Abstract
The genera of “long-bodied” opisthoproctids, Bathylychnops, Ioichthys, Dolichopteryx, Dolichopteroides and the first described Duolentops gen. nov. (type species: D. minuscula), were revised. An overview of diagnostically significant characters was given and new diagnoses of the genera were compiled. The characteristics of a number of species (I. kashkini, D. minuscula, D. andriashevi, D. longipes, D. parini, D. pseudolongipes, D. trunovi, and D. vityazi) were specified. D. pseudolongipes was first discovered in the western Pacific Ocean. A new species, Dolichopteryx nigripes sp. nov., from the South Pacific was described. A new key for identification of the genera and species of “long-bodied” opisthoproctids has been compiled.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Representatives of the family Opisthoproctidae are some of the most grotesque recent fish and are traditionally featured in popular literature when describing the bizarre inhabitants of the deep sea. Indeed, the appearance of these fish, inhabiting the meso- and partly bathypelagic from the boreal latitudes of the Atlantic and Pacific to the Subantarctic, is very peculiar. They are characterized by an exceptional specialization of the organs of vision, the variants of which are very diverse within the family (Partridge et al., 2014), and deep reductive transformations of the skeleton (Cohen, 1964). The genera of opisthoproctids can, with a certain degree of convention, be divided into two groups: “long-bodied” and “short-bodied”, characterized by 40–85 and 30–40 vertebrae, respectively. The first group includes the genera Bathylychnops Cohen, 1958, Dolichopteryx Brauer, 1901, Dolichopteroides Parin et al., 2009 and Ioichthys Parin, 2004; the second group: Macropinna Chapman, 1939, Opisthoproctus Vaillant, 1888 (including Monacoa Whitley, 1943), Rhynchohyalus Barnard, 1925 and Winteria Brauer, 1901. The conditionality of this division is explained by the fact that the genus Winteria apparently is morphologically closest to the common ancestor of all opisthoproctids, and the genus Rhynchohyalus is a morphologically intermediate form between “long-bodied” and “short-bodied” opisthoproctids. At the same time, the “long-bodied” genera of opisthoproctids represent, most likely, a monophyletic group, the common feature of which is a tendency to an increase in the number of vertebrae, displacement of the vertical fins in the caudal direction, and elongation of the body.
Despite the recent revision (Parin et al., 2009), the taxonomy of “long-bodied” opisthoproctids remains poorly developed, which is explained by the rarity of these fish in collections and their extremely fragile constitution, which is why they usually come to the hands of researchers badly damaged. A number of species of the genus Dolichopteryx remain known only by the juveniles; on the other hand, there are species for which, on the contrary, early stages of development have not been described. A number of characters used in generic and species diagnostics are subject to ontogenetic variability or are present only at certain ontogenetic stages, which greatly complicates the identification of species. Parin et al. (2009) recognized 16 valid species within four genera of “long-bodied” opisthoproctids, in addition to which two other forms were indicated by them in an open nomenclature. Over the past ten years, the author have discovered several new specimens from this group of fish in various museum collections, work with which has revealed a number of inaccuracies in the descriptions and/or interpretations of previous authors, as well as a number of errors in the key in the last revision of the group of Parin et al. (2009), making species identification difficult. As a result, the author undertook a re-examination of all available specimens for this group and the available literature data, the results of which are reported in this paper.
MATERIALS AND METHODS
The list and label data of the studied specimens are given in the descriptive part of the paper. The measurement and calculation technique is standard (Hubbs and Lagler, 1958) with the following additions: the horizontal diameter of the eyeball was measured at the extreme points of the anterior and posterior edges of the eyeball, the horizontal length of the bony orbit—from the posterior edge of the lateral ethmoid to the posterior bone edge of the orbit that is most distant from it. Gill rakers were counted either on the completely extracted arches or after trimming the surrounding tissues, which allows for a complete visualization of the branchial arch. For better visualization of the rakers, the extracted arches were stained with alizarin red S. The nomenclature of eye structures was taken from a previously published paper (Pearcy et al., 1965) and adapted to the Russian language; subscleral lenses are called thickenings formed under the cornea of the eye; “tapetum” (Wagner et al., 2009), a reflective layer of crystal-containing cells in the bottom of the secondary globe. The following abbreviations have been used: SL—standard length; D, A, P, V, C—dorsal, anal, pectoral, pelvic and caudal fins, respectively; r. br, sp. br—number of branchiostegal rays and gill rakers in the outer row on the first arch; p. c—number of pyloric caeca, n—number of examined specimens; uncat.—uncatalogued specimens; R/V, FRV—research vessels and fishery research vessels, respectively; IKMT—Isaacs-Kidd midwater trawl, HCD—hamseros cone dredge, st.— oceanographic station; IO RAS—Institute of Oceanology RAS, Moscow; ZIN—Zoological Institute RAS, St. Petersburg; ZMMSU—Zoological Museum of Moscow State University; MNHN—National Museum of Natural History, Paris (France).
RESULTS AND DISCUSSION
Overview of Characters Used for Generic Diagnosis of “Long-Bodied” Opisthoproctids
Structure of the eyes (Fig. 1). The eyes of all opisthoproctids undergo significant morphological transformations, which, together with other structural modifications, determine the exclusively unique appearance of these fish. For “long-bodied” opisthoproctids, two types of eyeball structure are characteristic: vesicular (“pouchlike” in the English-language literature (Fukui and Kitagawa, 2006a, 2006b; Fukui et al., 2008)) (Figs. 1a–1c, 1i) and cylindrical, or telescopic (Figs. 1d – 1h). Pouchlike eyes are characteristic of species from the genera Bathylychnops, Ioichthys and Duolentops gen. nov., telescopic: Dolichopteryx and Dolichopteroides. The indication of the presence of pouchlike (Fukui and Kitagawa, 2006b; Fukui et al., 2008) or weakly telescopic, obliquely upward (Parin et al., 2009) eyes in D. parini is apparently explained by their inaccurate illustration in the original description of this species (Kobylyanskii and Fedorov, 2001, p. 125, figure), since the authors listed above did not have their own material on this species. Meanwhile, the text of the original description clearly indicates that the eyes of D. parini are “telescopic” (Kobylyansky and Fedorov, 2001, p. 126), and their structure in the specimen of this species studied by the author does not differ in any way from that of other representatives of the genus Dolichopteryx sensu novo (Figs. 1d, 1g).
Eyeball of Ioichthys kashkini, paratype SL 66.3 mm (a, b); Duolentops minuscula, specimen SL 30 mm (c); Dolichopteryx parini, specimen SL 170 mm (d, g); D. andriashevi, paratype SL 41.4 mm (e, f); Dolichopteroides binocularis, specimen SL 55 mm (h); Bathylychnops exilis, specimen SL 420 mm (i) (a–e, h, i—laterally; f, g—ventrolaterally). Legend: ap—aperture of the globular body, permeable to light; apk—aphakic space, c.g—globular body (anterior corneal evagination, secondary globe), cor—corneal lenses (“additional lenses”); dsc, vsc—dorsal and ventral subscleral lenses, respectively; myo—median dorsal myosepta, pce— posterior corneal evagination, rd—retinal diverticulum, scl—sclerotized corneal constriction, so—suborbital accumulation of melanophores, ta–white (luminous?) tissue at the lower edge of the eye. Scale, mm: a—2; b, g—4; c, e–f, h—1; d, i—8.
The eyeball in all species of “long-bodied” opisthoproctids has corneal evaginations, the structure of which differs significantly in representatives of different genera. They are most simply arranged in Ioichthys, an eye of which is practically devoid of areas of additional corneal sclerotization. The anterior corneal evagination in Ioichthys is large, bean-shaped, located at the anterior edge of the lens of the eye and occupies about half of its vertical diameter. Its anterior and superior sections are transparent, while the posterior inferior section contains a retinal diverticulum surrounded by a pigment membrane (Fig. 1a). The pupil opening is framed by a light, probably somewhat sclerotized border (the most developed along the posterior edge of the anterior corneal evagination), the lower part of which Parin (2004, p. 438) apparently called the “skin eyelid”. The posterior evagination is a simple thickening of the cornea; it is well pronounced in the holotype SL 232 mm, but barely outlined in the paratype SL 66 mm. Although Parin (2004) points out that the posterior evagination is larger than the anterior one, this is not so, since he apparently did not take into account the unpigmented region in the anterior and superior parts of the anterior evagination, together with which the length of this evagination is more than two-thirds of the vertical diameter of the lens of the eye.
In Duolentops gen. nov., the structure of the anterior corneal evagination is similar to that described above for Ioichthys; the retinal diverticulum looks like a vertically elongated band along the anterior edge of the lens of the eye with a well-formed light lenticular thickening in the inferior part (Fig. 1c), apparently serving as an additional lens or homologous to the “tapetum” of Dolichopteryx. Ioichthys and Duolentops gen. nov. differ from all other genera of “long-bodied” opisthoproctids by the presence of an aphakic space in the eye in front of the anterior-superior edge of the lens (in Bathylychnops and genera with telescopic eyes, it is not developed), it is most pronounced in Ioichthys. The corneal evaginations in these genera do not yet form a separate globular body on the eyeball (“secondary globe” in the terminology of Pearcy et al., 1965).
In Duolentops gen. nov., in the lower part of the eyeball under the lens there is a thick, oval, clearly circumscribed light lenticular subscleral thickening, with a deep depression on the outer surface (Fig. 1c). It is also depicted in figures in the original descriptions of both species (Fukui and Kitagawa, 2006a, 2006b). A structurally similar lenticular formation is also present in the upper-posterior part of the orbit (when viewed from the side, it is partially covered by the bones of the skull) in the specimen of D. minuscula the author examined (Fig. 1c). In the papers of Fukui and Kitagawa (2006a, 2006b) such a structure was not described. Posterior corneal evagination in Duolentops gen. nov. is not developed.
In Dolichopteryx, the anterior corneal evagination is thin, in the juveniles it is absolutely transparent; there is a retinal diverticulum in its posteroventral portion; together they form a separate globular body, displaced to the lateral surface of the lens of the eye. The area of the anterior wall of the globular body is transparent, and a light thickening is observed at the bottom of the diverticulum (Fig. 1e). According to Wagner et al. (2009), this structure, which they called a “tapetum”, provides reflection of light rays penetrating through the transparent part of the cornea onto the light-sensitive cells of the retinal diverticulum. Dolichopteryx lacks a secondary lens that focuses light (Brauer, 1908; Pearcy et al., 1965; Frederiksen, 1973; Wagner et al., 2009). Under the anterior evagination, the posterior corneal evagination is located; it is well pronounced in Dolichopteryx and represents a bean-shaped or oval protrusion of the cornea, covered by the overgrowth of the pigment membrane of the eye. The ventral position of the posterior evagination relative to the anterior one is explained by the telescopic shape of the eyes and their upward orientation in their default position. Between the anterior and posterior evaginations, a constriction of the sclera is formed, wherefore they have the shape of an “hourglass” (Figs. 1f, 1g).
During ontogenesis, the structure of the corneal evaginations and accompanying formations becomes more complex in Dolichopteryx. At SL 30–60 mm, the globular body is already fully formed, but the lower corneal evagination is still poorly developed; the sclera between the superior and inferior evaginations is thickened and determined by a region of milky-white color with a silvery tint in oblique lighting (Fig. 1f), but does not yet form a constriction, which appears in the adult fish with SL 170 mm (Fig. 1g). In the adult fish the retinal diverticulum occupies the entire volume of the globular body. In all the examined juveniles, the globular body is located in the anterior half of the lateral surface of the eye, while in the posterior half in a large specimen of D. parini. It is unclear whether the globular body is displaced in the caudal direction with growth or whether this is a species-specific feature.
In D. anascopa and D. parini, along the lower edge of the eye, an oval (D. anascopa) or semilunar (D. parini) region of milky-white, non-sclerotized, superficially located tissue is developed (Figs. 1d, 1g). The functional significance of this formation has not been clarified.
Unfortunately, the material on Dolichopteroides examined by the author is characterized by very poor preservation of the eyes. In juvenile SL 55 mm the sheaths of the eye are lost; at the level of the middle of the lens (on its lower half), there is a small globular formation on the lateral side (Fig. 1h). Of two adult fish, the remains of the left eye were preserved in a specimen from the Walvis Ridge: the lens is extruded, any sclerotized formations on the membranes of the eye are not detected, the presence and nature of corneal evaginations are not possible to determine. Parin et al. (2009) indicate the presence of a secondary lens for Dolichopteroides.
The eyes of Bathylychnops have the most complex structure, they are characterized by the maximum development of lenticular sclerotized formations, which has no analogues among other vertebrates (Fig. 1i). The eye of an adult Bathylychnops has a large, well-separated globular body located anteroventral to the lens of the eye and completely covered with a pigment membrane, in the anteroventral part of which there is a transparent “window” allowing light to pass through. Behind this “window”, on the lateral surface of the globular body, at its inferior edge, in the middle of the length of the latter, there is a large globular corneal sclerotized formation, which is a secondary lens (Pearcy et al., 1965). At the posterior edge of the globular body, the cornea forms another, approximately one and a half times larger lenticular formation (corneal lens), and there is a third one at the posterior edge of the lens of the eye, commensurate with the second one, but more elongated along the vertical axis. The second lenticular formation is connected to the third one by a thickened scleral bridge; from its anterodorsal and anteroventral corners, bands of sclerotized tissue also extend, covering the back of the globular body; another band extends dorsal to the second corneal lens from the sclerotized bridge between the second and third lenses to the anterior edge of the eyeball. The third corneal lens forms scleral thickenings at its anterior edge and at its superior end. Together, these scleral thickenings connect the corneal lenses like a skeleton, possibly participating in the accommodation of the corneal lenses.
No interspecies variability in the structure of the eyes in Bathylychnops was revealed. Although the smaller B. brachyrhynchus specimens at the author’s disposal have less developed sclerotized formations than the very large B. exilis specimen, this seems to reflect ontogenetic variability. The author does not have juvenile specimens of this genus, but, according to the literature (Pearcy et al., 1965; Badcock, 1988), corneal lenses are not yet developed in the juveniles of Bathylychnops. Cohen (1958) notes that all three corneal lenses are developed in fish larger than SL 112 mm, but in two specimens SL 107 and 110 mm studied by Pearcy et al. (1965), the second and third formations were not formed yet.
