Abstract
The morphology and distribution of Diplacanthopoma japonicum and Pycnocraspedum microlepis have been specified. The species D. japonicum was recorded for the first time in the waters off Northwestern Australia. The possibility of synonymy of the nominal species D. japonicum and D. nigripinne is assumed. Five specimens of P. microlepis caught on the Kyushu-Palau Ridge make it possible to clarify the variability of some diagnostic characters of the species. A key has been compiled to identify species of the genus Pycnocraspedum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In the collections of national fish-searching expeditions, now stored at the Institute of Oceanology (IO) RAS, Moscow, several specimens of rare species of Ophidiiformes from the genera Diplacanthopoma Günther, 1887 and Pycnocraspedum Alcock, 1889 were found. Information on the species of these genera is extremely limited, their identification is difficult, and the nature of intraspecific variability is practically not studied. In light of this, the description of the new material is of undoubted interest, especially since the new specimen of D. japonicum (Steindachner et Döderlein, 1887) significantly expands the known range of the species, and P. microlepis (Matsubara, 1943) is represented in the studied collection by a series of five specimens (more than was described in the literature for all previous time).
The traditional division of the order Ophidiiformes into suborders of viviparous (Bythithoidei) and oviparous (Ophidioidei) ophidiiforms (Cohen and Nielsen, 1978; Nielsen et al., 1999) has been repeatedly called into question (Howes, 1992; Prokofiev, 2004a, 2004b). Based on the results of studying the complex of anterior vertebrae associated with the swim bladder (Prokofiev, 2004b), the family Neobythitidae was distinguished from the combined family Ophidiidae sensu by Cohen and Nielsen (1978) as part of the former subfamilies Neobythitinae and Brotulotaeniinae and the tribe Sirembini in the classification of Cohen and Nielsen, but with the exception of the genus Hypopleuron Smith et Radcliffe, 1913, which was assigned to a separate subfamily within Ophidiidae s. str. (Prokofiev, 2004a, 2004b). A more detailed morphological analysis (Ohashi, 2014) fully confirmed the identification of the family Neobythitidae within the indicated boundaries, showing its sister relationship with the complex, including Carapidae, Hypopleuron, and Ophidiidae s. str. Based on this, the author considers it incompetent to unite Ophidiidae and Neobythitidae in one family and oppose them to Carapidae, which continues to appear in the most recent reports (Nelson et al., 2016). Such a union is formally admissible only when all families of oviparous species of Ophidiiformes are merged into one, which is hardly justified.
MATERIALS AND METHODS
The described specimens belong to the collection of the IO RAS. The study methodology is generally accepted for the group (Cohen and Nielsen, 1978). The calculation of the number of rays in the unpaired fins and vertebrae was performed according to radiographs made by the author; when counting the vertebrae, the urostyle was not taken into account. Counts that differ on different sides of the same fish are separated by a “/”. “Pseudocaudal” is the name given to the condition that occurs during the regeneration of the lost caudal end of the body, when C is replaced by the regenerating rays of the vertical fins (Iwamoto, 1970). The pores of the seismosensory canals of the head with large gaping holes are called nostril-shaped, according to Andriashev (1955). The following abbreviations have been used in the text: D, A, P, V, and C—dorsal, anal, pectoral, ventral, and caudal fins; vert.—number of vertebrae (abdominal (vert. abd.) + caudal), TL and SL—total and standard length, lc—head length, H and HA—maximum body depth and its depth at the level of the beginning of A; aD, aA, and aV—predorsal, preanal, and preventral distances, respectively; lP and lV—length of Р and V, ao—snout length, oo—horizontal diameter of the orbit, io—width of the bony interorbital space, lmx and hmx—length of the upper jaw and height of the maxillary plate, LFT—large fishery freezer trawler.
RESULTS AND DISCUSSION
Diplacanthopoma japonicum (Steindachner et Döderlein, 1887)
(Fig. 1)
Material. IO RAS no. 03620, 1 specimen TL 245+ mm, 09°01′12′′ S 130°59′30′′ E, LFT Akademik Berg, bottom trawl, depth 540 m.
