2017–2019. (4.7666.2017/BCh). The powder X-ray diffraction studies were performed using the equipment at the Baikal and Krasnoyarsk Centers for Collective Use of the Siberian Branch of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Avdeeva
Supplementary material
Rights and permissions
About this article
Cite this article
Golovnev, N.N., Molokeev, M.S. & Sterkhova, I.V. Structure and Thermal Decomposition of Nd(III), Gd(III) and Tb(III) 2-Thiobarbiturates. Russ. J. Inorg. Chem. 64, 1146–1151 (2019). https://doi.org/10.1134/S0036023619090134
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023619090134