The formation of corneal evaginations and the transformation of the anterior one into a globular body, enclosing the retinal diverticulum, which contains photoreceptor cells, is a general specialization of “long-bodied” opistoproctids. From the least specialized structural plan observed in Ioichthys, all other variants of the transformation of these structures observed in other genera can be deduced. At the same time, it cannot be said that the variants of the structure characteristic for different genera demonstrate unidirectional specialization and progressive complication of various structures of the eye. The globular body is the most complex in Bathylychnops, but the eyeball in this genus retains a primitive pouchlike structure. Pearcy et al. (1965) consider the specializations in the eye structure of Bathylychnops as a specialization to stereoscopic vision independent from other opisthoproctids. According to them, stereoscopy in Dolichopteryx is provided by telescopic, upward-directed eyes, while the globular body in this genus is more primitive and devoid of additional sclerotization. As shown by Brauer (1908) and Wagner et al. (2009), light entering the globular body in Dolichopteryx does not fall directly on the retina, but is reflected from the “tapetum”, i.e. a situation unique for vertebrates is observed when the image is obtained not by refraction, but by reflection. By analogy with the bipartite eyes of some pelagic crustaceans, it is assumed that the cylindric eyeball of Dolichopteryx provides predominantly the visualization of objects with the sun rays penetrating into the mesophotic zone, while the globular body is responsible for the visualization of bioluminescent sources (Land, 2000; Wagner et al., 2009). In this case, the different eye structure in Bathylychnops and Dolichopteryx is not associated with the achievement of the same function in different ways, as suggested by Pearcy et al. (1965), but with differently directed specializations of vision in different genera. Lack of information about the microscopic structure of the eye structures in Duolentops gen. nov. does not allow speaking about the possible functional specializations of vision in this genus, however, the development of subscleral lenses in it, obviously, also provides an expansion of the field of view while maintaining pouchlike eyes. Subscleral lenses in Duolentops gen. nov. are developed much better than a weakly isolated globular body, which fundamentally distinguishes this genus from Bathylychnops.
Vomerine dentition. Teeth on the vomer are found in all opisthoproctids, with the exception of Ioichthys; their loss in the latter is regarded as a specialization of this genus (Parin, 2004). In Bathylychnops, the teeth on the vomer are arranged in one row, and not in several rows, as in other genera. Thus, the following evolutionary tendency in the development of this character takes place: (Dolichopteryx + Dolichopteroides + Duolentops gen.nov.) → Bathylychnops → Ioichthys.
Structure of the gill rakers (Figs. 2, 3). Gill rakers are present on all arches, but only the first arch is used for diagnostic purposes. The rakers of subsequent arches in different species are characterized in general by the same characters as the rakers of the first arch, but on each subsequent arch they become smaller and smaller, their number decreases, and the characters of morphological specialization are smoothed out. Previously, it was shown that the number of outer rakers is an important species character (Parin et al., 2009); however, the differences in the morphology of rakers have not yet been paid due attention. In representatives of all genera of “long-bodied” opisthoproctids, the gill rakers are arranged in two rows on each arch; rakers in the outer row are always better developed than in the inner row. The outer and inner rows of rakers are widely separated; the anterior surface of the branchial arch located between them is very wide; the bases of the rakers are connected by a dense cord of connective tissue; the skeletal elements of the branchial arch are covered with a thick, easily detachable epithelium (probably with a developed gelatinous layer under it). Gill rakers are ossified very weakly or not at all, even in the largest fish. In the juveniles of Ioichthys and Dolichopteryx studied by the author, the gill rakers of the outer row are flattened, but rather narrow and elongated (ciliform), spaced apart (Figs. 2a, 2c); in adult representatives of these genera, the rakers become short and wide (linguliform) and touch at the edges (Fig. 2e). The rakers of the inner row are reduced (to a lesser extent in Ioichthys), ciliform or papillary (Figs. 2b, 2d, 2f), more or less spaced. In the adults of Bathylychnops (the juveniles have not been studied in this respect) the gill rakers of the outer row remain ciliform, they are narrower and longer than in juveniles of the above genera; the rakers of the inner row are also better developed, in both rows the opposite rakers are bent so that they form a sort of the walls of the canal, the bottom of which is formed by the anterior surface of the branchial arch (Figs. 2g, 2h). On the contrary, in Dolichopteroides the maximum degree of shortening and widening of the gill rakers is observed, which is formed already at the early stages of ontogenesis (Figs. 2i, 2j). Rakers of the inner row in Dolichopteroides are reduced to the greatest extent in comparison with other genera. The cover tissue of the inner surface of epi- and ceratobranchiale-1 in the adults of D. binocularis forms the dorsal and ventral cariniform folds (Figs. 2k, 2l), not found in representatives of other genera. A completely unique specialization in the structure of the gill rakers is observed in Duolentops gen. nov.: rakers of the outer row in this genus are sharply hypertrophied and subdivided into two rows. The outer row is formed by 14 long, thick, weakly flattened rakers (the last one is very small) located on cerato- and hypobranchiale-1 and directed forward, like in most other teleost fishes (Fig. 3a). Inwards and slightly above the rakers of this row, there are two more large rakers (Figs. 3b, 3c), located behind the ceratobranchial row and oriented parallel to the surface of ceratobranchiale-1: one of them is attached to the junction of the epi- and ceratobranchiale, and the second one is attached to the epibranchiale-1 just above this joint. Rakers of the inner row in Duolentops gen. nov., thick and finger-shaped (Fig. 3b), remain relatively well developed.
Structure of the gill rakers of the outer (a, c, e, g, i, j) and inner (b, d, f, h, k, l) rows of the first branchial arch of Ioichthys kashkini, paratype SL 66.3 mm (a, b); Dolichopteryx vityazi, specimen SL 57.5 mm (c, d); D. parini, specimen SL 170 mm (e, f); Bathylychnops brachyrhynchus, specimen SL 308 mm (g, h); Dolichopteroides binocularis, specimen SL 55 (i), 233 (j, k), 210 (l) mm; pd, pv—dorsal and ventral cariniform folds, respectively. Scale, mm: a, b—1.5; c, d, i—1; e, f, j–l—2.5; g, h—3.
Differences in the structure of the gill rakers in different genera and/or different ontogenetic stages obviously reflect differences in nutrition and/or peculiarities of manipulation with food objects. Weak musculature, small mouth and very weak jaws suggest feeding on small crustaceans and coelenterates: copepods, euphausiids, and siphonophores have been noted in the digestive tract of B. exilis and Dolichopteryx spp. (Cohen, 1964; Fitch and Lavenberg, 1968; Stein and Bond, 1985). It has already been suggested that particles of food objects crushed by the tongue or individual small animals are retained by the rakers and then sent to the crumenal organ, which is formed by the epibranchial skeletal elements of the fourth and fifth branchial arches and overgrown soft tissues that produce a large amount of mucus. The role of the crumenal organ (or one of the roles) is to form a dense food lump, which the fish then swallows (Greenwood and Rosen, 1971; Stein and Bond, 1985). It is possible that the role of thin and elongated rakers is mainly reduced to retaining rather large food particles, while wide and closely approximated rakers, being covered with a layer of mucus during life, contribute to the retention and movement of very small objects to the crumenal organ. The functional significance of a peculiar modification of the rakers of the outer row of the first arch in Duolentops gen. nov. is not entirely clear, but possibly also associated with the transport of food items to the crumenal organ.
Number of vertebrae (myomeres). According to the number of vertebrae, the genera of “long-bodied” opisthoproctids are divided into three groups: multivertebral (Bathylychnops), relatively low-vertebral (Dolichopteryx, Duolentops gen. nov.) and with an intermediate number of vertebrae (Dolichopteroides, Ioichthys). In species of Bathylychnops the number of vertebrae varies from 67 to 85 and is species-specific. In Dolichopteroides the number of vertebrae is 58–60, and in Ioichthys it is 53–58. In species of Dolichopteryx, the number of vertebrae ranges within 41–48, in Duolentops gen. nov.: 40–46 (Parin et al., 2009). The increase in the number of vertebrae is in direct correlation with the degree of elongation of the body. Multiple vertebrae (over 67) should certainly be considered an advanced condition. In bathylagid and microstomatid fishes the number of vertebrae varies from 35 to 52, in argentinids their number is slightly higher: 48–67 (43 in the dwarf monotypic subgenus Prosoarchus) (Cohen, 1964; Kawaguchi and Butler, 1984; Kobylyansky, 1990, 2006; Hatooka, 2002). “Short-bodied” genera of opistoproctids have 30–40 vertebrae (Rhynchohyalus, which is most similar to Dolichopteryx in body shape, has the greatest number of them), in the morphologically least modified genus Winteria there are 33–36 vertebrae (Haedrich and Craddock, 1968; Aizawa, 2002). If the opisthoproctids originate from some low-specialized low-vertebrate microstomatid fish (like Nansenia), then the following evolutionary trend can be assumed: Winteria → Rhynchohyalus → (Dolichopteryx + Duolentops gen.nov.) → Ioichthys → Dolichopteroides → Bathylychnops.
Length of P. As a rule the rays of fins in the museum specimens are broken off; therefore, this character can be estimated only in some specimens. In Bathylychnops and apparently in Ioichthys both rays of P and V are comparatively short; in Duolentops gen. nov. and in most species of Dolichopteryx (except for D. anascopa and D. parini) V is noticeably longer than P, the latter do not reach beyond the base of V. In D. anascopa and D. parini the rays of P and V are of approximately the same length, the ends of P reach beyond the vertical of the beginning of D. Finally, in Dolichopteroides the rays of P are significantly elongated, much longer than the rays of V, with a significantly greater elongation of the trunk region than in Dolichopteryx, extending beyond the base of A or even the base of C (Roule and Angel, 1930; Beebe, 1933; Trunov, 1997). In terms of the proportions of paired fins, Dolichopteroides stands out sharply among other genera of opistoproctids.
Position of D and V. Parin et al. (2009) identified the genus Dolichopteroides on the basis of strongly posteriorly displaced V and greater antedorsal distance (more than 75 versus 70% SL or less). The validity of this genus on the basis of these characters was subsequently challenged (Stewart, 2015). Indeed, taking into account the noticeable variability of the position of D and V in species of the genus Dolichopteryx (the beginning of V in different species is located at a distance of 3–9 myomeres from the vertical line of the beginning of D), the difference between Dolichopteroides and Dolichopteryx in this character does not seem significant. In addition, the differences in the value of the antedorsal distance given by Parin et al. (2009) are inconsistent already based on the descriptions of the species in this paper: in one of the paratypes of D. andriashevi it reaches 79.4% SL (Parin et al., 2009. P. 844). According to the author’s observations and data published by various authors (Cohen, 1964; Trunov, 1997; Fukui and Kitagawa, 2006a, 2006b; Fukui et al., 2008; Parin et al., 2009; Stewart, 2015; Mizusawa et al., 2015), there are no differences between Dolichopteroides and Dolichopteryx in the relative value of antedorsal distance (78–82 and 70–82% SL, respectively) at the genus level. However, these genera differ in the mutual arrangement of the bases of D, A and V (in Dolichopteroides the bases of V and A are at least partially located under the base of D, which is never observed in Dolichopteryx (only the base of A can be located partially under the base of D)). In addition, the caudal displacement of D and V in Dolichopteroides correlates with significantly greater body elongation and an increase in the number of vertebrae than in any species of Dolichopteryx (according to these characters, Dolichopteroides is second only to species of the genus Bathylychnops). Therefore, this feature can reasonably be considered an independently emerging specialization of the genus Dolichopteroides.
Number of procurrent rays of C. In Ioichthys the number of procurrent rays of C (5 or 6) is less than that observed in other representatives of “long-bodied” opisthoproctids (7–14, less than 9 rays were noted only in Bathylychnops chilensis: Parin et al., 2009).
Neoteny. The size of mature fish in most genera of “long-bodied” opisthoproctids apparently exceeds 100 mm, although such fish are caught very rarely. The adults of Dolichopteryx and Dolichopteroides reach lengths over 200 mm; of Ioichthys, 232–253 mm; of Bathylychnops, up to 580 mm SL. In contrast, the maximum known sizes of Duolentops gen. nov. do not exceed 66.2 mm SL, fish about 50 mm SL have mature eggs (Fukui and Kitagawa, 2006a, 2006b). The appearance of species of Duolentops gen. nov. is similar to that in specimens of Dolichopteryx of postlarval juvenile stages, they also retain juvenile melanophore pigmentation (both species) and may have peritoneal markings (D. minuscula). It is obvious that the species of Duolentops gen. nov. are neotenic forms.
Pigmentation (Figs. 1b, 4–7). Until now, insufficient attention has been paid to the use of pigmentation features for the taxonomy of opisthoproctids. Significant differences in pigmentation of juvenile and adult forms are characteristic for “long-bodied” opisthoproctids. According to the author’s observations, the features of juvenile pigmentation are specific for each genus and can often be used for species diagnostics.
Peculiarities of pigmentation in Ioichthys kashkini, paratype SL 66.3 mm (a, b); Dolichopteryx andriashevi, paratypes SL 41.4 (c, d, f, i) and 52.4 (e) mm; D. vityazi, specimen SL 51 (g, l, m) and 31 (j, k) mm; D. trunovi, holotype SL ~ 80 mm (h): (a) ventral surface of the snout; (b, e–g) posterior half of the body laterally; (c, d) infraorbital accumulation of melanophores (◀) in oblique light (a silvery tint of the epidermis under the eye is shown) (c) and in direct light (d); (h) pigment marking on the anterior part of the stomach (◀), (i) pigmentation of the branchial arches; (j–m) pararectal pigmentation ((j, l) lateral view; (k, m) ventral view); an—anus. Scale, mm: a—10, b—5; c, d, i, j, k—1; e–g—4, h—2; l, m—1.5.
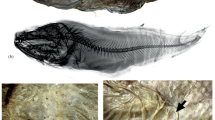
Pigmentation of the caudal part of the body of Duolentops minuscula, specimen SL 30 mm (a) (▶)—position of the adipose fin pressed to the lateral surface of the body and poorly distinguishable in the photograph) and the structure of the pyloric caeca: (b) Dolichopteryx parini, specimen SL 170 mm; (c) Duolentops minuscula, specimen SL 30 mm; (d) D. trunovi, holotype SL ~ 80 mm. Scale, mm: a—1.5, b—5; c, d—1.