Description. P 29/31, V 1; vert. abd. 20. The caudal end of the fish was lost during life, forming a “pseudocaudal” (Figs. 1a, 1b), and therefore a correct calculation of the number of rays in the vertical fins and the total number of vertebrae is impossible. The head is massive, wide; the snout is somewhat flattened, the interorbital space is depressed between orbits, the upper bony edge of orbits is elevated. The dorsal profile of the head rises sharply from the eyes to the occiput; the maximum body depth falls on the predorsal region, 1.55 times less than the length of the head. Two nostrils; the anterior one is smaller, opens with a very short tubule above the upper jaw at an equal distance from the snout top and the anterior edge of the orbit. The posterior nostril is located higher than the anterior nostril and opens directly in front of the anterior edge of the orbit as a large oval opening with raised dorsal, anterior and ventral edges. The mouth is large, the upper jaw ends behind the vertical of the posterior edge of the eye; the maxillary plate is free, strongly widened posteriorly, its posterior edge is slightly concave; there is a well-developed supramaxilla. When the mouth is closed, most of the maxilla is covered from the outside by wide infraorbital bones, leaving only the ventroposterior end of the maxillary plate visible. The dentition of the premaxilla is visible from the outside with the mouth closed. On the jaws, vomer and palatine bones, there are small conical teeth arranged in strips; the vomerine tooth spot is in the form of a wide V-shaped transverse strip; the basibranchial dental plates are absent. There are two pores in the supraorbital canal, the first is very large, nostril-shaped, opens at the top of the snout medially to the anterior nostril; the posterior pore is small, opening on the dorsal surface of the head somewhat behind the vertical from the posterior edge of the orbit. There are six nostril-shaped pores in the infraorbital canal, of which the first is the smallest and is located above the others, the third and sixth are the largest; the anterior three are brought together, the fifth is located above the posterior end of the maxilla, the sixth is in front of the anterior edge of the preopercle at the level of the lower edge of the orbit. The temporal canal has two large pores located above the opercle. There are seven nostril-shaped pores in the preoperculo-mandibular canal; the first pair of pores is widely separated, the fifth pore is located above the mandibular joint, the last two pores are gigantic in size, commensurate with the diameter of the pupil, open on the preopercle. Coronal and supratemporal commissures are absent. The skin of the top and sides of the head is completely covered with sparse hair-like dark-colored papillae and larger and sparse (but forming noticeable concentrations on the dorsal surface of the snout and around the eyes) narrow valve-shaped dark-colored neuromasts (Fig. 1c). At the snout top, near the anteroventral edge of the first supraorbital pore and under the anterior nostril, there are several short thick skin outgrowths, probably vestiges of the rostral (ethmoid) commissure (Balushkin and Prokofiev, 2005). The body lateral line is represented by easily shed free neuromasts. The preopercle has uneven smooth posterior edge, covered with skin, without spines. The opercular lobe is narrowed and pointed towards the top, the opercular spine is curved upwards, its end reaches the posterior edge of the opercle. A wide cutaneus lobe rounded at the free end (“Diplacanthopoma-flap,” according to: Prokofiev, 2004b) is well developed above the upper edge of the opercle (Fig. 1d). There are three (1 + 2) developed gill rakers on the first branchial arch, in addition to them there are 16 (3 + 1 + 12) tuberculate ones. The pseudobranch is rudimentary, represented by three short and wide elements. The tongue is massive, its end is free.
The beginning of D is located noticeably behind the base of P, the insertion of V is under the preopercle, the ends of the rays of V noticeably extend beyond the vertical of the base of P. The base of P forms a very short and wide lobe with a fleshy outgrowth at the upper edge, the end of adpressed P reaches the vertical of the anus. The anus is located at the beginning of A. The origin of A is located on the vertical of the 31st ray of D. The body is covered with medium-sized cycloid scales, the scales on the head are completely absent. There are about 18 transverse rows of scales in the predorsal region.
Coloration of preserved fish after long-term storage in formalin is light brown, whitish on the lower surface of the head, chest and belly; distal half of P and distal edges of vertical fins are distinctly darker, V is pale, almost white. The oral cavity is light, the branchial cavity is dark.
Measurements. In mm: lc 85, H 55, HA 47, aD 95, aA 155, lP 51, lV 39, ao 18, oo 15, io 24, lmx 35, hmx 13. In % lc: ao 21.2, oo 17.7, io 28.2, lmx 41.2, hmx 15.3.
