Abstract
The Arctic staghorn sculpin Gymnocanthus tricuspis is a demersal fish that plays an important role in the Arctic ecosystem as a link between benthic invertebrates and higher trophic levels. In the Kara Sea, the highest density concentrations exceeding six thousand individuals per square km were found in shallow waters (depth 20–30 m) adjacent to the northwestern part of Yamal Peninsula, in autumn of 2019. Sculpins aged from 1+ to 7+ years, TL 41–177 mm long, and weighing 0.6–72.5 g were registered in trawl catches, with small size fish (TL = 41–80 mm, weight <10 g, age 3–4+ years) being predominant in those catches. Sexual dimorphism was pronounced in external morphology and body size. Similar to other species of this genus, the proportion of females sharply increased among fish over 110 mm long, reaching 100% for fish130 mm long. Individual fecundity of females 134–164 mm long varied from 2385 to 3353 eggs (2994 average). This benthophagous species showed clear age-related diet composition. Diet of young fish consisted mainly of polychaete worms, and it changed to a more diverse diet for older fish in which bivalves, isopods and amphipods prevailed. Arctic staghorn sculpin is considered an indicator-species, and the results of the present study may help monitor health of the Kara Sea ecosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Arctic staghorn sculpin Gymnocanthus tricuspis (Reinhardt, 1830) is a typical representative of the demersal ichthyofauna of the Arctic, Subarctic, and adjacent Atlantic and Pacific waters. It is a common or even a dominant species in some parts of its wide range (Andriashev, 1954; Allen and Smith, 1988; Andriashev and Chernova, 1994; Sheiko and Fedorov, 2000; Mecklenburg et al., 2013, 2018; Parin et al., 2014; Tuponogov and Kodolov, 2014; Orlov et al., 2020a). Due to its high abundance, the sculpin is an important element of the Arctic food webs (McConnaughey and McRoy, 1979; Livingston et al., 2017; Gray et al., 2017), serving as a food source for some predatory fish, water birds and marine mammals (Finley and Evans, 1983; Atkinson and Percy, 1991, 1992; Elliott et al., 2008; Gleason, 2012; Walkusz et al., 2012; Gray et al., 2017). It is also considered to be one of the indicators of the state of ecosystems of the Arctic seas (Mecklenburg et al., 2007).
The distribution and biology of sculpin are best studied in the eastern Chukchi and western Beaufort Seas, and in Canadian Arctic waters due to extensive research undertaken by the United States and Canada, and in the Barents Sea due to regular Norwegian–Russian joint research.
Such information for waters of the Siberian Arctic (the Kara, Laptev, East Siberian, and western Chukchi Seas), remained extremely limited and fragmented until recently due to harsh climatic conditions that prevent regular research here (Andriashev, 1954; Chuchukalo, 2006; Dolgov, 2012; Chernova, 2015; Orlov et al., 2019, 2020b, 2020c, 2020d). Current warming of the Arctic has led to reduction of ice cover in recent years. This allowed to intensify scientific research in the region and to obtain, among other things, new information about the distribution and biology of sculpin in those parts of its range (Stasko et al., 2016; Gray et al., 2017; Forster et al., 2020; Yamazaki et al., 2020). However, published data on sculpin in the Kara Sea are limited mostly to information on its occurrence in the area, capture depths, maximum length and age, and some general biological characteristics (Semushin and Novoselov, 2009; Dolgov, 2013; Chaus and Karamushko, 2017; Dolgov et al., 2018; Orlov et al., 2020e).
The main objective of this paper is to characterize the distribution of sculpin and its biological characteristics (size–age and size–sex structures, sexual dimorphism, growth, and diet) in the Kara Sea. In this paper, we tried to identify the differences in distribution and life history of sculpin in the Kara Sea and to compare those to other parts of its range. Furthermore, we tried to relate any of these differences to specific climate and oceanographic conditions (Prishchepa, 2008). Since sculpin is considered an indicator–species of good status of the Arctic fish communities (Mecklenburg et al., 2007), the results of the present study may help monitor the health of the Kara Sea ecosystem.
MATERIALS AND METHODS
Survey design. Sculpin sampling was carried out during the transarctic expedition on board the R/V Professor Levanidov (VNIRO, Moscow, Russia) in the southern part of the Kara Sea from 15 to 29 September 2019 (Orlov et al., 2020d, 2020e, 2021). During this period, 55 research stations (Supplement 1) with bottom trawl hauls and bottom temperature measurements were completed (Supplement 2).
A large-mesh bottom trawl “DT 27.1/24.4” was deployed. Trawl mesh size was 8.0 cm in the wings and body, 6.0 cm in the intermediate, 3.0 cm in the codend, and the codend was equipped with a 10 mm mesh liner. Spherical boards with an area of 4.3 m2 were used. Contact of the gear with the seabed and parameters of the trawl opening were monitored with third–wire net controller SIMRAD FS 20/25 (Kongsberg Simrad Mesotech Ltd., Norway). Bottom trawl hauls were carried out in depths of 18 to 533 m; the wire length correspondingly varied from 40 to 1050 m. The trawling speed varied in the range from 3.0 to 3.5 knots, averaging 3.17 ± 0.12 knots. The horizontal opening of the trawl at the specified parameters of the speed and length of the wires varied from 6.0 to 16.0 m, averaging 12.36 ± 1.38 m. The vertical opening was in the range from 3.0 to 3.9 m, averaging 3.09 ± 0.20 m.
An SBE 25 CTD operated together with Carousel Deck Unit SBE 33 (Sea-Bird Scientific, USA) was used for the bottom temperature measurements.
Biological sampling. Captured individual sculpin were frozen, delivered to VNIRO, and then transferred to the Kamchatka Branch of the Pacific Institute of Geography of the Far Eastern Branch of the Russian Academy of Sciences for subsequent processing in the laboratory. A total of 206 individuals (including 82 females 41–177 mm long, weighed 0.54–72.52 g and 124 males 43–131 mm long, weighed 0.61–30.46 g) were examined (age determinations, stages of gonad maturity, and diet composition) using widely accepted methodology (Laevatsu, 1965; Pravdin, 1966). Length was measured with a measuring ruler with an accuracy of 1 mm, and body weight was measured on laboratory electronic scales CAS MWP 150 (CAS Corporation, South Korea) with an accuracy of 0.01 g.
Otoliths were broken across the area of the central hollow with a scalpel, then calcined on an electric stove and coated with glycerin (lightly polished if necessary) (Tokranov and Orlov, 2007). Subsequently, data of all length measurements using the size–age key were recalculated to age (Chugunova, 1959).