Dolichopteryx andriashevi, paratype SL 41.4 mm (a, b); D. longipes, holotype SL 46 mm (c); D. nigripes sp. nov., holotype SL 120 mm (d–f) and D. vityazi, holotype SL 61 mm (g), and specimen SL 51 mm (h, i): a, c, d, g—general view, laterally; b, h— eye orientation; e—radiogrpah; f, i—pigmentation of fins and sides of the body in its posterior half, lateral view; (▶)—position of peritoneal markings in the P–V interval; D, A, V—dorsal, anal and ventral fins, respectively; m.p—remnants of peritoneal markings. Scale, mm: b, h—1; f—14, i – 3.5.
The genus Ioichthys, least specialized in eye structure, apparently also possesses juvenile pigmentation, which is closest to that of the supposed ancestor of the group. In the juvenile of I. kashkini with SL 66.3 mm (Figs. 1b, 4a), melanophore pigmentation on the head is distributed almost everywhere, excluding the area in the region of suspensorium in the interval between the anterior edge of the orbit and the posterior edge of the lower jaw (but its distribution in the postorbital part of the head has not been sufficiently clarified due to poor preservation of the skin here: Fig. 1b). On the body, melanophores are first grouped along the edges of myomeres, and by the middle of its length they pass to their surface, forming a wide longitudinal stripe on the caudal peduncle covering its entire area (Fig. 4b). The ventral surface of the body is completely pigmented with large, densely located melanophores; there are paired peritoneal markings between P and the anus.
The available descriptions of the juvenile stages of Bathylychnops (Badcock, 1988; Parin et al., 2009) (the author does not have own material) indicate a great similarity in the pigmentation of the juveniles in this genus and Ioichthys, with the exception of the presence of an unpaired dorsal row of pigment markings in Bathylychnops, located along midline (Badcock, 1988. Fig. 1). The presence of a dorsal row of markings not seen in any other opisthoproctids seems to represent an autapomorphy of Bathylychnops. The juveniles of Bathylychnops have paired peritoneal markings, and the pigmentation of myomeres and myosepta, judging by the Badcock’s picture (Badcock, 1988. Fig. 2), is similar to that of Ioichthys (the distribution of melanophores on the head of the juveniles of Bathylychnops has not been described or depicted in the literature).
In the juvenile Dolichopteryx melanophore pigmentation on the head forms small, clearly circumscribed areas (on the dorsal surface of the snout, on the upper and lower jaws, on the ventral surface of the head, and under the eye). Its total area is much less than the area of non-pigmented areas. One should note a patchy accumulation of melanophores in the infraorbital region, which apparently exists in the juveniles of all “long-bodied” opisthoproctids (unknown for Bathylychnops, but is present in Ioichthys) and possibly is functionally associated with adaptations of vision, since only in this region of the head the epidermis has a pronounced shiny silvery tint (Figs. 4c, 4d). Pigmentation of the body of the fry of Dolichopteryx (Figs. 4e – 4g, 6i) has its own specific characters. There are no dorsal pigment markings in the fry of this genus and, apparently, there is absolutely no pigmentation of the dorsal median myosepta located between the occiput and the base of D (but in the adult stage it has dark pigmentation in D. parini (Fig. 1d), the juveniles of which are not known). On the caudal peduncle, at the base of C, there is a more or less formed basicaudal accumulation of melanophores, from which the dorsal and ventral pigment stripes, consisting of one or several rows of punctate melanophores and/or intense silvery markings contoured by melanophore pigmentation, extend forward above and below the mid-lateral line. The length and degree of development of the dorsal and ventral pigment stripes are subject to interspecific variability, sometimes the dorsal stripe is very weakly expressed, but it is always present on the caudal peduncle. The presence of markings of silvery pigment in the composition of these stripes is probably an autapomorphy, but these markings are not expressed in all species of the genus (Fig. 7d). Melanophore pigmentation of the ventral surface of the body is developed differently in different species; peritoneal markings are present or absent in different species, pigment markings on the stomach and/or intestines may also be present (Fig. 4h), the number and location of which is species-specific. The presence or absence of melanophore pigmentation on the branchial arches is also diagnostic (Figs. 4i, 6b, 6h).
In the literature there are often indications of the presence of luminous organs on the abdomen in Dolichopteryx. Previously, peritoneal markings (Parr, 1937) or infracarinal muscles and surrounding tissues along the mid-abdominal line (Beebe, 1932; Stein and Bond, 1985) were often mistaken for them. However, no indisputable morphological evidence of the presence of a luminous organ or at least the presence of bacteria in the band of the mid-ventral line has been presented so far (Stein and Bond, 1985). The holotype of D. andriashevi “on the lower surface of the abdomen under the skin has a large milky-white marking of irregular L-like shape” (Parin et al., 2009, p. 844, Fig. 5c), which is interpreted as a luminous organ, but in others specimens of this species it has not been found. Nevertheless, there are still grounds to assume the possibility of bioluminescence of specimens at least at certain ontogenetic stages. All the juveniles of Dolichopteryx with SL 30–68.5 mm studied by the author have a paired whitish peritoneal fold with a pronounced silvery tint and dense melanophore pigmentation represented by very small dash-shaped melanophores around the terminal intestine immediately in front of the anus. In some fishes chromatocytes are apparently fixed more or less straightened, due to which the melanophore pigmentation looks continuous (Figs. 4j, 4k); in others they are contracted, and the melanophores look very small and scattered (Figs. 4l, 4m), but the pigment marking is clearly defined at the superior posterior end of the fold (Fig. 4l). The intestine in the area of this fold is clearly thickened. In the juvenile of D. pseudolongipes SL 100 mm, the peritoneum is intensely black, but its pararectal region is milky-white, without melanophore pigmentation (Fig. 7b). With a high degree of probability this structure in the juvenile of Dolichopteryx can be associated with bioluminescence. In Duolentops gen. nov. and in the juvenile of Ioichthys there are the clusters of pararectal melanophores, but the milky-white coloration of the peritoneum is not observed (in the juveniles of Bathylychnops, this character has not been studied). No traces of this structure were found in the adult fish.
The preservation of the juvenile of Dolichopteroides available for the author does not allow establishing any peculiarities of pigmentation with the exception of the absence of melanophores on the branchial arches and the dorsal median myosepta. The juveniles of D. binocularis have been described earlier (Roule and Angel, 1930; Beebe, 1933): they have a well-developed stripe of melanophores below the mid-lateral line, extending from the caudal peduncle almost to the base of P, and a short strip above this line, only barely extending beyond the end of the base of D. In the juvenile of SL 58 mm depicted by Roule and Angel (1930, pl. IV, Fig. 94) the ventral surface has dense punctate melanophore pigmentation, but there are no separate peritoneal markings. However, they are shown in Beebe’s picture (Beebe, 1933, Fig. 16) and described for D. binocularis by Parr (1937, p. 34), who cites them as “five glandular luminous bodies in midventral series in advance of ventral fins”. Possibly, such discrepancies are due to the presence of more than one species in the composition of Dolichopteroides. In any case, the pigmentation features of D. binocularis are fully within the range of variation known for species of Dolichopteryx.
On the adult stage representatives of all the aforementioned genera are apparently dark in color. In the museum specimens the skin is usually torn off, and only fragments of the epidermis, which have a dark coloration (Stein and Bond, 1985), or remnants of dark-colored scaly pockets (Kobylyanskii and Fedorov, 2001) remain. In the adults of Ioichthys, the entire head and scale pockets are black, the ventral surface is silvery (Parin, 2004). In the specimens of B. brachyrhynchus from the waters of West Africa and from the Nazca Ridge studied by the author some differences in pigmentation are observed, possibly related to the fact that the specimen from the Nazca Ridge is larger (308 versus 192 mm SL). In fish from West African waters, the entire head is covered with a continuous melanophore speck, individual melanophores are larger and scattered on the lateral and ventral surfaces of the head; body skin has diffuse melanophore pigmentation represented by multiple very small brownish melanophores. In the specimen from the Nazca Ridge, the entire dorsal surface of the head and the lateral sides of the snout are completely covered with diffuse brownish pigmentation, there is no melanophore pigmentation on the ventral surface, but diffuse subcutaneous melanophore pigmentation remains in the abdominal region; the skin on the body is torn off. The dorsal median myosepta in the specimen from the Nazca Ridge has scattered melanophore specks, while in fish from West African waters, the melanophore pigmentation merges into a solid dark brown coloration. The rays of all fins in the fish from the Nazca Ridge are not pigmented, while the specimen from the waters of West Africa has dotted melanophores on the rays of all fins (it is unclear for P, the rays of which are broken off to the base). A large specimen of B. exilis is completely devoid of skin; on the head continuous dark pigmentation is developed only on the dorsal surface of the snout and around the eyes; rays of all fins are not colored; the median dorsal myosepta is not pigmented. A common character of all the specimens of Bathylychnops studied by the author is a solid black coloration of the branchial cavity and a continuous dark pigmentation of the anterior surface of the branchial arches (the rest of the branchial arches are not pigmented).
In two specimens of Dolichopteroides from the Walvis Ridge and from the Indian Ocean, which the autor studied, the skin was completely lost, but in the specimen from the Indian Ocean, small fragments of it were preserved at the bases of D and A (the skin is black). In both specimens, in the posterior half of the caudal peduncle, there are a diffuse punctate subcutaneous melanophore pigmentation, subdivided into dorsal and ventral (above and below the myocomm), and larger and scattered subcutaneous melanophores – at the bases of the vertical fins (in the region of their pterygiophores). On the head, the black color of the snout top (around the mouth) stands out sharply. The branchial cavity is black, punctate melanophore pigmentation is present on the branchial arches at the bases of the gill rakers. Author’s two fish sharply differ from each other in the pigmentation of the dorsal median myosepta, which is not colored in the specimen from the Walvis Ridge, but is entirely pigmented in the fish from the Indian Ocean. In addition, in fish from the Walvis Ridge, the rays of all fins are not colored, and in fish from the Indian Ocean, the rays of V are darkened. These differences in pigmentation may support the hypothesis of the collective nature of species of D. binocularis in its current understanding (Parin et al., 2009).
Unfortunately, the author possesses a single specimen of an adult Dolichopteryx belonging to the species D. parini, which, apparently, differs significantly in color from other species of the genus. It is characterized by black coloration of paired fins. By analogy with the situation observed in other groups of mesopelagic fish, it should be expected that the pigmentation of the fins is formed already at the early postlarval stages and is retained in the adult fish. Among other species of Dolichopteryx, the black coloration of V was noted only for D. pseudolongipes and D. nigripes sp. nov. (known only by the juveniles), in all other species of the genus, all fins are not colored. The descriptions available in the literature (Beebe, 1933; Parr, 1937; Cohen, 1964; Fukui and Kitagawa, 2006a, 2006b; Fukui et al., 2008; Parin et al., 2009; Mizusawa et al., 2015), apparently, refer only to the fry stages. Although Cohen (1964, p. 58) indicates that the largest specimen of D. longipes at his disposal “between 85 and 95 mm SL” had “well-developed” eggs, his description of the pigmentation of D. longipes corresponds to juvenile specimens of this species. Therefore, it is currently not possible to estimate the limits of intra- and interspecific variability in the pigmentation of the adults of Dolichopteryx. It cannot be ruled out that such characters of D. parini as a completely pigmented dorsal median myosepta, a light oral cavity, and a relatively weak pigmentation of the branchial cavity (the underside of the gill cover with dark melanophore pigmentation) are a species character.
In all representatives of “long-bodied” opisthoproctids the peritoneum of the adult fish is intensely black and seen through the wall of the abdominal cavity along the mid-ventral line. The outer surface of the peritoneum has a pronounced iridescent silver tint. Along the mid-ventral line under the skin from isthmus to A, thin paired bands of milky-white color stretch (during life, enclosed in a transparent gelatinous sheath (Stein and Bond, 1985), which dissolved in the fish the author studied), that are infracarinal muscles (Fig. 5b). Stein and Bond (1985) do not exclude the ability of these structures to bioluminescence.
Separately, one should dwell on the peculiarities of pigmentation of representatives of the genus Duolentops gen. nov., known from specimens SL 28.0–66.2 mm (Fukui and Kitagawa, 2006a, 2006b; Parin et al., 2009). These fish are mature at SL of about 5–6 cm (Fukui and Kitagawa, 2006a, 2006b), but the smallest specimens indicated in the paper of Parin et al. (2009) may have not yet reached maturity (the state of the gonads of a specimen of D. minuscula with SL 30 mm studied by the author was not identified due to its poor preservation and risk of destruction). However, the pigmentation of a specimen of Duolentops rostrata with SL 35.8 mm, despite the schematic picture (Parin et al., 2009. Fig. 3), corresponds to the pigmentation of the holotype of this species with SL 66.2 mm (Fukui and Kitagawa, 2006a, Fig. 1). Unlike all other genera, pigmentation of Duolentops gen. nov. in the adult stage retains juvenile features, which is due to the neotenic nature of these species. The general character of pigmentation of the head and body of Duolentops gen. nov. in many respects is similar to that in specimens of Dolichopteryx at postlarval stages of development; however, there is a diffuse melanophore pigmentation that occupies all or most of the area of the caudal peduncle and is combined with a pronounced silvery tint of individual sections of the epidermis (instead of small, clearly delimited shiny markings) (Fig. 5a) on the caudal peduncle instead of the basicaudal accumulation of pigment with dorsal and ventral pigment stripes extending from it, consisting of dotted melanophores and specks of silvery tissue, bordered by melanophore pigmentation. The dorsal and ventral pigment stripes extend further forward, almost to the base of P (only the ventral stripe is shown for D. rostrata SL 35.8 mm by Parin et al. (2009, Fig. 3)), in addition to which there may be separate stellate melanophores. In the specimen of D. minuscula SL 30 mm studied by the author these stripes consist of separate dotted melanophores, and the epidermis along these stripes has areas of a silvery tint, but there are no specks of silvery pigment which are isolated or merging into a stripe. Peritoneal markings are present (D. minuscula) or absent (D. rostrata), the dorsal row of markings and pigmentation of the dorsal median myosepta are absent. A patchy accumulation of melanophores under the eye in Duolentops gen. nov., unlike Dolichopteryx, more or less extends forward from the vertical of the anterior orbital edge. This may be a diagnostic feature, but it should be noted that in D. minuscula, the degree of manifestation of this accumulation is subject to noticeable individual variability. Previously, the presence of the “stripe under the eye” as a feature of similarity with Duolentops gen. nov. was postulated for D. parini (Fukui and Kitagawa, 2006a, 2006b; Parin et al., 2009); however, in Duolentops gen. nov. this stripe is a character of juvenile pigmentation that is lost in adult state (no juveniles of D. parini is known). In the adults of D. parini a diffuse brownish melanophore pigmentation on the lateral sides of the snout is indeed developed (the same as on the dorsal surface of the snout), but it is not homologous to the infraorbital accumulation of melanophores in the juveniles of Dolichopteryx and Duolentops gen. nov.