Comparative notes. Species of the genus Diplacanthopoma are rare in collections, and their taxonomy is completely undeveloped. Eight nominal species have been described (the name D. alcockii Goode et Bean, 1896, according to Cohen and Nielsen, 2002, is a nomen nudum): D. brachysoma Günther, 1887 (Western Atlantic); D. brunneum Smith et Radcliffe, 1913 (Philippines); D. japonicum (Southern Japan); D. jordani Garman, 1899 (tropical Eastern Pacific); D. kreffti Cohen et Nielsen, 2002 (Northwestern Australia); D. nigripinne Gilchrist et von Bonde, 1924 (South Africa); D. raniceps Alcock, 1898 (Andaman Sea) and D. riversandersoni Alcock, 1895 (Arabian Sea). It was proposed to divide these species into two groups according to eye size (Cohen and Nielsen, 2002): small-eyed (oo 14.8–18.9% lc), including D. japonicum, D. kreffti, and D. riversandersoni, and large-eyed (oo 20.0–25.4% lc)—all the rest. This division seems to be very conditional, and the limits of intraspecific, including ontogenetic variability of this character remain unclear in any species. The studied specimen characterized by oo 17.7% lc falls into the group of small-eyed species. It differs well from the only representative of the genus previously known in Australian waters (D. kreffti, the shelf of northwestern Australia) in a noticeably shorter and deeper upper jaw (lmx and hmx 41.2 and 15.3 versus 49.3 and 10.6% lc), which is mostly free (covered by a fold of skin in D. kreffti). According to Cohen and Nielsen (2002), D. kreffti also differs from D. japonicum in a smaller number of rays of D and A (117 and 86 versus 132–134 and 100–101, respectively), but these characters are unidentifiable in the described fish due to the loss of the caudal end of the body. However, the number of rays of D before the beginning of A in the studied specimen (31) is also less than in the holotype of D. kreffti (37). The studied specimen noticeably differs from the holotype and the only known specimen of D. kreffti in the smaller width of the interorbital space and the length of P, but the greater length of V (28.2, 60.0, and 45.9 versus 34.6, 68.6 and 36.9% lc) and the greater number of developed rakers on the first gill arch (four versus three).
The studied specimen cannot be identified with D. riversandersoni, since it has noticeably more rays of P (29–30 versus 24) and the maxillary plate not so widened posteriorly (hmx 15.3 versus 18.2% lc) (Alcock, 1895; Cohen and Nielsen, 2002). All characters of the studied fish are in good agreement with the data given by different authors (Steindachner and Döderlein, 1887; Machida, 1988; Cohen and Nielsen, 2002; Nakabo, 2002) for D. japonicum, on the basis of which the author considers the Australian specimen to be conspecific to this species.
At the same time, it should be noted that the South African species D. nigripinne occupies an intermediate position between the large-eyed and small-eyed species, having oo 20% lc according to the original description (Gilchrist and von Bonde, 1924; Cohen and Nielsen, 2002). Given the variability for this character of 15.9–18.9% for D. japonicum, this value may well represent a particular case of individual variability. The holotype of D. nigripinne, like many other types of Gilchrist, was vandalized by some L.T. Hogben (Pietsch, 1972). The second specimen of this species was briefly characterized by Cohen (1986). Its measurements were not given, and the number of rays in D and A is given as ca.120 and ca.70, respectively. This is significantly less than that known for D. japonicum and among all species of the genus approaches only D. raniceps, but the number of rays of Р (29) is beyond the values known for D. raniceps (21–26) (Cohen and Nielsen, 2002). At the same time, Cohen (1986) gives only an approximate number of rays and does not give the number of vertebrae. Species of Diplacanthopoma are characterized by a sharp thinning of the caudal end of the body, which is often lost during life and replaced by a pseudocaudal (Cohen and Nielsen, 2002). If the approximate count of the number of rays in the vertical fins of the second South African specimen is explained by the presence of a pseudocaudal (the drawing of the fish given in the publication does not give complete confidence that this is not the case), then no other characters different in D. japonicum and D. nigripinne remain. These species may well be conspecific. Unfortunately, the specimen in question was also lost even before the inventory of the collection when it was moved to another building in 2007 (O. Gon, personal communication, October 2019).
Distribution. Until now, the species has been known from two records off the Pacific coast of Japan (near Tokyo and Cape Urado) (Steindachner and Döderlein, 1887; Machida, 1988; Cohen and Nielsen, 2002; Nakabo, 2002). A new find in the Timor Sea between the Tanimbar Islands and the northwestern coast of Australia significantly expands the known range of the species; however, if the assumption of the synonymy of D. nigripinne with D. japonicum is correct, then it can be distributed at bathyal depths throughout the tropical and subtropical Indo-West Pacific.