To elucidate the sexual dimorphism in external morphological characters, eight morphometrics were analyzed in 30 males and 30 females (78–129 and 103–161 mm total length (TL), respectively): the height of the first (hDI) and second (hDII) dorsal fins, the height of the anal fin (hA), the length of the base of the first (lDI) and second (lDII) dorsal fins, the length of the anal fin base (lA), and the length of the pectoral (lP) and pelvic (lV) fins, the absolute values of which were attributed to the TL (Tokranov, 1993). At the same time, variations in the coloration of females and males were evaluated, as well as the presence of urogenital papilla in males.
Gonadal maturity state of sculpin specimens were examined at macroscopic level based on a six–stage scale (Sakun and Butskaya, 1968): I—juvenile, II—immature, III—developing, IV—mature (pre–spawning), V—spawning (ripe), VI—spent (post–spawning).
Stomachs were dissected in the laboratory and stored in 10% formalin to fix stomach contents. Stomach contents were sorted to the lowest possible taxonomic level, weighed, and counted with subsequent calculation of index of stomach fullness estimated as the weight of food divided by fish body weight and multiplied by 105 (Manual…, 1974).
Data analysis. The abundance and biomass per unit of the surveyed area (in individuals/km2 and kg/km2) for each i-trawling were calculated using the formulas: \(\frac{{{{N}_{i}}}}{{{{A}_{i}}}} = \frac{{{{N}_{i}}}}{{1.852{{v}_{i}}{{t}_{i}}0.001{{h}_{i}}}}\) and \(\frac{{{{M}_{i}}}}{{{{A}_{i}}}} = \frac{{{{M}_{i}}}}{{1.852{{v}_{i}}{{t}_{i}}0.001{{h}_{i}}}}\), where Ni is a number (individuals) and Mi is the weight of fish in the catch of i-trawling (kg); Ai is the area swept during trawling (km2); vi is the trawling speed (knots);ti is the duration of haul (h); hi is the horizontal opening of the mouth of the trawl (m); 1.852 is the number of km in 1 nautical mile, 0.001 is the conversion factor from m to km.
The values of the total abundance and biomass for the surveyed area were obtained as follows: \({\text{Abundanc}}{{{\text{e}}}_{{\left( {{\text{total}}} \right)}}} = \sum \frac{{{{N}_{i}}}}{{{{A}_{i}}}}{{S}_{i}}\) and \({\text{Biomas}}{{{\text{s}}}_{{\left( {{\text{total}}} \right)}}} = \) \(\sum \frac{{{{M}_{i}}}}{{{{A}_{i}}}}{{S}_{i}}\), where Ni is a number (individuals) and Mi is the weight of fish in the catch of i-trawling (kg); Ai is the area caught during i-trawling (km2). Si is the area of Thiessen polygon for each station.
An individual Thiessen polygon was calculated for each station (QGIS, 1981; Schumann, 1998). The shallow part of the shelf was not surveyed. Therefore the inner polygon boundaries were set along the contour of a 20-m isobath. The isobath boundary was obtained according to IBCAO Version 3.0 (Jakobsson et al., 2012). For 55 research stations, a total area of 511.28 thousand km2 was surveyed (Supplement 3).
For data curation and storage, Microsoft Access 2016 Database Management System was used. Statistical processing was performed in Microsoft Excel 2016, using Analysis ToolPack. Calculations of the parameters of the Von Bertalanffy growth function (VBGF) and a graph of the relationship between the length of fish and their age were carried out using the PAST 4.02 software (Hammer et al., 2001).
Basic statistics and Student t-test were used for processing of biological sample data (morphometry, age, diet) (Lakin, 1980). Geostatistical data processing, including the calculations of polygon areas and the drawing of spatial distribution maps, was performed in the QGIS Desktop 3.16 (https://qgis.org/). The temperature distribution was interpolated by the Kriging (QGIS module: SAGA, https://docs.qgis.org/2.8/en/docs/user_manual/processing_algs/saga/index.html). The study area calculations were performed in the coordinate system WGS (World Geodetic System, 1984), EPSG: 4326 (https://epsg.io/4326). The maps presented in this paper were plotted in the Asia North Albers Equal Area Conic projection (ESRI: 102025, https://epsg.io/102025).
RESULTS
Distribution and relative abundance. During the autumn period in the Kara Sea, sculpin were encountered at depths from 18 to 255 m (Fig. 1а) at a bottom temperature from –1.5 to 3.0°C (Fig. 1b).
The frequency of occurrence of sculpin was low and amounted to 21.8% for all stations. In the open part of the sea on the traverse of the Taimyr Peninsula near the islands of the Nordenskjold Archipelago, this species was absent from catches. In the southwestern Kara Sea, two areas were characterized by high sculpin occurrence.
The first area was located between the Gyda Peninsula and the Novaya Zemlya archipelago. The depths here ranged from 18 to 255 m, and the bottom temperature ranged from –1.5 to 0°C. The number of sculpin varied widely from 42.2 to 7255.7 individuals/km2, and the biomass varied from 0.82 to 40.16 kg/km2. The number of fish along transects decreased with increasing depth. The maximum number of fish was recorded to the northeast of the Yamal Peninsula, at the outlet of the Gulf of Ob at a depth of 18 m. The bottom temperature in that location was –1.0°C.
The second area was located off the western coast of the Yamal Peninsula, at the exit of Baydaratskaya Bay. The depths in that area ranged from 20 to 217 m, and bottom temperatures ranged from –1.0 to 3.0°C. The number of sculpin ranged from 90.0 to 6179.5 individuals/km2, and the biomass varied 1.59 to 304.30 kg/km2. Again, the maximum sculpin values were characteristic of the minimum depths.
In general, catch rates increased with a decrease in the depth of trawling. On average, for stations with sculpin in catches, sculpin numbers were highest at depths 20–30 m (4681.6 individuals/km2; Fig. 2a). Furthermore, with increasing depth, the relative number decreased. The relationship between relative abundance and bottom temperature could not be identified. High average numbers of sculpin were found associated with both negative and positive values of bottom temperature (Fig. 2b).
The total number of sculpin for the surveyed area in the Kara Sea was 111.73 million individuals, the biomass was 1.95 thousand tons.
Biological description. The maximum TL of sculpin in the Kara Sea in September 2019 was 177 mm, maximum body weight was 72.52 g, and maximum age was 7+ years. In general, within the surveyed area, this species was represented by individuals with a TL of 41–177 mm (average 75.4 ± 1.6) and a body weight of 0.54–72.52 g (average 8.3 ± 0.7) aged from 1+ to 7+ years (Fig. 3). However, fish with a TL of 41–80 mm (68%) and a body weight of less than 10 g (78.3%) dominated.