Characterizing the differences in juvenile pigmentation of “long-bodied” opisthoproctids from phylogenetic positions, it can be assumed that the pigmentation of Ioichthys is closest to that of the putative ancestral form, and all other variants can be derived from it. In Bathylychnops a dorsal row of pigment markings is formed as an apomorphy, apparently through the concentration of diffuse melanophore pigmentation of the median dorsal myosepta observed in Ioichthys. In contrast, in Dolichopteryx, Duolentops gen. nov. and probably in Dolichopteroides such pigmentation is lost, but instead of more or less diffuse melanophore pigmentation of myomeres, dorsal and ventral pigment stripes are formed, which (at least in Dolichopteryx), in addition to the melanophores proper, include specks of bright silver pigment, the presence of which is likely an apomorphy. Position of Duolentops gen. nov. based on the characteristics of pigmentation is not entirely clear. The specific characters of pigmentation of this genus can be regarded as a morphologically intermediate state between that observed in the fry of Ioichthys and Dolichopteryx. However, it cannot be excluded that the pigmentation of Duolentops gen. nov. corresponds to an intermediate state between the pigmentation the juveniles and the adults of Dolichopteryx. There are no such fish in the author’s material, and they are not specifically described in the literature. Therefore, at present as an autapomorphic character in pigmentation of Duolentops gen. nov. one can assume only a better development of the infraorbital accumulation of melanophores in species of this genus, more or less extending onto the lateral surface of the snout. However, the phylogenetic relevance of this character, given its interspecies and intraspecific variability, is obviously very low.
In the adult stage the pigmentation of all representatives of the “long-bodied” opisthoproctids (excluding the neotenic genus Duolentops gen. nov.) is similar; of diagnostic significance (apparently only at the species level) can be differences in the pigmentation of the branchial cavity, fins, and, possibly, the median dorsal myosepta and branchial arches.
Pyloric caeca in opisthoproctids are 3–6 in number, short and thick. In most taxa, they are about the same size, arranged in a row, and oriented in the same direction (Fig. 5b). The exception is Duolentops gen. nov., with caeca of different sizes (Fig. 5c), and D. trunovi, in which one of the caeca is directed in the opposite direction (Fig. 5d). Probably these deviations from the general structural plan should be considered as apomorphies of these taxa.
Summarizing the above, it can be noted that, according to the analyzed characters, the most generalized genus is Ioichthys, which is the closest to the putative ancestral form in terms of the structure of the eyes and characters of juvenile pigmentation. However, this genus already demonstrates some features of specialization relative to other genera of “long-bodied” opisthoproctids (loss of vomerine dentition, reduction of procurrent rays of C in number, some lengthening of the body). Bathylychnops apparently is an early isolated independent lineage characterized by maximum lengthening of the body, the presence of dorsal pigment markings in juvenile stages, and the exceptional development of additional eye structures while maintaining the primitive pouchlike structure of the eyeball. The possibility of a sister relationship between Ioichthys and Bathylychnops cannot be ruled out. Another lineage is represented by Dolichopteryx characterized by a relatively small number of vertebrae, specific juvenile pigmentation, which includes areas of bright silvery tissue, telescopic eyes, and a well-developed secondary globe, but lacking corneal sclerotization. Neotenic Duolentops gen. nov. in characters of pigmentation is more similar to the juveniles of Dolichopteryx than to any other genera, but is more primitive in the structure of the eyeball (pouchlike) and secondary globe (weakly developed); the unique characters of this genus are the development of subscleral lenticular structures (Fig. 1c) and the specific structure of the outer row of rakers of the first branchial arch (Fig. 3). Dolichopteroides is close to Dolichopteryx, differing from representatives of the latter genus in greater elongation of the body region, close position of the bases of D, V and A, and a highly elongated P. A more detailed study of the morphology of “long-bodied” opistoproctids, including osteology and myology, currently impossible due to the limitedness and incomplete preservation of the material available to the author perhaps will significantly correct the assumptions made here.
Taxonomic Descriptions
Ioichthys Parin, 2004
Type species—Ioichthys kashkini Parin, 2004.
Diagnosis. Fish with a moderately elongated body, 53–58 vertebrae. The eyes are pouchlike, with a pronounced aphakic space in front of the lens (Fig. 1a); cornea with two bean-shaped evaginations (the posterior one is barely visible in juveniles), without areas of sclerotization. Vomer edentulous. Gill rakers in two rows on the epi- and ceratobranchiale of the first arch; in the outer row they are elongated in juveniles and short in adult fish, not hypertrophied; well developed in the inner row. V are attached in front of the vertical of the beginning of D; antedorsal distance is 70.9–72.5% SL; the beginning of A is located behind the vertical of the end of D. There are 5–6 procurrent rays of C. A fry of SL 66.3 mm has peritoneal pigment markings, but no dorsal ones, with diffuse melanophore pigmentation, starting along the borders of myomeres, but over the entire surface in the posterior half of the body; in adult fish the skin and peritoneum are black. Maximum known SL ~ 253 mm.
Composition and distribution. A single species from the tropical zone of the Indian Ocean was described in the composition of the genus (Parin, 2004). The same genus should include a juvenile from Californian waters, indicated by Moser (1996) as B. brachyrhynchus (Parin et al., 2009), and two adult specimens caught in Peruvian waters and described as Opisthoproctidae gen. et sp. indet. (Shinohara, 2009). The greater number of vertebrae in East Pacific fish compared to specimens of the type series of I. kashkini (56–58 versus 53–55) suggests that they belong to a separate species.
Ioichthys Kashkini Parin, 2004
Ioichthys kashkini: Parin, 2004, p. 438, figured (original description); Parin et al., 2009, p. 841, Fig. 2 (addition to the description of the paratype).
Material. IO RAS no. 2565, paratype SL 66.3 mm, 12°00′ N, 64°58′ E, R/V Vityaz, cruise 40, buoy C, HCD-180, 4075–0 m, sample no. 133, March 24, 1967. The holotype of this species (ZIN no. 53071, adult specimen SL 232 mm) was briefly examined by the author in 2005, during its transfer to the ZIN RAS at the request of N.V. Parin.
Description. The species is described in sufficient detail in the original description, therefore, only a description of the pigmentation of the juvenile of SL 66.3 mm is given here, for which only the presence and number of peritoneal spots were previously indicated (Parin et al., 2009).
The dorsal and dorsolateral sides of the snout are speckled with spaced melanophores of different sizes, which condense in front of the orbit; the surface of the frontalia between and behind the orbits is also covered with numerous but scattered melanophores; meningeal pigmentation is represented by rare melanophores of different sizes. The upper jaw is densely pigmented with brownish melanophores merging into a continuous strip. Under the eye there is an extensive accumulation of rather large scattered melanophores, passing to the lower surface of the head (Figs. 1b, 4a); epidermis under the eye with a pronounced silvery tint; the lateral side of the snout between the posterior edge of the lower jaw and the vertical line of the anterior edge of the orbit apparently without melanophore pigmentation. In the postorbital part of the head melanophore pigmentation is traced, but due to the fact that the skin is mostly torn off, its distribution has not been clarified (there are dotted melanophores along the posterior edge of the orbit). There are rare dotted melanophores on the branchial arches at the base of the gill filaments. The lower surface of the head has dense melanophore pigmentation, most developed in the gular region, very shallow at the symphyseal edge of the lower jaw (Fig. 4a), and strongly sparse on the branchiostegal membrane. The ventral surface of the body from isthmus to the beginning of A has continuous dense melanophore pigmentation; individual melanophores here are mostly large, never merging. In the interval between the bases of P and V there are three pairs of dark peritoneal markings, one more pair is located directly between the bases of V and two pairs are located in the interval between V and A. On the sides of the body there are separate melanophores grouping along the boundaries between the myomeres and along the median myocomma. Towards the middle of the body (before the beginning of V), they become more numerous and pass to the surface of myomeres, forming a median longitudinal stripe, and completely cover its surface on the caudal peduncle (Fig. 4b). There is a vertical stripe of scattered melanophores along the cleithrum. At the bases of P, D, and A accumulations of brownish melanophores are developed; rays of all fins are not pigmented. The dorsal median myosepta between the occiput and D is covered with melanophores that merge along its upper edge into a longitudinal stripe; towards the vertebrae the pigmentation of the myosepta becomes uniformly brownish.
Bathylychnops Cohen, 1958
Type species—Bathylychnops exilis Cohen, 1958.
Diagnosis. Fish with a greatly elongated body; 67–85 vertebrae. The eyes are pouchlike; the anterior corneal evagination forms a large separated secondary globe with a spherical additional lens; in the inferior and posterior parts of the eyeball behind the secondary globe there are two more corneal lenses (not developed in juveniles) (Fig. 1i). Lens eccentric (eye semi-telescopic); there is no aphakic space. Vomer with a single row of teeth. The gill rakers are thin, ciliform, in two rows on the epi- and ceratobranchiale of the first arch, not hypertrophied in the outer row, well developed in the inner row. V are attached in front of the vertical of the beginning of D; the antedorsal distance is 72.4–76.6 (80.2)% SL; the beginning of A is located behind the vertical of the end of D. There are 7–12 procurrent rays of C. Postlarval pigmentation is represented by an unpaired median dorsal row of pigment spots and the paired peritoneal spots along the mid-ventral line; parallel pigment stripes on the sides of the body, consisting of melanophores and specks of silvery tissue, are absent (Cohen, 1960; Badcock, 1988; Parin et al., 2009); adult fish with monotonous dark pigmentation of the skin, peritoneum is black; SL up to 580 mm (Parin et al., 1995).
Composition and distribution. Three species: type one, B. brachyrhynchus (Parr, 1937) and B. chilensis Parin, Belyanina et Evseenko, 2009. B. exilis is known from the transitional waters of the North Pacific Ocean (Stein and Bond, 1985; Fujii, 1985; Parin et al., 1995; Aizawa, 2002), indications of the finding of this species in the Atlantic (Harrisson, 1967; Aizawa, 2002; Parin et al., 2009) refer to B. brachyrhynchus (Badcock, 1988). The latter seems to have a wide-tropical range in all oceans (Badcock, 1988; Parin et al., 2009). B. chilensis is known only from the southeastern Pacific Ocean at 33°–34° S (Parin et al., 2009).
Notes. Parin et al. (2009) give the number of the rays of D and A as differences between B. chilensis and B. exilis (12–13 and 11–12, respectively, versus 14–16 and 13–14). In the specimen of B. exilis examined by the author there are 13 rays of D, 12 rays of A. According to Stein and Bond (1985), the number of the rays of D and A in B. exilis is 13–16, respectively (on average 14, n = 16) and 10–14 (on average 12, n = 15). Thus, there are no grounds for distinguishing species by the number of the rays in vertical fins.
Material. B. exilis, 1 specimen SL 420 mm, 47°09′ N, 153°52′ E, depth 420–460 m, FRV Mlechny Put, trawl no. 61, March 17, 1990. B. brachyrhynchus, 1 specimen, SL 308 mm, Nazca Ridge, FRV Professor Mesyatsev, cruise 13, trawl 94 , November 10, 1983, collected by A.N. Kotlyar; 2 specimens, SL 192 mm (head length 40 mm) and detached head 33 mm long, West Africa, R/V Professor Vodyanitsky, cruise 5, trawl no. 11.
Duolentops Prokofiev, gen. nov.
Type species—Dolichopteryx minuscula Fukui et Kitagawa, 2006.
Diagnosis. Fish with a relatively short body and deep caudal peduncle; 40–46 vertebrae. The eyes are pouchlike; the anterior corneal evagination is bean-shaped, stretched along the anterior edge of the lens and expanded ventrally, with a light (sclerotized?) thickening in the lower part; there is an aphakic space in front of the lens (Fig. 1c); the posterior corneal evagination is not developed. In the lower part of the eyeball under the lens there is a large lenticular formation, macroscopically circumscribed from the membranes of the eye (Fig. 1c). Vomer with several rows of teeth. The gill rakers on the first arch are thickened, finger-shaped, strongly hypertrophied in the outer row; the two upper rakers of the outer row are attached medial to the line connecting the bases of the subsequent rakers on ceratobranchiale-1, and are inclined downward parallel to the longitudinal axis of ceratobranchiale-1, lying behind the outer row of ceratobranchial rakers (Fig. 3). The rakers of the inner row are well developed. V are attached noticeably in front of the vertical of the beginning of D; the antedorsal distance is 72.3–83.2% SL; the beginning of A is located behind the vertical of the end of D, less often on the same vertical. The length of the caudal peduncle is equal to its depth. There are 9–11 procurrent rays of C. Caudal peduncle with diffuse melanophore pigmentation and reflective areas of silvery tissue; dotted rows of melanophores extend forward above and below the myocomma; the area of melanophore pigmentation under the eye continues forward (to a greater or lesser extent) along the lateral sides of the snout; peritoneal markings are present or absent. Dwarf neotenic forms, maximum known SL 66.2 mm.
Etymology. The genus name is derived from the Latin words “duo” (double), “lens” (lentil, in modern morphological terminology—lens of the eye) and “ops” (eye) and reflects a characteristic feature of the genus: the presence of a lenticular subscleral thickening under the lens of the eye.
Composition and distribution. In addition to the type species from the Indo-West Pacific, the Atlantic species Dolichopteryx rostrata Fukui et Kitagawa, 2006 should be attributed to the new genus. The author did not have the opportunity to directly study the specimens of this species, but such characters of it noted in the text of the original description and/or shown in the original picture (Fukui and Kitagawa, 2006b, Fig. 1) as pouchlike eyes with a lenticular structure under the lens, the dwarf size of the holotype with mature eggs (SL 66.2 mm), and the characteristic pigmentation of the caudal portion of the body allow this species to be attributed to the genus described.