Pycnocraspedum microlepis (Matsubara, 1943)
(Fig. 2)
Material. IO RAS no. 03619, 5 specimens TL 185–330 mm, SL 167–315 mm, 26°05′ N 135°49′ E, depth 320–340 m, January 7, 1982.
Description. D 95–98, A 70–71, P 23–27, V 2, C 10; vert. 13 + 39–41 = 52–54. The head is of moderate size, 4.0–4.6 times in SL, 1.8–2.0 times in preanal distance. The snout is blunt, widely rounded, equal to or slightly shorter than the horizontal diameter of the eye, which is 1.3 (SL 167 mm) or 1.6 (SL 213–315 mm) times less than the width of the interorbital space. There are two nostrils lying on the same horizontal line at the level of the middle of the eye. The lower jaw is shorter than the upper one, the dentition of the premaxillaries is visible when the mouth is closed; the upper jaw ends far behind the posterior edge of the orbit, its plate is high with a concave posterior edge; the supramaxilla is well developed. The jaws, vomer, palatine bones, and basibranchial dental plates have very small teeth arranged in strips. There are two basibranchial plates, both medial; the anterior one is 3–4 times as long as the posterior one, 3.3–3.9 times as long as head. There are two pores in the supraorbital canal, the first one is large, nostril-shaped, located behind the snout top directly above the upper lip; the second one is small, opens at the level of the nostrils. There are nine pores in the infraorbital and preoperculo-mandibular canals, five mandibular pores, and four preopercular pores. The temporal canal opens with one large pore located on a free cutaneus lobe (“Diplacanthopoma-flap”) above the upper edge of the branchial aperture. Coronal and supratemporal commissures are absent. The body lateral-line canal is distinct, trough-shaped, fragmented and disappearing in the posterior quarter of the body. The preopercle has three or four short spines; the opercular spine is short, far does not reach the posterior edge of the opercular lobe. The first branchial arch has four developed rakers, in addition to them there are 3–4 + 3 + 10–15 dental plates (in front of, between, and behind the developed rakers). A pseudobranch, as a rule, is absent, but in 1 specimen, four short spaced elements were found. There are 18 pyloric caeca (in 1 specimen).
The beginning of D is located in front of the vertical of the base of P, the insertion of V is under the preopercle; the ends of the rays of V are filiform; the end of adpressed P does not reach the anus. The anus is located at the beginning of A. The body is covered with medium-sized cycloid scales reaching the snout top on the head.
Coloration of preserved fish is brown (around the places where the scales have fell off it is light), the body canal of the lateral line is distinctly darkened, and the vertical fins are blackish. The oral cavity is light, the branchial cavity is dark. The stomach is blackish in the anterior half, light in the posterior one.
Measurements. In % SL: lc 22.2–25.2, H 19.2–21.6, HA 18.0–20.2, aD 18.0–22.2, aA 43.6–44.9, aV 16.2–19.1, lP 16.0–19.7 (in 1 specimen: 14.9), lV 12.1–18.8, ao 3.9–4.4, oo 4.2–4.8, io 6.0–7.1, lmx 12.0–14.3, hmx 4.5–5.1. In % TL: SL 90.3–95.5.
Comparative notes. The genus includes a single Atlantic species, P. phyllosoma (Parr, 1933), known only from a metamorphosing specimen, and four Indo-Pacific species: P. armatum Gosline, 1954 (Hawaii); P. fulvum Machida, 1984 (Okinawa Trench); P. microlepis (South Japan) and P. squamipinne Alcock, 1889 (East Africa to New Caledonia; possibly a combined group) (Nielsen et al., 1999; Prokofiev, 2005). The assignment of the described specimens to the species P. microlepis is based on the number of rays in the vertical fins, spines of the preopercle, and pyloric caeca; the absence of lateral basibranchial dental plates and relatively short P not reaching the anus (Gosline, 1954; Machida, 1984; Nakabo, 2002; Prokofiev, 2005; Teena et al., 2021). The studied fishes differ from the available descriptions of P. microlepis only in greater variability in the number of the rays of P (23–27 versus 25–28) and preopercular spines (3–4 versus 3) (Matsubara, 1943; Machida, 1984; Nakabo, 2002). P. microlepis differs from all other Indo-Pacific representatives of the genus in a larger number of pyloric caeca (18–20 versus 12–13), similar to that of the Atlantic P. phyllosoma (21). In addition, it differs from P. armatum in short P, which do not reach the anus (in P. armatum they extend behind the anus), and in the more posterior position of the beginning of D (behind the vertical of the posterior edge of the preopercle, while in P. armatum it is on this vertical) (Gosline, 1954), and from P. fulvum in the absence of lateral basibranchial dental plates and pseudobranch, a more developed preopercular spination, and a greater number of rays in the vertical fins (Machida, 1984; Nakabo, 2002). To identify the species of Pycnocraspedum, the following key can be proposed.