Male sculpins were represented in catches by individuals with TL of 43–129 mm (average 71.8 ± 1.7) and body weight of 0.61–30.46 g (average 5.7 ± 0.5), aged from 1 + to 6+ years (average 3.07 ± 0.11). Female TL ranged from 41–177 mm (average 78.1 ± 2.4), body weight was from 0.54–72.52 g (average 10.3 ± 1.1), and age ranged 1+ to 7+ years (average 3.14 ± 0.11).
The ratio between TL, cm and weight (W, g) of sculpin of the Kara Sea is well approximated (R2 = 0.99) by the power function W = 2.3 × 10–6 TL3.382 and is characterized by positive allometric growth (Fig. 4).
During the first three years of life, the length and weight of males and females of this species are quite similar, but from the fourth year onwards, females grow much faster. Length and especially weight of fish of the same age differ significantly between sexes 4+ years of age, reaching an average difference of almost 17 mm and 20 g at the age of 6+ years (Fig. 5).
An attempt to calculate the parameters of the VBGF (Table 1) did not produce a good model fit, despite the high coefficients of determination. The obtained Linf values turned out to be significantly lower than the observed data (Supplement 4). The relationship between TL, mm and age (A, years) are best described by the linear function (Fig. 6): A = 0.046 TL + 0.447 (R2 = 0.978) for all specimens analyzed, A = 0.059 TL – 0.338 (R2 = 0.983) for males, and A = 0.045 TL + 0.470 (R2 = 0.982) for females.
Of the individuals studied, most were immature (76.2% of males 88–90 long with age up to 3 years and 85.6% of females shorter 114 mm with age up to 4 years). All adult males and the vast majority of females had gonads at the initial stage of maturation (stage III). Although the materials at our disposal do not allow us to analyze the rate of sexual maturation of the sculpin, apparently, the maturation of males of this species in the Kara Sea occurs at a length over 90 mm and the age of 3 years, while females mature a year later at a length of 110–115 mm.
Among the small sculpins (up to 80 mm), the sex ratio was approximately equal. Individuals with a length of 81–110 mm were mostly males, the relative number of which reached 63.6–73.9%. Among larger fish with a length of over 110 mm, the proportion of females begins to increase sharply, reaching 100% with a length of 130 mm (Supplement 5). In general, females of the sculpin somewhat prevailed (by 1.3 times) over males. Among the individuals studied, three mature females with a length of 134, 140, and 164 m and weight of 34.6, 37.4, and 66.9 g had pre-spawning ovaries (stage IV) weighed 4.77, 3.79, and 6.55 g respectively. Individual fecundity varied from 2385 to 3353 (average 2994) eggs with a diameter of about 2.0 mm (since the fish were frozen for almost a year, more accurate measurements could not be made due to the fact that their eggs were frozen and exfoliated).
Sexual dimorphism. The sculpin exhibits sexual dimorphism in the coloration of individuals of different sexes. Its males are brighter than females. They have larger and more contrasting spots and stripes on the body and fins. In addition, males have an urogenital papilla, which is clearly visible already at a length of over 41 mm.
In addition to the difference in color and the presence of urogenital papillae in males, there are significant differences in the size of some fins in individuals of different sexes (Table 2). Of the eight characters analyzed (the height of the first hDI and second hDII dorsal fins, the height of the anal fin hA, the length of the base of the first lDI and second lDII dorsal fins, the length of the anal fin lA base, the length of the pectoral lP and pelvic lV fins), statistically significant differences were found in three of them: hDI, HDII, and lV in males were greater than in females. The length of the pelvic fin was especially distinguished by the degree of difference, the relative values of which in male sculpins were 19.3–33.3% and in females 15.7–19.3% of TL, i.e. practically did not coincide.
Sexual dimorphism also was manifested in different sizes of males and females. Males were much smaller than females (Fig. 3) and matured at an earlier age, which resulted in a significant decrease in their proportion in the spawning population. In addition, males were characterized by a shorter life span compared to females, and therefore the proportion of the males decreased significantly with an increase in the size of fish (see Supplement 5).
Diet composition. The sculpin is a benthophagous species with a relatively diverse diet composition that included representatives of 10 different taxonomic groups of invertebrates (Table 3). However, the bulk of its diet (about 99% by weight) was formed by only four groups of prey: polychaete worms (Polychaeta, mainly the family Phyllodocidae), isopod crustaceans (Isopoda, mainly Saduria entomon), various amphipods (Amphipoda), and bivalve mollusks (Bivalvia). With increasing sculpin size, diet changed significantly. The main prey of small individuals (41–80 mm) was polychaete worms (more than 78% of the diet). The bulk of the diet of the largest specimens (over 120 mm) were bivalves (41.5%) and isopod crustaceans (24.2%), while the value of polychaete worms reduced (almost 18%). Along with the change in the prey composition, as a sculpin grows, the size of prey consumed increased as well. In the diet of individuals with a length of 41–60 mm, the size of amphipods varied from 2 to 8 mm (average 5.2), in fish with a TL over 100 mm they were from 12 to 37 mm (average 24.5) long. The sizes of isopods in the diet of fish with a TL less than 100 mm varied from 10–14 mm (average 11.3), and in individuals over 120 mm long, isopod prey ranged from 12–25 mm (average 17.4).
DISCUSSION
Distribution and life history of the sculpin remains poorly understood, and published data for the Kara Sea are mostly limited by scarce and fragmentary information about presence of the species, capture depths, maximum length and age, and some general biological features (Prishchepa, 2008; Semushin and Novoselov, 2009; Dolgov, 2013; Chaus and Karamushko, 2017; Dolgov et al., 2018; Orlov et al., 2020e).
Distribution and relative abundance. In the Pechora Sea, the sculpin is extremely scarce in coastal areas and forms the main concentrations in the open part of the sea (Semushin et al., 2019). On the contrary, near the coast of Novaya Zemlya in the eastern part of the Barents Sea, it forms concentrations with maximum density in shallow areas (Johannesen et al., 2017). This species is widespread in the Kara Sea, except in deep–water areas (Dolgov et al., 2018), but its maximum concentrations in various years were observed in the southwestern part of the sea, near the northwestern coast of the Yamal Peninsula; in Baydaratskaya Bay off the western coast of the Yamal Peninsula; and off the southeastern coast of Novaya Zemlya and Vaigach Island (Prishchepa, 2008; Semushin and Novoselov, 2009; Chaus and Karamushko, 2017; Dolgov et al., 2018). In 2019, maximum catches were recorded in the mouth of Baydaratskaya Bay and to the north of the Yamal Peninsula. Since all studies were conducted during the summer–autumn period, inter-annual differences in spatial distribution might be associated with changing temperature and/or forage conditions related to changes in climate.