Duolentops minuscula (Fukui et Kitagawa, 2006)
Dolichopteryx minuscula: Fukui, Kitagawa, 2006a, p. 114, Figs. 1–3 (original description).
Dolichopteryx sp. cf. longipes: Prokofiev, 2014, p. 379.
Material. 1 specimen SL 30 mm, 23°34′ N, 128°35′ E, R/V Vityaz, cruise 57, st. 7175, IKMT no. 11, sample 21, fishing horizon 500 m, 1600 m wire out, fishing time 22.00–23.05, February 8, 1975.
Description. D 10, A 8, P 12, V 9, C x + 10 + 9 + x; r. br 2, sp. br 0 + 14 (1 + 1 + 0) (two upper rakers are displaced medially and form an additional row inwards from the outer row of the ceratobranchial rakers (Fig. 3)), rakers in the inner row on the first arch 4 + 1 + 11 = 26; myomeres about 45; p. c 3, of which the middle caecum is much smaller than the lateral ones (Fig. 5c). The ends of P and V are broken off. There are 9 myomeres between the verticals of the beginnings of V and D. The beginning of A is located behind the end of D. The adipose fin is present, located above the end of А.
Some measurements, in % SL: head length 27.1, maximum and minimum body depth 11.9 and 10.2, respectively, caudal peduncle length 10.2; antedorsal, anteventral and anteanal distances 74.6, 59.3 and 84.75, respectively; snout length 11.2, horizontal eyeball diameter 3.7, length of lenticular formation under the lens 2.5, horizontal bony orbit length 5.9, lower jaw length 5.9.
Pigmentation. Previously the specimen was stained with alizarin red S, due to which superficial pigmentation is currently poorly visible, but traces of longitudinal dark stripes remain on the dorsal and lateral surfaces of the snout. The dorsal surface of the snout tip with isolated subdermal melanophores. Directly under the eye there is a longitudinal row of rather large subdermal melanophores, adjacent under the superficial pigmentation, below which there is a row of the same melanophores extending anteriorly to the quadrate-mandibular articulation; another short row of subdermal melanophores is located in front of the rays of the branchiostegal membrane. The epidermis under the orbit has an intense silvery-iridescent tint. Meningeal pigmentation is represented by scattered rather large melanophores. The branchial arches are not pigmented. At the bases of paired and vertical fins there are isolated dotted melanophores. The caudal peduncle bears a diffuse superficial brownish pigmentation and separate scattered dotted melanophores; epidermis on the sides of the body in the region of the bases of the vertical fins and on the caudal peduncle with a pronounced silvery tint (Fig. 5a); further forward below the myocomma and to the base of P a longitudinal silvery strip bordered by brownish melanophores runs. Ventral surface has scattered dotted melanophores, with four stripe-like peritoneal markings in the interval between the bases of P and V and with a fifth marking located on both sides of the anus. Median dorsal myosepta between occiput and D and rays of all fins is not pigmented.
Notes. Formerly the above described specimen was mentioned by the autor (Prokofiev, 2014) as a form of the complex “D. longipes ”on the basis of similar values of meristic characters and the supposed absence of an adipose fin in this specimen, although non-telescopic eyes and an unusual formula of gill rakers in the outer row on the first arch were noted, excluding the identification of this specimen with D. longipes. However, actually this specimen has an adipose fin, but it is crumpled and dried to the upper edge of the caudal peduncle, which is why it is difficult to detect (Fig. 5a). By all the characteristics available for comparison, the described specimen fully corresponds to the original description of D. minuscula, with the exception of the structure of the first branchial arch. In the original description (Fukui and Kitagawa, 2006a), 5–6 + 1 + 10–11 rakers were indicated for this species in the outer row on the first arch, and no peculiarity in their structure was mentioned. Since other characters characterizing D. minuscula are also quite peculiar, and an existence of another species differing in the structure of the first branchial arch only in the same area seems frivolous, the author believes that most likely Fukui and Kitagawa (2006a, 2006b) mistakenly indicated the the outer rakers as the ones of the inner row, the number of which in the specimen the author studied corresponds to that given by the cited authors.
The described specimen is the fourth finding of this rare species, previously known only from three specimens of the type series (Fukui and Kitagawa, 2006a; Parin et al., 2009) caught in the Pacific waters of Japan between 29° and 39° N and 143°–150° E and in the Indian Ocean south of the island of Mauritius. The fishing area of the author’s specimen, originating from the waters between Ryukyu Islands and the Philippines, lies within the proposed range of the species.
Dolichopteroides Parin, Belyanina et Evseenko, 2009
Type species—Dolichopteryx binocularis Beebe, 1932.
Diagnosis. Fish with a noticeably elongated body; 58–60 vertebrae. The eyes are cylindrical, telescopic; the presence of an additional lens is indicated (Parin et al., 2009), other corneal sclerotizations apparently are absent. Vomer with multiserial teeth. Gill rakers are short, flattened, linguliform at all age stages; in two rows on the epi- and ceratobranchiale of the first arch, not hypertrophied in the outer row, strongly reduced in the inner row. Rays of P are strongly elongated, extending beyond the end of the base of A. D, V and A are strongly displaced caudally, the antedorsal distance is 78.2–82.0% SL; V are attached near or behind the vertical of the origin of D; beginning of A located under middle—posterior third of the base of D. There are 11–13 procurrent rays of C. Postlarval pigmentation is represented by two pigment stripes extending forward from the base of C above and below the myocomma, of which the dorsal stripe is very short, the ventral one is long, there are peritoneal markings; adult fish apparently are of a monochromatic dark color, the peritoneum is black. Maximum known SL 266 mm (Stewart, 2015).
Composition and distribution. Monotypic genus with a circumglobal wide-tropic range; Parin et al. (2009) do not exclude the composite nature of the only known species.
Material. IO RAS uncat.: 1 specimen SL 233 mm, Walvis Ridge, 36°21′ N, 07°38′ E, depth 1150–1200 m, FRV Evrika, trawl no. 17, October 14, 1975, collected by I.А. Trunov; 1 specimen SL 210 mm, 01°12′ N, 56°28′ E, R/V Rift, st. 27, bottom trawl, site depth 800–810 m, fishing horizon—bottom, fishing time 07.25– 07.57, sample no. 40, March 6, 1983; ZMMSU no. 22700, 1 specimen SL 55 mm (Central East Atlantic, 00°35′ N 07°02′ E, depth 340–350 m, FRV Fiolent, cruise FAO-1, trawl no. 11, January 5, 1976.
Dolichopteryx Brauer, 1901
Type species—Dolichopteryx anascopa Brauer, 1901.
Diagnosis. Fish with a moderately elongated body; 41–48 vertebrae. The eyes are cylindrical, telescopic; the anterior corneal evagination forms a well-separated secondary globe without an additional lens, the posterior evagination is located under the anterior one; there are no additional corneal lenses (Figs. 1d–1g). Vomer with multiserial teeth. Gill rakers are flattened, elongated and more spaced in juveniles, short, linguliform in adult fish, in two rows on the epi- and ceratobranchiale of the first arch, not hypertrophied in the outer row (Figs. 2c–2f). V are attached in front of the vertical line of the beginning of D (by 3–9 myomeres); antedorsal distance is 70.6–82.1% SL (usually 72–77%); the beginning of A is located below or behind the end of D. There are 9–14 procurrent rays of C. Postlarval pigmentation is represented by two pigment stripes extending anteriorly from the base of C above and below the myocomma (Figs. 4e–4g); peritoneal markings are present or absent, there may be areas of pigmentation on the stomach and/or intestines; dorsal pigment markings are absent. Adult fish with dark-pigmented scale pockets, peritoneum is black; the median dorsal myosept between the occiput and D may also be pigmented. Maximum known SL 217 mm (D. parini: Mecklenburg et al., 2002).
Composition and distribution. The genus contains eight described species, one of which is known from the Atlantic Ocean (D. longipes (Vaillant, 1888)), two from the Indo-West Pacific (D. anascopa Brauer, 1901 and D. vityazi Parin, Belyanina et Evseenko, 2009) and four from the Pacific Ocean only (D. andriashevi Parin, Belyanina et Evseenko, 2009, D. nigripes Prokofiev, sp.nov., D. parini Kobyliansky et Fedorov, 2001; D. pseudolongipes Fukui, Kitagawa et Parin, 2008); D. trunovi Parin, 2005 apparently have a circumglobal range in the moderately high latitudes of the Southern Hemisphere (between 41° and 53° S) (Parin, 2005); at least one other undescribed species is known from the California Current in the East Pacific (Moser, 1996; Parin et al., 2009). All species are known from few finds, and therefore the boundaries of their ranges cannot be established. Most species (except D. parini and D. trunovi) are associated with tropical and subtropical waters; the range of D. parini apparently is confined to the transition zone of the North Pacific waters.
Notes. D. anascopa and D. parini noticeably differ from other species of the genus in strongly elongated P, extending beyond the beginning of D, in the more anterior position of A (under the middle of the base of D, while under its end or more often behind it in the other species) and the presence of a milky-white tissue at the lower edge of the eye. More detailed morphological studies are needed to clarify the relationship of these species and the degree of their taxonomic isolation from other representatives of the genus.
Dolichopteryx andriashevi Parin, Belyanina et Evseenko, 2009
(Fig. 6a)
Dolichopteryx andriashevi: Parin et al., 2009, p. 843, Fig. 5 (original description).
Dolichopteryx cf. longipes № 2: Parin et al., 2009, p. 845, Fig. 6.
Material. In addition to the five original specimens listed in the work of Parin et al. (2009. P. 843, 845), 1 specimen was studied, SL 37 mm, 01°07′ N, 187°00′ E, R/V Vityaz, cruise 26, st. 3798, site depth 5620–5600 m, HCD-160, 1000–0 m (angle 65°, 2100 m wire out), fishing time 08.50–11.10, sample no. 86, November 30, 1957.
In the original description Parin et al. (2009. P. 843) formally designated only the holotype (ZMMSU no. 22214 SL 56.5 mm, st. 4490). The status of three other fish from the collections of the R/V Vityaz (st. 3721, SL 52.4 mm; st. 4490, SL 41.4 mm; st. 5040, SL 68.5 mm) was not directly indicated in the original description. The diagnosis of the new species was made for all 4 specimens, so all of them should be included in the type series, which was confirmed orally by one of the authors of the species (S.A. Evseenko). The specimen SL 39.5 mm from st. 3835 described by Parin et al. (2009. P. 845) as D. cf. longipes no. 2, is not type.
Description. D 10–12, A 8–10, P 12–13, V 9, C x–xii + 10 + 9 + x–xii; r. br 2, sp. br 5–10 + 14–18 = 20–25; vertebrae (myomeres) 45–47; p. c 5–6 (arranged in a row). The eyes are telescopic, directed vertically upward (Fig. 6b). P is clearly shorter than V; in juvenile SL ~ 50 mm the tips of the rays of P only barely extend beyond the first pair of peritoneal markings in the P–V interval; the tips of the rays of V reach or almost reach the base of C. There are three or four myomeres between the verticals of the beginnings of V and D. The beginning of A is located under the end of D. The adipose fin is absent.
Some measurements, in % SL: head length 24.8–29.6, maximum and minimum depth of body 7.6–10.3 and 6.1–7.1, respectively, caudal peduncle length (9.5) 12.0–13.1; antedorsal, anteventral, and anteanal distances 73.6–79.4, 66.3–69.5, and 78.1–84.5, respectively; snout length 10.6–14.5, horizontal diameter of the eyeball 3.3–3.9, horizontal length of the bony orbit 6.3–7.6 (9.5), length of the lower jaw 5.1–6.7 (7.7).
Pigmentation. A wide longitudinal stripe consisting of small brownish melanophores is extended along the upper edge of the snout from the top to the orbits, slightly not reaching the latter. On each side at the symphyseal end of the lower jaw there is a stripe-like cluster of melanophores. There are a longitudinal row (one melanophore wide) of melanophores from the quadrate-mandibular joint to a narrow patchy cluster of melanophores immediately under the eye and a short stripe of larger melanophores in front of the branches of the branchiostegal membrane. The epidermis under the orbit has an intense silvery-iridescent tint (Fig. 4c). Meningeal pigmentation is poorly expressed. The branchial arches are pigmented with intense black melanophores (Fig. 4i). The base of P is densely speckled with brownish melanophores. Spotted accumulations of melanophores are found at the base of V. The bases of D and A have small, scattered melanophores. From the basicaudal accumulation of melanophores and patches of silvery tissue the pigment stripes extend forward above and below the myocomma. The ventral pigment stripe consisting of dash-shaped specks of silvery tissue outlined by melanophores, larger on the caudal peduncle, which merge anteriorly of V into a solid silvery strip with underlying melanophores arranged in a row extends anteriorly to the base of P. The dorsal lateral pigment stripe consists of dotted melanophores only; in some specimens it is very short, consists of only a few melanophores that do not extend anterior to the procurrent rays of C (Fig. 4e). For specimens from st. 3721 and 3798, the dorsal stripe is much better expressed and reaches anteriorly to the vertical of the beginning of V (Fig. 4f). There are three well-defined transversely elongated paired peritoneal markings in the interval between the bases of P and V and one more less sharp, rounded paired peritoneal marking between the bases of P (Fig. 6a); the abdominal surface bears large, sparsely located melanophores, more numerous anterior to the third peritoneal marking. The intestine in front of the anus is enclosed by two whitish folds of the peritoneum, bearing dense melanophore pigmentation (Figs. 4j–4m); in the holotype, there is also an area of milky-white tissue of an irregular L-like shape in front of the bases of V. The dorsal median myosepta between the occiput and D and the rays of all fins are not pigmented.
Notes. D. cf. longipes no. 2, described by Parin et al. (2009) in the open nomenclature, which supposedly differs from the Atlantic D. longipes by a greater number of gill rakers and from D. andriashevi by a smaller number of them (20 versus about 18 and 23–25, respectively) should be included in D. andriashevi. The difference in the number of gill rakers calculated by Parin et al. for this specimen (at present, both of the first branchial arches have been lost) and for the type series D. andriashevi is well within the limits of variability established in closely related species (D. longipes, D . vityazi). A specimen from st. 3835 has all the characteristic features of D. andriashevi (four peritoneal markings in the P–V interval; there is melanophore pigmentation on the branchial arches; the beginning of A is located under the end of D) and can undoubtedly be identified with this species.