1 (2) Five or six developed gill-rakers………… ……………….…….………..…...................…. P. armatum
2 (1) Four developed gill-rakers…………………..… 3
3 (4) Two preopercular spines; lateral basibranchial plates are present (P. fulvum, not known for P. phyllosoma) ………………………..……………………………….… 5
4 (3) Three to four preopercular spines; lateral basibranchial plates are usually absent (as an exception, a reduced plate can be found on one side (Prokofiev (2005)) …….............................................……………. 7
5 (6) D 83, A 63; pyloric caeca 13; Pacific Ocean south of Japan (Okinawa Trench) …............. P. fulvum
6 (5) D 97, A 71; pyloric caeca 21; Atlantic Ocean off the Bahamas………..................……… P. phyllosoma
7 (8) Pyloric caeca 12 or 13; head 3.0–3.9 times in SL .……………………....................…….. P. squamipinne
8 (7) Pyloric caeca 18–20; head 4.0–4.6 times in SL …………………………....................……. P. microlepis
Distribution. The species is known from the Pacific waters of Japan from the Kumano Nada Sea to Tosa Bay and from the Kyushu-Palau Ridge (Nakabo, 2002), has also been reported for the East China Sea (Nielsen et al., 1999). Our specimens were caught on the Kyushu-Palau Ridge.
REFERENCES
Alcock, A., Natural history notes from H.M. Indian marine survey steamer “Investigator”. Ser. II. No. 18. On a new species of viviparous fish of the family Ophidiidae, Ann. Mag. Nat. Hist. Ser., 1895, vol. 16, pp. 144–146.
Andriyashev, A.P., Review of eel-like lycods [Lycenchelys Gill (Pisces, Zoarcidae) and related forms] of the seas of the USSR and adjacent waters, Tr. Zool. Inst. Akad. Nauk SSSR, 1955, vol. 18, pp. 349–384.
Balushkin, A.V. and Prokofiev, A.M., A new species of the genus Cataetyx (Ophidiiformes: Bythitidae) from Heracles Banks (the South Pacific Rise), J. Ichthyol., 2005, vol. 45, no. 7, pp. 541–545.
Cohen, D.M., Bythitidae, in Smith’s Sea Fishes, Johannesburg: McMillan South Africa, 1986, pp. 354–356.
Cohen, D.M. and Nielsen, J., Guide to the identification of genera of the fish order Ophidiiformes with a tentative classification of the order, NOAA Tech. Rep. NMFS Circ. 417, Seattle: NOAA; NMFS, 1978. https://doi.org/10.5962/bhl.title.63242
Cohen, D.M. and Nielsen, J., Diplacanthopoma kreffti (Pisces, Bythitidae), a new species from the Northwest Australian shelf, with comments on the name D. alcockii Goode and Bean, 1896, 1896, Arch. Fish. Mar. Res., 2002, vol. 50, no. 1, pp. 11–15.
Gilchrist, J.D.F. and von Bonde, C., Deep-sea fishes procured by the S.S. “Pickle” (Pt. 2), Rept. Fish. Mar. Biol. Surv. Union of South Africa, 1924, vol. 3, no. 7, pp. 1–24, pls. 1–6.
Gosline, W.A., Fishes killed by the 1950 eruption of Mauna Loa. II. Brotulidae, Pac. Sci., 1954, vol. 8, no. 1, pp. 68–83.
Iwamoto, T., Macrourid fishes of the Gulf of Guinea, in The R/V Pillsbury Deep-Sea Biological Expedition to the Gulf of Guinea, 1964–65. Ser. Stud. Trop. Oceanogr., Miami: UM, 1970, no. 4. pt. 2, pp. 316–431.