In the northern Bering Sea, the relative abundance in beam trawl catches was 688 individuals/km2, and in otter trawl catches, 1857 individuals/km2 (Cui et al., 2009). In the Chukchi Sea, the sculpin has a significantly smaller abundance of 0.1–19.4 individuals/km2 (average 9.3) (Norcross et al., 2013). Sculpin number and biomass off the coast of the Novosibirsk Islands were 6.6 individuals/km2 and 0.011 kg/km2 (Chernova, 2015). In the Kara Sea in 2019, the average abundance was 4682 individuals/km2. In 2012–2016 (Chaus and Karamushko, 2017), sculpin biomass off southwestern Yamal was <200 kg/ km2, while in 2019 it was 304.3 kg/km2. North of Yamal in 2012–2016, the maximum biomass was >200 kg/km2, while in 2019 it was 40.2 kg/km2. It is known that sculpin abundance in 2012–2015 in the eastern Barents Sea significantly decreased compared to 2004–2007 (Johannesen et al., 2017). Therefore we hypothesize that the main reason for the inter-annual differences observed is the redistribution of sculpin to the Kara Sea from the Barents Sea, due to the general warming of the Barents Sea (Wiedmann et al., 2014; Fossheim et al., 2015; Eriksen et al., 2017).
The sculpin belongs to the group of shallow–water representatives of the genus Gymnocanthus, whose main habitat depths lie within 0–70 m (Majewski et al., 2013; Yamazaki et al., 2013, 2020; Johannesen et al., 2017; Dolgov et al., 2018). In the northern Bering, Chukchi, East Siberian, Kara, and Barents Seas, sculpin have been usually recorded at depths shallower than 100 m (Mecklenburg et al., 2007; Wienerroither et al., 2011; Longshan et al., 2012; Chernova, 2015; Dolgov et al., 2018). In the Kara Sea in 2019, sculpin were recorded within the depth range of 18–255 m, which is somewhat shallower than known from the literature (maximum 372 m) (Prishchepa et al., 2008). The densest concentrations of this species in the Kara Sea were previously observed at depths of 100–160 m in 2007 (Prishchepa et al., 2008) and of 2–30 m in 2012–2016 (Chaus and Karamushko, 2017), while we found them at depths 20–30 m. These differences might be also due to climate changes mentioned above.
The sculpin has adapted to the harsh conditions of the Arctic climate due to the presence of antifreezing proteins (Brand and Fischer, 2016), however, it is considered a species with a smaller range of tolerant hydrography. Other authors believe that this species is adapted to inhabit waters with wide limits of temperature and salinity (Majewski et al., 2013). Within the species range, it occurs in a temperature range from ‒1.8 to 12.5°C (usually close to 0°C) and salinity of 16–35‰ (Prishchepa et al., 2008; Chernova, 2015; Dolgov et al., 2018). The bimodality of the distribution of the sculpin depending on the bottom temperature in the Kara Sea in 2019 may be due to the different temperature tolerance of juvenile and adult individuals who live in different bathymetric ranges (Andriashev, 1954; Chaus and Karamushko, 2017).
Biological description. The sculpin is a relatively small, short-lived species, with a maximum length in Russian Arctic waters reaching 250 mm (Knipovich, 1926; Esipov, 1952), although specimens up to 299 mm are found off the coast of Greenland (Andriashev, 1954; Dolgov, 2004; Love et al., 2005; Mecklenburg et al., 2007). The maximum known length for the Kara Sea is 195 mm (Chaus and Karamushko, 2017). In our catches in 2019, TL did not exceed 177 mm. These differences may be due to different surveyed areas and depths, since the size differentiation of individuals of this species by depth is characteristic in various other parts of its range (Andriashev, 1954). Some potential sampling bias might also affect these differences.
In the majority of the species’ range, sculpin catches are represented by individuals with TL 20 to 170 mm with modal size class 40–60 mm (Fechhelm et al., 1984; Atkinson and Percy, 1991, 1992; Mecklenburg et al., 2007; Stasko et al., 2016; Gray et al., 2017; Forster et al., 2020). The largest individuals are likely characteristic of waters off Newfoundland (132–268 mm), Svalbard (45–200 mm, average 145 mm, modal classes 60 and 150–160 mm), and the Barents Sea (30–210 mm, modal class 90–150 mm) (Ennis, 1968; Wienerroither et al., 2011; Brand, Fischer, 2016). The sculpin in the Kara Sea had a larger size compared to individuals from the Chukchi and Beaufort Seas and the waters of the Canadian Arctic, but was considerably smaller than fish from the waters of Svalbard, Barents Sea and Newfoundland. Such geographic differences might be due to different habitat conditions of sculpin in various parts of its range, as well as to the selectivity of fishing gear (Ennis, 1968; Fechhelm et al., 1984; Atkinson and Percy, 1991, 1992; Mecklenburg et al., 2007; Wienerroither et al., 2011; Brand, Fischer, 2016; Stasko et al., 2016; Gray et al., 2017; Cui et al., 2019; Forster et al., 2020).
Comparison of the relationship between the length and body weight of sculpin in the eastern Chukchi Sea and the western Beaufort Sea (Forster et al., 2020) with our data showed similar growth characteristics that might be due to similar habitat conditions in those areas.
The lifespan of female sculpin can be 9 years, and that of males 8 years in the Kara Sea (Dolgov et al., 2018), compared with maximum age of 7 years in the Beaufort Sea and 6 years in the Chukchi Sea (Forster et al., 2020). The longevity of this species in the Kara Sea, according to our data (7+ years), is close to previously published data. In the Chukchi Sea the catches of sculpin were dominated by fish under the age of 4 years inclusively (Gleason, 2012). Our catches were dominated by somewhat older individuals that might be associated with different gear selectivity or different age compositions of sculpin in both areas compared.
Individuals from the Kara Sea up to the age of 5 years grow noticeably slower as compared to the Chukchi and Beaufort seas (Forster et al., 2020). Upon reaching the age of 5 years, the size and age characteristics of individuals from the Kara, Chukchi, and Beaufort seas become almost equal. The oldest individuals aged 6–7 years from the Kara Sea are considerably larger than the fish of the same age from the Chukchi and Beaufort seas. These differences might be due to different habitat and/or forage conditions.