Dolichopteryx longipes (Vaillant, 1888)
(Fig. 6c)
Material. MNHN no. 1887–0136, holotype SL 46 mm, Morocco, 29°01′59'″ N, 12°28′59″ E, 1163 m, R/V Talisman, st. 47, 1883.
Description. D ~ 9, A ?, P ~ 13–14, V ~ 9; myomeres ~ 48; r. br 2, sp. br 6 + 16 = 22; p. c. 6. The eyes are telescopic, directed vertically upward. There are three myomeres between the verticals of the beginnings of V and D. The beginning of A is located behind the end of D, the interval between the verticals of the end and the beginning of A is commensurate with the interval between the verticals of the beginnings of V and D. The adipose fin is absent.
Some measurements, in % SL: head length 30.4, snout length 15.2, maximum and minimum depth of body 9.8 and 6.0, respectively; antedorsal, anteanal and anteventral distances 71.7, 80.4, and 63.0, respectively.
Pigmentation is strongly faded, but at the base of C the transverse basicaudal accumulation of melanophores is quite distinguishable, from which the longitudinal pigment stripes parallel to each other extend forward (above and below the myocomma). The dorsal pigment stripe is much weaker than the ventral one, disappears under the base of D; the ventral one stretches forward almost to the beginning of the body. There are three large transverse peritoneal markings on the abdomen in the interval between the bases of P and V, the first of which is located at the level of the pyloric caeca, there is no marking between the bases of P. The rays of all fins and the remnants of the membrane between them are not pigmented. No pigmentation on the head and branchial arches.
Notes. The author agrees with the opinion (Parin et al., 2009) that the range of D. longipes should be limited to the Atlantic Ocean; however, from the differences between Atlantic and Indo-Pacific fishes in the number of the rays of D and sp. br (in both cases lower in Atlantic fishes) and the relative position of D and A given by these authors, only the latter is real. According to Cohen (1964), Atlantic D. longipes has 10–11 (on average 11, n = 6) rays; thus, their numbers in D. longipes and D. andriashevi overlap (9–11 and 10–12, respectively), and the differences in mean values (which could be assumed based on the material studied by Parin et al. and the author) are doubtful. The number of gill rakers on the first arch in the holotype of D. longipes (22) is significantly greater than that given for this species by Parin et al. (18); thus, this character also overlaps widely in the compared species (18–22 versus 20–25 in D. andriashevi). Nevertheless, D. longipes differs well from D. andriashevi in the lower number of peritoneal markings in the P–V interval (three versus four, there is no marking between the bases of P), the absence of melanophore pigmentation of the branchial arches, and a more posterior position of A (noticeably behind the end of D, and not under its end, like in D. andriashevi).
Dolichopteryx nigripes Prokofiev, sp. nov.
(Figs. 6d–6f)
Dolichopteryx longipes (non Vaillant): Wagner et al., 2009, pp. 109, 113, Fig. 1 (24°00′ S 175°30′ W, 600–800 m).
Material. MNHN no. 2000-0446, holotype SL 120 mm, New Caledonia, 25°40′59″ S, 167°10′59″ E, 1030–1320 m, November 15, 1996.
Diagnosis. A species of the genus Dolichopteryx without an adipose fin, with three peritoneal markings between the bases of P and V, with the beginning of A located in front of the end of D, with black pigmentation of V.
Description. D 11, A 8, P 12, V 10, C xiv + 10 + 9 + x; ~ 45 myomeres, vertebrae (according to the radiograph) 45 (Fig. 6e); r. br 2, sp. br ~ 7 + ~ 16(18) = ~ 23(25) (branchial arches of the holotype are severely damaged by the collection number attachment thread passed through them, due to which several of the uppermost and lowermost rakers could be underestimated). The eyes are telescopic, directed upwards. The wall of the abdominal cavity has been torn out, due to which the position of the beginning of V in the holotype cannot be precisely determined, but, apparently, they were attached slightly in front of the vertical of the beginning of D; the internal organs are mostly lost. In the specimen depicted by Wager et al. (2009, Fig. 1a), the base of V is three myomeres in front of the beginning of D. The beginning of A is located under the posterior third of the base of D. The adipose fin is absent. Judging by a specimen of Wagner et al. (2009, Fig. 1a), P is shorter than V, does not go beyond the second pair of peritoneal markings; the tips of V extend slightly beyond the base of С.
Some measurements, in % SL: head length 29.2, maximum and minimum depth of body 10.0 and 7.1, respectively, caudal peduncle length 11.7, antedorsal and anteanal distances 77.5 and 81.2, respectively; snout length 12.5, horizontal diameter of the eyeball 3.9, horizontal length of the bony orbit 6.7, length of the lower jaw 8.2.
Pigmentation. The snout tip, jaws and lower surface of the head are not pigmented; there are several rare small melanophores under the eye, the epidermis here has a pronounced silvery tint; pigmentation of the meningeal membranes is diffuse, brownish, with separate larger punctate melanophores. At the base of C, there is a wide transverse basicaudal marking, in the upper part forming only a short projection anteriorly, and in the lower part extending in the form of a stripe of dotted melanophores and specks of silvery tissue anteriorly, at least to the level of the beginning of V (Fig. 6f). The ventral wall of the abdominal cavity is preserved in the interval between the bases of P and V in the form of a tissue strip; the remains of three peritoneal markings are visible on it (Fig. 6f). The specimen depicted by Wager et al. (2009, Fig. 1a) has three peritoneal markings, of which the first one is 1.4 times closer to the second one than to the base of P, the second and third ones are located at an equal distance from each other and respectively from the first marking and from the beginning of the base of V; there is no marking between the bases of P. In the interval between the beginnings of V and A there are apparently no peritoneal markings. Membrane between rays of V from the third to the seventh is intense black (except for nearly the base); at the base of the rays of V there is a dense accumulation of dark melanophore specks (Fig. 6f). The rest of the fins are not colored (at a magnification a poorly visible melanophore speck is traced in the middle rays of P).
Etymology. The specific epithet is derived from the Latin words “niger” (black) and “pes” (leg) and reflects the characteristic feature of the species (black pigmentation of V); indeclinable noun.
Comparison. The new species is undoubtedly close to D. longipes and D. andriashevi, but it differs well from both species in the black coloration of V. In addition, it differs well from the Atlantic D. longipes by the more anterior position of the beginning of A (slightly in front of the vertical of the end of D versus noticeably behind it) and possibly by a slightly smaller number of vertebrae (myomeres) (45 versus 46–48), and from D. andriashevi by three (versus four) peritoneal markings in the P–V interval, the absence of melanophore pigmentation of the branchial arches and possibly by very weak development of melanophore pigmentation on the snout and under the eye. The new species can be easily distinguished from all other species of the genus by the absence of an adipose fin and a combination of other characters (short and light P, black V, sp. br about 23–25, there are peritoneal markings at SL up to 120 mm).
Dolichopteryx vityazi Parin, Belyanina et Evseenko, 2009
(Fig. 6g)
Material. ZMMSU no. 22215, holotype SL 61 mm, 07°35′′ N, 162°0′ E, 1000–0 m. Non-type material (described for the first time): 4 specimens SL 31–57.5 mm, Arabian Sea, 09°06′ N, 64°00′ E, R/V Petr Lebedev, cruise 7, sample 7–25, Isaacs–Kidd trawl, fishing horizon 232–252 m, fishing time 12.36–13.36, April 9, 1969; 1 specimen SL 50 mm, 09°18′ N, 63°37′ E, R/V Petr Lebedev, cruise 7, sample 7–33, Isaacs–Kidd trawl, fishing horizon 280–330 m, fishing time 11.55–12.55, April 10, 1969.
Description. D 11–12, A 9–11, P 13–15, V 9–10, C ix–x + 10 + 9 + ix–x; r. br 2, sp. br 5–10 + 17–20 = 23–28; vertebrae (myomeres) 44–48; p. c 6. The eyes are telescopic, directed upward and slightly forward (Fig. 6h). P apparently is much shorter than V, the ends of the rays of P slightly extend beyond the first pair of peritoneal markings in the P–V interval; the ends of the rays of V are broken off in all the fish studied, reaching at least the end of the base of A. There are three myomeres between the verticals of the beginnings of V and D. The beginning of A is located below or behind the end of D. The adipose fin is located behind the vertical of the end of А.
Some measurements, in % SL: head length 30.6–32.3, maximum and minimum body depth 8.7–9.9 and 5.8–7.4, respectively, length of caudal peduncle 13.9–16.1; antedorsal, anteventral, and anteanal distances 72.6–76.5, 69.4–72.6, and 79.7–82.7, respectively; snout length 12.9–14.5, horizontal diameter of the eyeball 3.2–4.3, horizontal length of the bony orbit 8.1–8.8, length of the lower jaw 7.8–8.7.
Pigmentation. The dorsal surface of the snout has an oval marking of small brownish melanophores that do not extend posteriorly beyond the vertical of the mandibular joint. In the area of the upper jaw and along the oral edge of the lower jaw there is a stripe of completely or partially merging melanophores. A small patchy accumulation of large subdermal melanophores is located under the eye; the epidermis above them has an intense silvery-iridescent tint. In larger fish there is only a row of several very small melanophores near the quadrate-mandibular joint; the lower edge of the posterior process of the quadratum and symplecticum is outlined by a narrow dashed stripe of dark pigment, but a short row of larger melanophores in front of the rays of the branchiostegal membrane is well developed. The smallest of the studied specimens has a short stripe of small dotted melanophores in front of the mandibular joint, further backward extending along the lower edge of the suspensorium as a row of at first very small melanophores that gradually increase in the caudal direction, connecting with a row of melanophores in front of the branchiostegal rays. The meningeal pigmentation in larger fish is continuous; when viewed from above it forms a dark outline around the brain, but in the smallest specimen the melanophores are not partially fused here. The branchial arches are not pigmented. At the base of P there is only a small brownish speck of superficial pigmentation; at the base of V there is a well-developed spot-like accumulation of melanophores of different sizes. The bases of D and A have small scattered melanophores and diffuse brownish pigmentation. From the basicaudal accumulation of melanophores and areas of silvery tissue above and below the myocomma the pigment stripes consisting of specks of silvery tissue, outlined with brownish melanophores, extend forward to the gill opening. The dorsal and ventral pigment stripes are of the same length, but the specks in the ventral stripe (in the caudal half) are noticeably larger than in the dorsal stripe, strongly elongated longitudinally, and the silvery tissue in them is much better expressed than in small rounded specks of the dorsal stripe (Figs. 4g, 6i). Anterior to the third peritoneal marking the specks of the ventral stripe merge into a solid line, in the dorsal stripe they are isolated up to its anterior end (at supracleithrum). There are three well-defined peritoneal markings in the space between the bases of P and V; there is no marking between the bases of P in larger fish. In the smallest of the studied specimens, there are scattered rather large punctate melanophores between the bases of P, which merge in the upper lateral direction into small markings on both sides. The area of the intestine in front of the anus is covered by sheets of milky-white tissue with a striated structure and very fine melanophore pigmentation (but in the smallest specimen it is noticeably more dense) and a small black pigmented marking near the dorso-posterior margin (Figs. 4j–4m). Small melanophores are present at the bases of the rays of V; rays of other fins are completely unpigmented (Fig. 6i). The dorsal median myosepta between occiput and D is not pigmented.
Notes. The species was previously known only from a holotype caught in the Pacific Ocean east of the Caroline Islands. The new material comes from the Arabian Sea, which implies that this species is widespread in the tropical Indo-West Pacific. Although the Indian Ocean material shows noticeable variability in a number of morphological characters, it is quite consistent with the diagnosis of this species (Parin et al., 2009). It should be noted that the difference in the orientation of the eyes in the studied material was constant for D. vityazi and D. andriashevi. In the first species they are oriented upward, but moderately inclined forward (Fig. 2h), while in the second one they are directed strictly upward (Fig. 2b). It is known that the eyes of opisthoproctids can turn 90° from the “up” position to the “forward” position (Robison and Reisenbichler, 2008). However, in preserved specimens they always occupy a strictly defined position, which indicates the possible existence of a “default” position, to which the eyes are brought, including after the death of the fish. It is possible that the differences in the “default” position have some taxonomic significance: out of the 6 specimens of D. andriashevi and 6 specimens of D. vityazi the author examined, only in 1 specimen of D. vityazi SL 51 mm, differences were observed in the degree of inclination of the right and left eyes, while in all other fish their orientation was exactly the same (as shown in Figs. 2b and 2h).
Dolichopteryx pseudolongipes Fukui, Kitagawa et Parin, 2008
(Fig. 7)
Material. MNHN no. 1970-0038, 1 specimen SL 100 mm, 00°07′ N, 152°40′ E, 510 m, R/V Koriolis, March 1, 1969, IO RAS uncat., 2 specimens SL 85 and 111.5 mm, 00°17′ N, 89°29′ W (near the Galapagos Islands), 500−0 m, R/V Akademik Kurchatov, cruise 4, st. 313. The specimens from the collection of the IO RAS have now completely dried up (for a short description see: Parin et al., 2009, p. 845). The description is based on the specimen MNHN no. 1970-0038.
Description. D ~ 10, A 8, P 15, V 11, C xii + 10 + 9 + x; myomeres ~ 42–44, vertebrae (according to the radiograph) 43 (Fig. 7c); r. br 2, sp. br 10 + 25 = 35. Eyes telescopic, directed upwards. There are three vertebrae between the bases of V and D. The beginning of A is located under the posterior third of D. Along the dorsal edge of the body, from the occiput to the beginning of D and between the end of D and the base of C, a narrow unpigmented skinfold extends (in some places damaged in the examined specimen). The adipose fin (damaged) is located in the thickness of this fold, approximately 1.5 and 2.0 times closer to the first additional ray of C than to the end of A and D, respectively (Fig. 7d).