Machida, Y., Pycnocraspedum fulvum, in Fishes of the Okinawa Trough and the Adjacent Waters, Tokyo: JFRCA, 1984, vol. 1.
Machida, Y., An additional specimen of an imperfectly known bythitid fish, Diplacanthopoma japonicum (Bythitidae, Ophidiiformes), Rep. Usa Mar. Biol. Inst. Kochi Univ., 1988, no. 10, pp. 69–73.
Matsubara, K., Ichthyological annotations from the depth of the Sea of Japan. I–VII, J. Sigenkagaku Kenkyusyo, 1943, vol. 1, no. 1, pp. 37–82, pl. 1.
Nakabo, T., Ophidiiformes, in Fishes of Japan with Pictorial Keys to the Species, Tokyo: Tokai Univ. Press, 2002, vol. 1, pp. 436–451.
Nelson, J.S., Grande, T.C., and Wilson, M.V.H., Fishes of the World, Hoboken: John Wiley and Sons, 2016. https://doi.org/10.1002/9781119174844
Nielsen, J.G., Cohen, D.M., Markle, D.F., and Robins, C.R., Ophidiiform fishes of the world. An annotated and illustrated catalogue of pearlfishes, cusk-eels, brotulas and other ophidiiform fishes known to date, FAO Species Catalogue, Rome: FAO, 1999, vol. 18.
Ohashi, S., Comparative morphology and phylogenetic systematics of the family Ophidiidae and related taxa (Teleostei: Ophidiiformes), Theses PhD, Hokkaido: HUSCAP, 2014. http://hdl.handle.net/2115/55362. Version 03/2022.
Pietsch, T.W., Ergebnisse der Forschungsreisen des FFS “Walter Herwig” nach Südamerika. XIX. Systematics and distribution of ceratioid fishes of the genus Dolopichthys (family Oneirodidae), with the description of a new species, Arch. Fishereiwiss, 1972, vol. 23, pp. 1–28.
ProkofIev, A.M., On the taxonomic position and relationships of Hypopleuron caninum Smith & Radcliffe, 1913 (Teleostei: Ophidiiformes), Ob”ed. Nauch. Zh., 2004a, vol. 106, no. 14, pp. 64–69.
Prokofiev, A.M., The structure and taxonomic significance of the complex of anterior abdominal vertebrae in representatives of the order Ophidiiformes (Pisces, Paracanthopterygii), and questions of classification of the order, Estestv. Tekhn. Nauki, 2004b, vol. 11, no. 2, pp. 129–142.
Prokofiev, A.M., On some rare species of Ophidiiformes from the South Atlantic and Indo-West Pacific with a description of the new genus Megacataetyx gen. novum (Teleostei: Ophidiiformes), Estestv. Tekhn. Nauki, 2005, vol. 16, no. 2, pp. 111–128.
Steindachner, F. and Döderlein, L., Beiträge zur Kenntniss der Fische Japan’s (IV), Denksch. K. Akad. Wiss. Wien, Math.-Wiss. Cl., 1887, vol. 53, pp. 257–296.
Teena, J.T.K., Murugan, A., Kumar, A.T.T., and Lal, K.K., Redescription of a rare cusk eel, Pycnocraspedum squamipinne (Actinopterygii, Ophidiiformes, Ophidiidae), from Bay of Bengal, Acta Ichthyol. Piscat., 2021, vol. 51, no. 1, pp. 77–83. https://doi.org/10.3897/aiep.51.63469
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by S. Avodkova
Rights and permissions
About this article
Cite this article
Prokofiev, A.M. New Data on the Morphology and Distribution of Two Rare Species of Ophidiiformes: Diplacanthopoma japonicum (Bythitidae) and Pycnocraspedum microlepis (Neobythitidae). J. Ichthyol. 62, 1019–1024 (2022). https://doi.org/10.1134/S0032945222060224
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0032945222060224




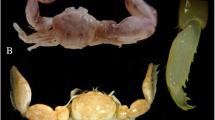

 ) “Diplacanthopoma-flap”—according to: Prokofiev, 2004b. Scale: 15 mm.
) “Diplacanthopoma-flap”—according to: Prokofiev, 2004b. Scale: 15 mm. 