Coefficients k of the VBGF in the Kara Sea and in the Chukchi and Beaufort Seas differ by 3–5 times (Forster et al., 2020). Since this coefficient characterizes the increase in size per unit of time, it is unlikely that the real growth rates in these areas differ so much. The values of the Linf obtained by us and those for the Chukchi and Beaufort seas (Forster et al., 2020) also differed very much (3.2–7.5 times). The values we calculated were considerably smaller than maximum observed values, while for the Chukchi and Beaufort Seas they were much larger. The main reason for this phenomenon, in our opinion, is that the VBGF model is not a good fit for the growth pattern of this particular species. It has been shown that for some species of the genus Gymnocanthus (e.g., the threaded sculpin G. pistiliger), the growth is well described by the von Bertalanffy equation (Hoff, 2000; Shelekhov and Panchenko, 2007). However, in our case, the growth of species under consideration is better described by a linear function. It is known that in some fish species, growth can be linear not only during a certain stage of the life cycle but also throughout the whole life (Allen, 1976; Schreck and Moyle, 1990; Hopkins, 1992). However, it is unlikely that the growth rates of Arctic staghorn sculpin remain the same throughout its lifecycle, since the majority of animals are characterized by the most intensive growth prior sexual maturation, after which it slows down. The linear character of growth in our case is probably due to a small number of large mature individuals, which requires further research involving a larger sample with a reliable representation of large specimens.
The reproductive biology of the sculpin is limited by information on the timing of spawning, the diameter of the eggs, and the duration of the pelagic larval phase (Ennis, 1968; Norvillo and Zhuravleva, 1989; Panchenko, 2012; Yamazaki et al., 2020). In the Kara Sea, males of this species dominate in size groups up to 90 mm, then the male proportion decreases with increasing size and most fish over 130 mm long are females (Chaus and Karamushko, 2017). According to our data, males dominate among fish up to 110 mm long, in the 110–130 mm size class their proportion decreases, and among fish over 130 mm long, females dominate, amounting to 100%. This contradiction might be associated with different nature of the data being compared, e.g. a single year of data collection (2019) in our case and several years (2012–2016) in previous studies (Chaus and Karamushko, 2017).
In the waters of Newfoundland and the Barents Sea, sculpin spawning is assumed to be in autumn, since pre-spawning fish were caught in October and post-spawning individuals were captured in November (Ennis, 1968; Wienerroither et al., 2011). Presence of pre-spawning females in our catches in September may indicate that spawning in the Kara Sea occurs approximately in the same period.
In our catches in the Kara Sea, females of the sculpin somewhat prevail (by 1.3 times) over males; this might be due to the low individual fecundity of this species, which ranged from 2385 to 3353 eggs (average 2994). Our results are quite similar to previously published data for some other parts of the species’ range, where fecundity of females with a TL of 117–158 mm ranges from 2 to 4.5 thousand spherical eggs with a diameter of 1.70–2.04 mm, on average 1.92 ± 0.08 mm (Andriashev, 1954; Wienerroither et al., 2011; Dolgov et al., 2018; Yamazaki et al., 2020).
Sexual dimorphism. Data collected in this study on the differences in fin size and coloration in males and females correspond to previously published information regarding various representatives of the genus Gymnocanthus (Tokranov, 1993, 2016; Dolgov et al., 2018).
The existence of sexual dimorphism in the sizes of sculpin was reported for the northern seas of Russia, and the waters of Newfoundland (Ennis, 1968; Andriashev, 1954; Chaus and Karamushko, 2017; Dolgov et al., 2018). Our observations from the Kara Sea supplement existing information.
In the Barents Sea, there is faster growth of females after they reach a length of 70–80 mm (Wienerroither et al., 2011). In the Kara Sea, according to our data, the acceleration of the growth of females in comparison with males occurs somewhat later, with an average TL of 92 mm after the age of 4 years. This is likely due to harsher climatic and/or worse forage conditions of the Kara Sea as compared to the Barents Sea.
Diet composition. The sculpin is a typical benthophagus species that consumes mainly polychaete worms, amphipods, cumacean crustaceans, and bivalve mollusks (Andriashev, 1954; Green, 1983; Atkinson and Percy, 1991, 1992; Chuchukalo, 2006; Karamushko, 2008; Prishchepa, 2008; Dolgov, 2012; Gleason, 2012; Stasko et al., 2016; Gray et al., 2017).
This species inhabits various substrates from mud to gravel and rock (Mecklenburg et al., 2007). In the Kara Sea, the location of its main concentrations coincides with sandy seafloor (Kara Sea…, 2016), as well as with the places of maximum concentrations of polychaetes (Frolova, 2008). In addition, bivalve mollusks predominate in benthic communities in the Baydaratskaya Bay (Atlas..., 2011). Since polychaetes and bivalves form the bulk of the diet of the sculpin in the Kara Sea, the location of its main concentrations in the Baydaratskaya Bay, near the Yamal Peninsula and off southeastern Novaya Zemlya, is most likely due to the feeding behavior.
Sculpin feeding mainly on amphipods, polychaetes and bivalves is also noted in other parts of the range, but isopods as a main dietary component was not previously known. In the Canadian Arctic and the Chukchi and Beaufort Seas, isopod prey is replaced by cumacean crustaceans (Atkinson and Percy, 1991, 1992; Gray et al., 2017). In some areas a significant component in the diet of sculpin is planktonic prey, i.e. copepods, hyperiids, and larvacean Oikopleura vanhoeffeni (Atkinson and Percy, 1991, 1992; Gray et al., 2017). This is explained by its greater mobility near the seabed (Gleason, 2012). We hypothesize this feeding habit is likely due to the limited forage resources in the area. Planktonic organisms have not been found in the diet of the sculpin in the Kara Sea, which may indicate availability of benthic forage resources in the Kara Sea (Orlov et al., 2020d, 2020e) and no need to swim away from the bottom to feed on plankton.
In the Chukchi and the Beaufort seas, the leading role in the diet of individuals of all sizes belongs to polychaetes and amphipods, and only in fish over 76 mm long do shrimp and fish become notably important in the diet (Gray et al., 2017). In the Kara Sea, with the increase in the size of the sculpin, the proportion of polychaetes in the diet decreases, and that of bivalves and isopods, on the contrary, increases. At the same time, the proportion of amphipods is maximal in the diet of smallest and largest individuals. Dietary differences in these areas might be associated with different compositions of benthic fauna.
Sculpin with a TL of up to 90 mm consumed exclusively small bivalve mollusks (2–4 mm), swallowing them whole, while specimens with a TL over 120 mm mainly bit off siphons, legs and mantle fragments from larger mollusks. Pieces of prey found in stomachs could be due to biting parts of prey, or due to digestion process.
Individual sculpin are consumed by seabirds, eared seals, and whales (Finley and Evans, 1983; Elliott et al., 2008; Walkusz et al., 2012). They also are the prey of some fish species, including polar cod Boreogadus saida, Bering flounder Hippoglossoides robustus, walleye pollock Gadus chalcogrammus, and shorthorn sculpin Myoxocephalus scorpius (Atkinson and Percy, 1991, 1992; Gleason, 2012; Gray et al., 2017; our data). G. tricuspis and M. scorpius often inhabit the same biotopes (Mohr et al., 1957; Mecklenburg et al., 2007). It was assumed that they enter into competitive feeding relations (Brand and Fischer, 2016). However, it has been shown (Gray et al., 2017) that the diet composition of these two sculpins differs significantly. If the former consumes mainly bottom polychaetes and amphipods, then the latter’s diet consists mainly of crabs, hyperiids, shrimp, and fish.