Some measurements, in % SL: head length 34.0, maximum and minimum body depth 9.0 and 5.5, respectively, caudal peduncle length 15.0; antedorsal, anteventral, and anteanal distances 71.0, 68.0, and 78.0, respectively; snout length 15.5, horizontal length of the bony orbit and eyeball 8.3 and 7.0, respectively, lower jaw length 6.1.
Pigmentation. The upper surface of the snout with scattered brownish melanophore pigmentation; the upper jaw is blackish. Under the eye there is a highly diffuse accumulation of rare dotted melanophores. A narrow stripe of melanophores extends from the quadrate-mandibular articulation along the lower edge of the suspensorium. There is a bright dark mark in front of the base of the branchiostegal rays. The meningeal membranes are dark pigmented. A scattered accumulation of punctate melanophores is present at the base of P. The rays of the fins are not pigmented, with the exception of V, at the bases of the rays of which a dense melanophore speck is developed, passing to the skin near the bases of the rays. Distal to it the rays and the interradial membrane of V are not pigmented. The base of D with dense accumulation of rather large melanophores; the base of A with rare dotted melanophores. Two longitudinal pigment stripes consisting of punctate melanophores extend from the base of C forward to the level of the D–V interval (Fig. 7d). In the posterior half of the caudal peduncle, they are very wide, reaching their maximum width near the base of C, where they almost merge with each other; anterior to the vertical of the base of the adipose fin melanophores become much more sparse and few in number; under the base of D they form only one longitudinal row. The dorsal and ventral pigment stripes are equally developed, do not contain markings of bright silvery tissue. The peritoneum is intensely black, seen through the ventral wall of the abdominal cavity; the area covering the rectal end of the intestine is milky-white with separate dotted melanophores (Fig. 7b); there are no peritoneal markings and melanophore pigmentation on the ventral surface of the abdominal wall.
The development and distribution of melanophore pigmentation in dried fish from the collections of the R/V Akademik Kurchatov corresponds to that described above for the New Caledonian specimen.
Notes. This species was previously known only from the waters of the East Pacific (from California and the Galapagos Islands) (Grey, 1952; Fitch and Lavenberg, 1968; Fukui et al., 2008; Parin et al., 2009). Its presence in the waters of New Caledonia somewhat unexpectedly and significantly changes the idea of the species’ range. However, although pronounced endemism is characteristic of the East Pacific mesobathypelagic ichthyofauna in general, there are cases when species considered to be East Pacific endemics were subsequently found in the West Pacific waters (Prokofiev and Pietsch, 2019). No significant differences were found between the New Caledonian and East Pacific specimens. The New Caledonian specimen has a slightly larger number of gill rakers (35 versus 31–33); however, the difference lies within the limits of intraspecific variability in other species of the genus. At the same time, it should be noted that there are differences between the specimens of the type series from the Californian waters and the fishes from New Caledonia and the Galapagos Islands in pigmentation. Fukui et al. (2008. P. 269) describe V as “blackish, except proximally at approximately one-fourth length of longest ray”. However, judging by the photograph of the holotype (Fukui et al., 2008. Fig. 2a) it has dark pigmentation at the base of V approximately on 1/6 of its length, then the proximal half of V is light, the distal half of V is black (unclear, entirely or only along the outer ray or several rays). This differs from the situation observed in the New Caledonian specimen and in fish from the Galapagos Islands, which have an accumulation of isolated point melanophores at the base of the rays of V, but otherwise these fins are entirely light. Grey (1952), who also described a specimen from the Galapagoses, also indicates chromatophore specks only in the proximal part of the rays of V. There may be morphological differences between fish from the California Transitional Region and from the equatorial waters of the Pacific Ocean, but it is not clear how they are significant. The pigment stripes, the characteristic shape of which distinguishes D. pseudolongipes from all other species of the genus (not clarified for D. parini, the juveniles of which are not known), are equally developed in the New Caledonian and Galapagos fishes that the author studied and, judging by the original description, in fishes of the type series.
Dolichopteryx parini Kobyliansky et Fedorov, 2001
Material. IO RAS uncat., 1 specimen SL ~ 170 mm, ocean side of the Kuriles, 47°09′ N, 153°52′ E, depth 420–460 m, FR/V Mlechny Put, trawl no. 61, fishing time 06.00–07.00, March 17, 1990.
Description. D 10, A 10, P 14, V 11, C x + 10 + 9 + ix; r. br 2, sp. br 8 + 23 = 31 (Fig. 2e); vertebrae (myomeres) 46; p. c 5, arranged in a row, with their apices directed backward (Fig. 5b). There are 11 + 13 rakers in the inner row on the first branchial arch (Fig. 2f); there is a slit behind the fourth branchial arch. The pseudobranch is well developed and consists of 18 elements. The eyes are telescopic, directed vertically upward. The tips of P reach the end of the base of A, the tips of V extend beyond the base of C. Eight myomeres between the verticals of the beginnings of V and D. The beginning of A is located under the middle of the base of D, the adipose fin is attached just behind the end of the base of А.
Some measurements, in % SL: head length 30.1, maximum and minimum depth of body 12.1 and 6.6, respectively, caudal peduncle length 13.3; antedorsal, anteventral and anteanal distances 76.5, 63.3 and 81.3, respectively; preadipose length 89.2, snout length 12.65, horizontal length of the bony orbit and eyeball, respectively 9.0 and 6.0, lower jaw length ~ 6.0.
Pigmentation. The dorsal and lateral surfaces of the snout with diffuse brownish pigmentation in the form of wide longitudinal stripes; the accumulations of melanophores under the eye and on the ventral side of the head, characteristic of juvenile stages, are absent. Traces of black scale pockets are preserved on the abdomen and in places on the sides of the body; besides them, the skin is light; the median dorsal myosepta is entirely black. Scraps of skin in the postorbital part of the head with diffuse melanophore pigmentation. The rays of D are dark, of A and C are pale; the rays of paired fins are dark to black (their color has probably faded somewhat). The adipose fin is brownish in the basal half, pale distally. The oral cavity is pale, the underside of the gill cover has dark melanophore pigmentation. Dotted melanophores are found on the gill rakers and on the anterior surface of the arches near the bases of the rakers. The peritoneum is black, on the outer surface with an iridescent tint seen through the abdominal wall.
Notes. The studied specimen was caught within the known range of the species and generally is in good agreement with the original description, with the exception of a noticeably larger number of sp. br (31 versus 26–28). However, it cannot be ruled out that several of the lowest rakers were not taken into account by Kobylianskii and Fedorov (2001), since their accurate calculation requires cutting the branchial membrane and the abductor hyohyoideum muscle, the performance of which is not mentioned in the cited work. In a specimen from the waters of Japan (Mizusawa and Fukui, 2009) sp. br 29 (7 + 22). In any case, their number in the studied specimen levels the hiatus for this character between D. parini and D. pseudolongipes. Considering that the first species is known only from mature fish, and the second one is known only from juveniles, it is advisable to discuss the question of the possible conspecificity of these species. D. parini is characterized by strongly elongated and dark pigmented paired fins, while in D. pseudolongipes at least P are entirely light. In all the specimens of the latter species studied by the author, the ends of P and V are broken off. The length of P (on the right side) in the holotype is 10.3%, and the length of V is 22% SL (Fukui et al., 2008); the lengths of P and V in the specimen studied by Grey (1952) are 15.1 and 18.3% SL, respectively. This is significantly less than that observed in D. parini (38.0–46.4 and 37.5–42.3% SL, respectively). Since there is another long-finned species of the genus (D. anascopa), known only from juveniles, there is a reason to believe that the length of paired fins (primarily P, since V are lengthened in juveniles of other species as well (Roule and Angel, 1930; Cohen, 1964)) in Dolichopteryx is not associated with growth. Finally, V in D. parini occupy a more anterior position than in D. pseudolongipes (eight vertebrae in front of the beginning of D versus only three ones in the compared species). With respect to the above, the author consider both species as valid.
Dolichopteryx trunovi Parin, 2005
Material. ZIN no. 36600, holotype SL ~ 80 mm, 53°01′ S, 109°30′ W, R/V Ob, fishing gear: RDT (4200 m wire out), April 28, 1958, collected by A.P. Andriyashev, Yu. Permitin.
Description. D 11, A 10, P 14, V 9, C x + 10 + 9 + ix; r. br 2, sp. br 4 + 11 = 15 (short and wide, flattened, almost triangular: Fig. 8a), rakers in the inner row on the first arch 3 + 7 (the same length as in the inner one, but somewhat narrower: Fig. 8b), on the second arch in the outer and the inner row—5 + 8 and 3 + 6, respectively; myomeres 48; p. c 4 (the three anterior ones are located in the transverse row, the last caecum is directed backwards parallel to the longitudinal axis of the stomach: Fig. 5d). Head is crushed, eyes are lost; the rays of all fins are distally broken off. There are 9 myomeres between the verticals of the beginnings of V and D. The beginning of A is located behind the end of D. The adipose fin is present, its base is located behind the vertical of the end of А.
Some measurements, in % SL: head length ~ 25, maximum and minimum body depth 8.75 and 5.6, respectively, caudal peduncle length 11.25; antedorsal, anteventral and anteanal distances 72.5, 58.75 and 85.0, respectively; snout length 11.25, horizontal length of the bony orbit ~ 5.9, lower jaw length 8.1.
Pigmentation. The specimen is skinless and completely depigmented. The head retains traces of a stripe of brownish pigment along the upper jaw, a small accumulation of small melanophores in the area of quadratum above the mandibular joint, and a narrow stripe of pigment along the lower edge of the suspensorium. Meningeal pigmentation is very weak, with several relatively large melanophores on the sides of the cerebral hemispheres. On the right side of the caudal peduncle the skin was preserved, but the pigmentation was strongly discolored, although single small specks of horizontal stripes above and below the myocomma were traced. At the bases of V spotty accumulations of melanophores are preserved. Very small isolated melanophores are found on the intestine and stomach; on the lateral sides of the stomach at the level of the 12th body myomere there is a small loose spot-like accumulation consisting of larger melanophores (Fig. 4h). The lower part of the abdominal wall is lost and it is difficult to judge the presence/absence of peritoneal markings. Peritoneum with large dense brownish specks merging paravertebrally. The dorsal median myosepta between occiput and D is not pigmented.
Notes. In the original description (Parin, 2005, p. 140) 4 + 7 = 11 gill rakers were indicated for the holotype (it was not specified exactly where, but, according to the study method adopted for the genus, their count should have been carried out in the outer row on the first arch). The branchial arches of the holotype are severely damaged; the epidermis on the ceratobranchiale of the first arch is partially behind the bone; nevertheless, there is no doubt that there are actually more rakers in the outer row on the ceratobranchiale-1 (11, not 7) (Fig. 8a). This is slightly greater than the values indicated by Trunov (1997) for South Atlantic fish (4 + 7–8 = 11–12). In addition, the holotype from the southeastern part of the Pacific Ocean differs from the South Atlantic fish by A more displaced backward (its origin is located behind the end of D, and not on the same vertical with it). The taxonomic significance of these differences is likely inconsiderable; variability in the latter characteristic was noted by the author in D. vityazi.
Dolichopteryx sp. indet. (prope anascopa Brauer, 1901)
Material. IO RAS uncat., 1 specimen SL ~ 90+ mm (heavily damaged, fragmented), 07°26′ N, 87°16′ E, 427 m, R/V Petr Lebedev, cruise 7, sample 7–14, Isaacs–Kidd trawl, fishing time 13.25–14.25, March 23, 1969.
Description. Head length ~ 27 mm. The snout is deep throughout. Longitudinal length of bony orbit 1.4 times as long as snout, 2.5 times as long as head length. The teeth on the vomer are multiserial; the teeth on the lower jaw are absent. Sp. br 10 + 20 = 30, flattened and slightly elongated. Gill filaments are very thin and long. The upper jaw and the oral edge of the lower jaw are blackish. There is a very small cluster of melanophores under the orbit. Meningeal membranes with diffuse brownish pigmentation. Chord along the entire length is pigmented with brownish chromatophores merging into a longitudinal strip. A fragment of the intestine has been preserved, in the initial part of which there is a patchy accumulation of brownish melanophores. Scraps of black membranous tissue remained on the intestine, probably the remains of peritoneum.
Notes. This specimen is extremely poorly preserved and its confident identification is impossible, since most of its features have been lost. Nevertheless, it differs from all the representatives of the genus studied by the author by unusually thin and long gill filaments. The short and high snout, the number of gill rakers, and the accumulation of melanophores in the preserved area of the intestine may suggest that this specimen belongs to D. anascopa; however, the listed characters are not enough to be fully convinced of this definition.
Key for Identification of the Genera and Species of “long-Bodied” Opisthoproctids
1(2) The eyes are pouchlike, without a separate globular body and an additional lens; there is an aphakic space (Figs. 1a–1c) .……………........................... 3
2(1) The eyes are telescopic (Figs. 1d–1h), and if they are pouchlike, then they have a large secondary globe containing an additional lens (Fig. 1i); no aphakic space …………………….….................................... 9
3(6) Vertebrae 53–58; subscleral lenticular thickening under the lens is absent; vomer toothless; procurrent rays of C 5–6; SL up to 253 mm…(Ioichthys) … ..................................………………..……………………. 4
4(5) Vertebrae 53–55; Indian Ocean …. I. kashkini
5(4) Vertebrae 56–58; Eastern Pacific …................ .............................................................. Ioichthys sp.