Comparison with congeners. The genus Gymnocanthus consists of six valid species (Wilson, 1973; Yamazaki et al., 2013). The Arctic staghorn sculpin is the smallest among its congeners (Esipov, 1952; Tokranov, 1987, 1993, 2016, 2017, 2019; Sheiko, Fedorov, 2000; Mecklenburg et al., 2002; Chuchukalo, 2006; Prishchepa, 2008; Panchenko, 2012; Tokranov and Orlov, 2012; Parin et al., 2014; Tuponogov, Kodolov, 2014; Chaus and Karamushko, 2017; Dolgov et al., 2018). Although its maximum sizes are similar to those of the threaded sculpin G. pistilliger and the intermediate sculpin G. intermedius, there are significant differences in body weight between them (Supplement 6). Along with the intermediate sculpin, G. tricuspis has the shortest lifespan among congeners, with the maximum age of males and females not exceeding 8 and 9 years, respectively. The individual fecundity of this species is almost an order of magnitude (7–12 times) lower than that of other congeners. This species belongs to the group of shallow-water representatives of the genus (Yamazaki et al., 2013, 2020), whose main habitat is shelf waters with depths usually up to 100 m, and more often shallower than 50 m (Fechhelm et al., 2004; Mecklenburg et al., 2007; Lin et al., 2014; Chernova, 2015). This species is a typical benthophagus fish, whose main food in various parts of its range is represented by benthic invertebrates such as polychaete worms, bivalve mollusks, and small crustaceans (mainly amphipods and isopods) (Atkinson and Percy, 1991, 1992; Gray et al., 2017; our data).
CONCLUSION
Since the reduction of the ice cover of Arctic seas due to global warming currently creates conditions for the expansion of fishing and the study of the biology of various fish species, the data we obtained on the distribution and biology of the Arctic staghorn sculpin can be used in the future to assess its role in the trophic chains of Arctic ecosystems. On the other hand, due to its wide distribution in the Arctic and relative high abundance, this species may serve as an indicator of the health of Arctic marine ecosystems that have become quite important during recent years due to increasing human activities (fishing, mining, shipping, tourism, etc.) and continuing climate change.
REFERENCES
Allen, R.L., Method for comparing fish growth curves, N.Z. J. Mar. Freshwat. Res., 1976, vol. 10, pp. 687–692.
Allen, M.J. and Smith, G.B., Atlas and Zoogeography of Common Fishes in the Bering Sea and Northeastern Pacific, US Dept. Commer. NOAA Tech. Rept. NMFS, 1988, vol. 66, pp. 1–151.
Andriyashev, A.P., Opredeliteli po faune SSSR: Tom 53. Ryby severnykh morey SSSR (Keys to the Fauna of the USSR: Volume 53. Fishes of the Northern Seas of the USSR), Leningrad: Izd. Akad. Nauk SSSR, 1954, pp. 1–566.
Andriashev, A.P. and Chernova, N.V., Annotated list of pisciformes and fishes of the seas of the Arctic region and adjacent waters, Vopr. Ikhtiol., 1994, vol. 34, pp. 435–456.
Atkinson, E.G. and Percy, J.A., Stomach content analysis of marine benthic fish from Arctic Canada, Can. Data Rept. Fish. Aquat. Sci., 1991, vol. 840, pp. 1–34.
Atkinson, E.G. and Percy, J.A., Diet comparison among demersal marine fish from the Canadian Arctic, Polar Biol., 1992, vol. 11, pp. 567–573. https://doi.org/10.1007/BF00237950
Atlas biologicheskogo raznoobraziya morey i poberezhiy Rossiyskoy Arktiki (Atlas of Biological Diversity of Seas and Coasts of the Russian Arctic), Spiridonov, V.A., Gavrilo, M.V., Krasnova, E.D., Nikolaeva, N.G., Eds., Moscow: WWF Russia, 2011.
Brand, M. and Fischer, P., Species composition and abundance of the shallow water fish community of Kongsfjorden, Svalbard, Polar Biol., 2016, vol. 39, pp. 2155–2167. https://doi.org/10.1007/s00300-016-2022-y
Chaus, S.A. and Karamushko, O.V., Some biological features of Gymnocanthus tricuspis in south-western area of Kara Sea, Tr. XVI Mezhdunarod. Nauch. Konf. “Problemy Arkticheskogo regiona” (Proc. XVI Internat. Sci. Conf. “Problems of the Arctic Region”), Chernyakov, S.M., Shapovalova, Yu.A., Eds., Murmansk: Poligrafist, 2017, pp. 118–122.
Chernova, N.V., Ichthyofauna of marine waters of Novosibirskie Islands (protected area of the Ust–Lensky Nature Reserve), Nauch. Tr. Gos. Prirod. Zapoved. Prisurskii, 2015, vol. 30, pp 271–276.
Chuchukalo, V.I., Pitaniye i pishchevyye otnosheniya nektona i nektobentosa v dal’nevostochnykh moryakh (Feeding and Feeding Relations of Nekton and Nektobenthos in Far Eastern Seas), Vladivostok: TINRO–Tsentr, 2006.
Chugunova, N.I., Rukovodstvo po izucheniyu vozrasta i rosta ryb (Guide to the Study of The Age and Growth of Fish), Moscow: Acad. Nauk SSSR, 1959.
Cui, X., Grebmeier, J.M., Cooper, L.W., et al., Spatial distributions of groundfish in the northern Bering Sea in relation to environmental variation, Mar. Ecol. Prog. Ser., 2009, vol. 393, pp. 147–160. https://doi.org/10.3354/meps08275
Dolgov, A.V., Composition, formation and trophic structure of the Barents Sea ichthyocene, Doctoral (Biol.) Dissertation, Moscow: Vseross. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2012.
Dolgov, A.V., Annotated list of fish-like vertebrates and fish of the Kara Sea, J. Ichthyol., 2013, vol. 53, pp. 914–922. https://doi.org/10.1134/S0032945213110039
Dolgov, A.V., Novoselov, A.P., Prokhorova, T.A., et al., Atlas-opredelitel’ ryb Karskogo morya (Atlas of the Kara Sea Fish), Murmansk: Polyar. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2018.
Ekologicheskiy atlas. Karskoye more (Kara Sea. Ecological Atlas), Moscow: Arkt. Nauch. Tsentr, 2016.
Ekosistema Karskogo morya (Ecosystem of the Kara Sea), Prishchepa, B.F., Ed., Murmansk: Polyar. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2008.
Elliott, K.H., Woo, K., Gaston, A.J., et al., Seabird foraging behaviour indicates prey type, Mar. Ecol. Prog. Ser., 2008, vol. 354, pp. 289–303. https://doi.org/10.3354/meps07221
Ennis, G.P., Occurrences of the staghorn sculpin (Gymnocanthus tricuspis) in Newfoundland waters, J. Fish. Res. Bd. Canada, 1968, vol. 25, pp. 2729–2731. https://doi.org/10.1139/f68-250
Eriksen, E., Skjoldal, H.R., Gjøsæter, H., and Primicerio, R., Spatial and temporal changes in the Barents Sea pelagic compartment during the recent warming, Progr. Oceanogr., 2017, vol. 151, pp. 206–226. https://doi.org/10.1016/j.pocean.2016.12.009
Esipov, V.K., Ryby Karskogo morya (Fishes of the Kara Sea), Leningrad: Akad. Nauk SSSR, 1952.
Fechhelm, R.G., Craig, P.C., Baker, J.S., and Gallaway, B.J., Fish Distribution and Use of Nearshore Waters in the Northeastern Chukchi Sea, Environmental Assessment of the Alaskan Continental Shelf. U.S, Department of Commerce, NOAA, OCSEAP Final Report, 1984, vol. 32. pp. 121–297.
Finley, K.J. and Evans, C.R., Summer diet of the bearded seal (Erignathus barbatus) in the Canadian High Arctic, Arctic, 1983, vol. 36, pp. 82–89. https://doi.org/10.14430/arctic2246
Forster, C.E., Norcross, B.L., and Spies, I., Documenting growth parameters and age in Arctic fish species in the Chukchi and Beaufort seas, Deep Sea Res. II, 2020, vol. 177, Article 104779. https://doi.org/10.1016/j.dsr2.2020.104779
Fossheim, M., Primicerio, R., Johannesen, E., et al., Recent warming leads to a rapid borealization of fish communities in the Arctic, Nat. Climate Change, 2015, vol. 5, pp. 673–677. https://doi.org/10.1038/nclimate2647
Frolova, E.A., Ecology of polychaet worms (Polychaeta) of the Kara Sea, Cand. Sci. (Biol.) Dissertation, Murmansk: Murmansk Marine Biol. Instit. of Kola Scientific Center, Russ. Acad. Sci., 2008.
Gleason, C.M., Physical environmental and biological correlates of otolith chemistry of Arctic marine fishes in the Chukchi Sea, M.Sci. Thesis, Fairbanks: University of Alaska, 2012.
Gray, B.P., Norcross, B.L., Beaudreau, A.H., et al., Food habits of Arctic staghorn sculpin (Gymnocanthus tricuspis) and shorthorn sculpin (Myoxocephalus scorpius) in the northeastern Chukchi and western Beaufort Seas, Deep Sea Res. II, 2017, vol. 135, pp. 111–123. https://doi.org/10.1016/j.dsr2.2016.05.013
Green, J.M., Observations on the food of marine fishes from Resolute Bay, Cornwallis Island, Northwest Territories, Astarte, 1983, vol. 12, pp. 63–68.
Hammer, Ø., Harper, D.A.T., and Ryan, P.D., PAST: Paleontological statistics software Package for education and data analysis, Palaeontol. Electron., 2001, vol. 4, pp. 1–9.
Hoff, G.R., Biology and ecology of threaded sculpin, Gymnocanthus pistilliger, in the eastern Bering Sea, Fish. Bull., 2000, vol. 98, no. 4, pp. 711–722.
Hopkins, K.D., Reporting fish growth: A review of the basics, J. World Aquacult. Soc., 1992, vol. 23, pp. 173–179. https://doi.org/10.1111/j.1749-7345.1992.tb00766.x
Jakobsson, M., Mayer, L.A., Coakley, B., et al., The international bathymetric chart of the Arctic Ocean (IBCAO) Version 3.0, Geophys. Res. Letters, 2012, vol. 39, Article L12609. https://doi.org/10.1029/2012GL052219
Johannesen, E., Mørk, H.L., Korsbrekke, K., et al., Arctic fishes in the Barents Sea 2004–2015: Changes in abundance and distribution, IMR/PINRO Joint Rept. Ser., 2017, vol. 1. P. 1–50.
Karamushko, O.V., Species composition and structure of the ichthyofaunal of the Barents Sea, J. Ichthyol., 2008, vol. 4, pp. 277–291. https://doi.org/10.1134/S0032945208040012
Knipovich, N.M., Key to fishes of the Barents, White and Kara Seas, Proc. Res. Inst. North Stud., 1926, vol. 27, pp. 1–183.
Laevatsu, T., Manual of Methods in Fishery Biology, FAO Manuals in fisheries science, Rome: FAO, 1965.
Lakin, G.F., Biometriya (Biometry), Moscow: Vysshaya Shkola, 1980.
Lin, L., Chen, Y., Liao, Y., et al., Composition of fish species in the Bering and Chukchi Seas and their responses to changes in the ecological environment, Acta Oceanol. Sinica, 2014, vol. 33, pp. 63–73. https://doi.org/10.1007/s13131-014-0490-x
Livingston, P., Aydin, K., Buckley, T., et al., Quantifying food web interactions in the North Pacific – a data-based approach, Environ. Biol. Fish., 2017, vol. 100, pp. 443–470. https://doi.org/10.1007/s10641-017-0587-0
Longshan, L.I., Yunchih, L.I., Jing, Z.H., et al., Composition and distribution of fish species collected during the fourth Chinese National Arctic Research Expedition in 2010, Adv. Polar Sci., 2012, vol. 23, pp. 116–127. https://doi.org/10.3724/SP.J.1085.2012.00116
Love, M., Mecklenburg, C.W., Mecklenburg, T.A., and Thorsteinson, L.K., Resource Inventory of Marine and Estuarine Fishes of the West Coast and Alaska, OCS Study MMS 2005–030 and USGS/NBII 2005–001, Seattle: US Geol. Survey, Biol. Res. Division, 2005.
Majewski, A.R., Lynn, B.R., Lowdon, M.K., et al., Community composition of demersal marine fishes on the Canadian Beaufort Shelf and at Herschel Island, Yukon Territory, J. Mar. Syst., 2013, vol. 127, pp. 55–64. https://doi.org/10.1016/j.jmarsys.2013.05.012
McConnaughey, T. and McRoy, C.P., Food-web structure and the fractionation of carbon isotopes in the Bering Sea, Mar. Biol., 1979, vol. 53, pp. 257–262. https://doi.org/10.1007/BF00952434
Mecklenburg, C., Mecklenburg, T., and Thorsteinson, L., Fishes of Alaska, Bethesda: Am. Fish. Soc., 2002.