6(3) Vertebrae 40–46; there is a clearly demarcated circumscribed lenticular thickening under the lens (Fig. 1c); the anterior edge of the vomer with multiserial teeth; principal rays of C 9–11; neotenic forms, maximum known SL 66.2 mm…(Duolentops gen. nov.) …............................................................................ 7
7(8) Short snout, 11.2–17.4% SL; peritoneal markings are present; vertebrae (myomeres) 45–46; Indo-West Pacific ………..……………………….... D. minuscula
8(7) Long snout, 19.6–25.4% SL; no peritoneal markings; vertebrae (myomeres) 40–42; North Atlantic ……………............................................. D. rostrata
9(10) The eyes are pouchlike, with a well-separated secondary globe containing an additional lens; fish SL > 112 mm have two corneal lenses behind the secondary globe; vertebrae 67–85; single row teeth on the vomer; juveniles have a longitudinal row of pigment spots along the dorsal edge of the body … (Bathylychnops) ……………………………................................... 11
10(9) The eyes are telescopic, with a well-separated secondary globe, but without an additional lens (present in Dolichopteroides (?): Parin et al., 2009) and without corneal lenses near the ventral and posteroventral edges of the eye; vertebrae 41–60; vomerine dentition multiserial; there are no dorsal pigment markings in juveniles ………………………................. 15
11(12) Vertebrae 65–73 ………… B. brachyrhynchus
12(11) Vertebrae 77–85 ……............................. 13
13(14) V are attached far in front of the vertical line of the beginning of D, the adipose fin is located above the end of A; Southeast Pacific……………… B. chilensis
14(13) V and D begin near the same vertical, adipose fin located above the anterior third or middle of A; North Pacific ………………….………………... B. exilis
15(16) Vertebrae 58–60; both the bases of V and A are at least partially located under the base of D; P is much longer than V…………………………………….…..…. ....................................... Dolichopteroides binocularis
16(15) Vertebrae 41–48, the base of V is always located noticeably in front of the vertical line of the beginning of D, P is much shorter than V or about the same length with them… (Dolichopteryx) ...……….. 17
17(18) The adipose fin is present; juveniles have no peritoneal markings (except for D. vityazi; not known for D. parini)………................................................ 19
18(17) The adipose fin is absent, juveniles have peritoneal markings …….…………........................... 27
19(20) P and V are strongly elongated, the beginning of A is located on the vertical line in the middle of the length of the base of D, there is an area of milky-white (not sclerotized) tissue at the lower edge of the eye ………………………............................................ 21
20(19) P is much shorter than V, the beginning of A is located in front of the end of the base of D or behind it, there is no specific tissue at the lower edge of the eye … ……………..………….…………………………………........ 23
21(22) Vertebrae (myomeres) 41–44, V 12; P, V and D are not pigmented; large oval area of white tissue at the lower edge of the eye; tropical Indo-West Pacific…………………………...................... D. anascopa
22(21) Vertebrae (myomeres) 46–47, V 10–11; P, V and D are pigmented; narrow semi-lunar area of white tissue at the lower edge of the eye; transition zone of the North Pacific …………............................ D. parini
23(24) Juveniles have peritoneal markings [sp. br 23–28; tropical Indo-West Pacific] ……….... D. vityazi
24(23) There are no peritoneal markings in juveniles …………………….………………………….............. 25
25(26) sp. br 31–35, California Transitional Region and Equatorial Pacific Ocean from New Caledonia to the Galapagos Islands ………………… D. pseudolongipes
26(25) sp. br 11–15, waters of the Southern Hemisphere between 41° and 53° S.………........... D. trunovi
27(28) Four peritoneal markings in the P–V interval; branchial arches with dotted melanophore pigmentation (Fig. 4i) [V are not pigmented; the beginning of A under the end of D] …………… D. andriashevi
28(27) Three peritoneal markings in the P–V interval; the branchial arches are not pigmented ………… 29
29(30) The beginning of A slightly in front of the vertical of the end of D; the membrane between the third–seventh rays of V is intense black (except for nearly the base); tropical South Pacific ……................ ..................................................... D. nigripes sp. nov.
30(29) The beginning of A behind the vertical of the end of D; V is not pigmented; Atlantic Ocean …..... ....................................................………… D. longipes
REFERENCES
Aizawa, M., 88. Opisthoproctidae barreleyes, in Fishes of Japan with Pictorial Keys to the Species, Nakabo, T., Ed., Tokyo: Tokai Univ. Press, 2002, vol. 1, pp. 287–288.
Badcock, J., Evidence for the assignment of Dolichopteryx brachyrhinchus Parr to the genus Bathylychnops Cohen, (Pisces, Opisthoproctidae), J. Fish. Biol., 1988, vol. 32, no. 3, pp. 423–432.
Beebe, W., Nineteen new species and four post-larval deep-sea fish, Zoologica (N.Y.), 1932, vol. 13, no. 4, pp. 47–107.
Beebe, W., Deep-sea fishes of the Bermuda oceanographic expeditions: family Argentinoidei, Zoologica (N.Y.), 1933, vol. 16, no. 3, pp. 59–70.
Brauer, A., Die Tiefsee-Fische. II. Anatomischer Teil, in Wissenschaftliche Ergebnisse der Deutschen Tiefsee-Expedition auf dem Dampfer “Valdivia” 1898–1899, Jena: Gustav Fischer Verlag, 1908, vol. 15.
Cohen, D.M., Bathylychnops exilis, a new genus and species of argentinid fish from the North Pacific, Stanford Ichthyol. Bull., 1958, vol. 7, no. 3, pp. 47–52.
Cohen, D.M., New records of the opisthoproctid genus Bathylychnops, with a notice of neoteny in the related genus Dolichopteryx,Copeia, 1960. 1960, no. 2, pp. 147–149.
Cohen, D.M., Suborder Argentinoidea, in Fishes of the Western North Atlantic, Part 4: Soft-Rayed Bony Fishes: Orders Isospondyli and Giganturoidei: Argentinoids, Stomiatoids, Pickerels, Bathylaconids, Giganturids, Mem. Sears Found. Mar Res. no. 1, Bigelow, H.B., Ed., New Haven: Yale Univ., 1964, pp. 1–70.
Fitch, J.E. and Lavenberg, R.J., Deep-water teleostean fishes of California, in California Natural History Guides, Berkeley: Univ. Calif. Press, 1968, no. 25.
Frederiksen, R.D., On the retinal diverticula in the tubular-eyed opisthoproctid deep-sea fishes Macropinna microstoma and Dolichopteryx longipes,Vidensk. Medd. Dansk Naturhistor. Foren., 1973, vol. 136, pp. 233–244.
Fujii, E., Family Opisthoproctidae, in The Fishes of the Japanese Archipelago, Masuda, H., Ed., Tokyo: Tokai Univ. Press, 1985, vols. 1–2, pp. 41–42.
Fukui, A. and Kitagawa, Y., Dolichopteryx minuscule, a new species of spookfish (Argentinoidei: Opisthoproctidae) from the Indo-West Pacific, Ichthyol. Res., 2006a, vol. 53, no. 2, pp. 113–120.
Fukui, A. and Kitagawa, Y., Dolichopteryx rostrata, a new species of spookfish (Argentinoidea: Opisthoproctidae) from the eastern North Atlantic Ocean, Ichthyol. Res., 2006b, vol. 53, no. 1, pp. 7–12.
Fukui, A., Kitagawa, Y., and Parin, N.V., Dolichopteryx pseudolongipes, a new species of spookfish (Argentinoidei: Opisthoproctidae) from the eastern Pacific Ocean, Ichthyol. Res., 2008, vol. 55, no. 3, pp. 267–273.
Greenwood, P.H. and Rosen, D.E., Notes on the structure and relationships of the alepocephaloid fishes, Am. Mus. Novit., 1971, no. 2473.
Grey, M., First record of the deepsea fish, Dolichopteryx longipes from the Pacific, with notes on Ophthalmopelton macropus,Copeia, 1952, no. 2, pp. 87–90.
Haedrich, R.L. and Craddock, J.E., Distribution and biology of the opisthoproctid fish Winteria telescopa Brauer, 1901, Breviora, 1968, no. 294.
Harrisson, C.M.H., On methods for sampling mesopelagic fishes, Symp. Zool. Soc. Lond., 1967, vol. 19, pp. 71–126.
Hatooka, K., 86. Argentinidae argentines, in Fishes of Japan with Pictorial Keys to the Species, Nakabo, T., Ed., Tokyo: Tokai Univ. Press, 2002, vol. 1, pp. 283.
Hubbs, C.L. and Lagler, K.F., Fishes of the Great Lakes region, Cranbrook Inst. Sci. Bull., 1958, no. 26.
Kawaguchi, K. and Butler, J.L., Fishes of the genus Nansenia (Microstomatidae) with descriptions of seven new species, Contrib. Sci. Los Angeles Cty. Mus., 1984, no. 352.
Kobylyanskii, S.G., Taxonomic status of Microstomatidae fishes and classification of suborder Argentinoidei (Salmoniformes, Teleostei), Tr. Inst. Okeanol. im. P.P. Shirshova,Akad. Nauk SSSR, 1990, vol. 125, pp. 148–177.
Kobylyanskii, S.G., Bathylagus niger sp. nova (Bathylagidae, Salmoniformes) a new species of Bathylagus from the subpolar waters of the Southern Ocean, J. Ichthyol., 2006, vol. 46, no. 6, pp. 413–417.
Kobylyanskii, S.G. and Fedorov, V.V., A new species of the genus Dolichopteryx—D. parini (Opisthoproctidae, Salmoniformes) from the mesopelagial zone of the Sea of Okhotsk and the Bering Sea, J. Ichthyol., 2001, vol. 41, no. 1, pp. 115–118.
Land, M.F., On the functions of double eyes in midwater animals, Philos. Trans. R. Soc. B, 2000, vol. 355, no. 1401, pp. 1147–1150.
Mecklenburg, C.W., Mecklenburg, T.A., and Thorsteinson, L.K., Fishes of Alaska, Bethesda, MA: Am. Fish. Sci., 2002.
Mizusawa, N. and Fukui, A., First Japanese record of a spook-fish, Dolichopteryx parini (Argentinoidei: Opisthoproctidae), from off the Pacific coast of Aomori Prefecture, Jpn. J. Ichthyol., 2009, vol. 56, no. 2, pp. 149–152.
Mizusawa, N., Takami, M., and Fukui, A., Redescription of the spookfish Dolichopteryx anascopa Brauer, 1901 (Argentinoidei: Opisthoproctidae), Ichthyol. Res., 2015, vol. 62, no. 2, pp. 236–239.
Moser, H.G., Opisthoproctidae: spookfishes, in The Early Stages of Fishes in the California Current Region, Lawrence, KY: Allen Press, 1996, pp. 216–223.
Parin, N.V., A new mesopelagic fish Ioichthys kashkini Parin, gen. et sp. nova (Opisthoproctidae) from the northwestern part of the Indian Ocean, J. Ichthyol., 2004, vol. 44, no. 7, pp. 485–488.
Parin, N.V., Dolichopteryx trunovi sp. nova—a new name for D. anascopa (nec Brauer, 1901) Trunov, 1997 (Opisthoproctidae, Argentinoidea), J. Ichthyol., 2005, vol. 45, no. 1, pp. 132–133.
Parin, N.V., Fedorov, V.V., Borodulina, O.D., and Bekker, V.E., New records of mesopelagic and epipelagic fishes in Pacific waters off the southern Kuril Islands, J. Ichthyol., 1995, vol. 35, no. 9, pp. 193–204.
Parin, N.V., Belyanina, T.N., and Evseenko, S.A., Materials to the revision of the genus Dolichopteryx and closely related taxa (Ioichthys, Bathylychnops) with the separation of a new genus Dolichopteroides and description of three new species (fam. Opisthoproctidae), J. Ichthyol., 2009, vol. 49, no. 10, pp. 839–851.
Parr, A.E., Concluding report on fishes. With species index for articles 1–7 (fishes of the third oceanographic expedition of the “Pawnee”), Bull. Bingham Oceanogr. Coll. Yale Univ., 1937, vol. 3, pp. 1–79.
Partridge, J.C., Douglas, R.H., Marshall, N.J., et al., Reflecting optics in the diverticular eye of a deep-sea barreleye fish (Rhynchohyalus natalensis), Proc. R. Soc. B, 2014, vol. 281, pp. 1–9. https://doi.org/10.1098/rspb.2013.3223
Pearcy, W.G., Meyer, S.L., and Munk, O., A “four-eyed” fish from the deep sea: Bathylychnops exilis Cohen, 1958, Nature, 1965, vol. 207, no. 5003, pp. 1260–1262.
Prokofiev, A.M., New and rare species of deep-sea pelagic fishes of families Opisthoproctidae, Melanostomiidae, Oneirodidae, and Linophrynidae, J. Ichthyol., 2014, vol. 54, no. 6, pp. 377–383. https://doi.org/10.1134/S0032945214040092
Prokofiev, A.M. and Pietsch, T.W., First record of the ceratioid anglerfish species Gigantactis microdontis (Teleostei: Lophiiformes: Gigantactinidae) in the western Pacific Ocean, Zootaxa, 2019, vol. 4664, no. 3, pp. 441–444.
Robison, B.H. and Reisenbichler, K.R., Macropinna microstoma and the paradox of its tubular eyes, Copeia, 2008, no. 4, pp. 780–784.
Roule, L. and Angel, F., Larves et Alevines de Poisons Provenant des Croisières du Prince Albert I de Monaco, Monaco: Imprimerie de Monaco, 1930, vol. 79, pp. 1–148.
Shinohara, G., Opisthoproctidae gen. et sp. indet, in Deep-Sea Fishes of Peru, Nakaya, K., Eds., Tokyo: Jpn. Deep Sea Trawlers Assoc., 2009, pp. 95.
Stein, D.L. and Bond, C.E., Observations on the morphology, ecology, and behavior of Bathylychnops exilis Cohen, J. Fish. Biol., 1985, vol. 27, no. 3, pp. 215–228.
Stewart, A.L., Family Opisthoproctidae spookfishes, in The Fishes of New Zealand, Roberts, C.D., Ed., Wellington: Te Papa, 2015, vol. 1, pp. 326–331.
Trunov, I.A., The species of the Opisthoproctidae family from the southern Atlantic Ocean, J. Ichthyol., 1997, vol. 37, no. 9, pp. 810–814.
Wagner, H.-J., Douglas, R.H., Frank, T.M., et al., A novel vertebrate eye using both refractive and reflective optics, Curr. Biol., 2009, vol. 19, no. 2, pp. 108–114.
Funding
The study of oceanic ichthyofauna and the comparative morphological analysis were carried out within the framework of the state assignment nos. 0149–2018–0009 and 0109–2018–0076, respectively; the study of ontogenetic variability and diagnostic significance of juvenile characters was supported by the Russian Science Foundation, grant no. 19–14–00026.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by S. Avodkova
Rights and permissions
About this article
Cite this article
Prokofiev, A.M. Revision of the Generic Classification of “Long-Bodied” Opisthoproctids (Opisthoproctidae) with a Description of New Taxa and New Finds. J. Ichthyol. 60, 689–715 (2020). https://doi.org/10.1134/S0032945220050094
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0032945220050094