Mecklenburg, C.W., Stein, D.L., Sheiko, B.A., et al., Russian-American long-term census of the Arctic: benthic fishes trawled in the Chukchi Sea and Bering Strait, August 2004, Northwest. Naturalist, 2007, vol. 88, pp. 168–187. https://doi.org/10.1898/1051-1733(2007)88[168:RLCOTA]2.0.CO;2
Mecklenburg, C.W., Byrkjedal, I., Christiansen, J.S., et al., List of marine fishes of the Arctic region annotated with common names and zoogeographic characterizations, Akureyri, Iceland: Conserv. Arctic Flora and Fauna, 2013.
Mecklenburg, C.W., Lynghammar, A., Johannesen, E., et al., Marine fishes of the Arctic region, vol. 1, Akureyri, Iceland: Conserv. Arctic Flora and Fauna, 2018.
Methods for Fish Biology, Schreck, C.B. and Moyle, P.B., Eds., Bethesda: Am. Fish. Soc., 1990.
Metodicheskoye posobiye po izucheniyu pitaniya i pishchevykh otnosheniy ryb v yestestvennykh usloviyakh (Manual for Analysis of Feeding and Food Relations of Fishes in Natural Conditions), Moscow: Nauka, 1974.
Mohr, J.L., Wilimovsky, N.J., and Dawson, E.Y., An Arctic Alaskan kelp bed, Arctic, 1957, vol. 10, pp. 45–52.
Norcross, B.L., Raborn, S.W., Holladay, B.A., et al., Northeastern Chukchi Sea demersal fishes and associated environmental characteristics, 2009–2010, Continent. Shelf Res., 2013, vol. 67, pp. 77–95. https://doi.org/10.1016/j.csr.2013.05.010
Norvillo, G.V. and Zhuravleva, N.G., Early postembryonal development of the Arctic staghorn sculpin Gymnocanthus tricuspis, J. Ichthyol., 1989, vol. 29, pp. 331–333.
Orlov, A.M., Benzik, A.N., Vedishcheva, E.V., et al., Fisheries research in the Chukchi Sea at the RV “Professor Levanidov” in August 2019: Some preliminary results, Tr. Vseross. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2019, vol. 178, pp. 206–220. https://doi.org/10.36038/2307-3497-2019-178-206-220
Orlov, A.M., Rybakov, M.O., Vedishcheva, E.V., Orlova, S.Yu., New data on the ichthyofauna of the four Russian Arctic seas (Chukchi, East Siberian, Laptev and Kara), Mater. XXI mezhdunar. nauch. konf. “Sokhraneniye bioraznoobraziya Kamchatki i prilegayushchikh morey” (Mater. XXI Internat. Sci. Conf. “Conservation of biodiversity of Kamchatka and Coastal Waters”), Petropavlovsk-Kamchatsky: Kamchatpress, 2020a, pp. 298–305.
Orlov, A.M., Benzik, A.N., Vedishcheva, E.V., et al., Preliminary results of fisheries research in the Laptev Sea at RV “Professor Levanidov” in September 2019, Tr. Vseross. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr, 2020b, vol. 179, pp. 206–225. https://doi.org/10.36038/2307-3497-2020-179-206-225
Orlov, A.M., Benzik, A.N., Vedishcheva, E.V. et al., Preliminary results of fisheries research in the East Siberian Sea at the RV “Professor Levanidov” in September 2019, Ibid, 2020c, vol. 179, pp. 187–205. https://doi.org/10.36038/2307-3497-2020-179-187-205
Orlov, A.M., Savin, A.B., Gorbatenko, K.M., et al., Biological studies in the Russian Far Eastern and Arctic seas in the VNIRO Transarctic expedition, Ibid., 2020d, vol. 181, pp. 102–143. https://doi.org/10.36038/2307-3497-2020-181-102-143
Orlov, A.M., Benzik, A.N., Rybakov, M.O., et al., Some preliminary results of biological studies in the Kara Sea at RV “Professor Levanidov” in September 2019, Ibid., 2020e, vol. 182, pp. 199–213. https://doi.org/10.36038/2307-3497-2020-182-199-213
Orlov, A.M., Gorbatenko, K.M., Benzik, A.N., et al., Biological research in the Siberian Arctic Seas in summer–autumn 2019 (cruise of the R/V Professor Levanidov), Oceanology, 2021, vol. 61, pp. 295–296. https://doi.org/10.1134/S0001437021020156
Panchenko, V.V., Growth and age of staghorn sculpins of the genus Gymnocanthus (Cottidae) in Peter the Great Bay and adjacent waters of Primorye (Sea of Japan), J. Ichthyol., 2012, vol. 52, pp. 226–238. https://doi.org/10.1134/S0032945212020117
Parin, N.V., Evseenko, S.A., Vasil’eva, E.D., Ryby morey Rossii: annotirovannyy katalog (Fishes of Russian Seas: Annotated Catalogue), Moscow: KMK Nauch. Pressa, 2014.
Pravdin, I.F., Rukovodstvo po izucheniyu ryb (Guide to the Study of Fish). Moscow: Pishchevaya Promyshlennost’, 1966.
QGIS, Quantum geographic information system, QGIS 3.16 Documentation, 24.1.16.81, Voronoi polygons, 1981, https://docs.qgis.org/3.16/en/docs/user_manual/ processing_algs/qgis/vectorgeometry.html?highlight=voronoi#voronoi–polygons, Version 12/2021.
Sakun, O.F. and Butskaya, N.A., Opredeleniye stadiy zrelosti i analiz polovykh tsiklov ryb (Determination of Gonad Maturity Degree and Analysis of Sexual Cycles of Fishes), Murmansk: Polyar. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 1968.
Schumann, A.H., Thiessen polygon, in Encyclopedia of Hydrology and Water Resources. Encyclopedia of Earth Science Series, Herschy R.W., Fairbridge R.W., Eds., Dordrecht, Boston, London: Kluwer Acad. Publ., 1998.
Semushin, A.V. and Novoselov, A.P., Species composition of ichthyofauna of Baidaratskaya Bay of the Kara Sea, J. Ichthyol., 2009, vol. 49, pp. 362–375. https://doi.org/10.1134/S0032945209050026
Semushin, A.V., Novoselov, A.P., Sherstkov, V.S., et al., Long-term changes in the ichthyofauna of the Pechora Sea in response to ocean warming, Polar Biol., 2019, vol. 42, pp. 1739–1751. https://doi.org/10.1007/s00300-018-2405-3
Sheiko, B.A. and Fedorov, V.V., Class Cephalaspidomorphi – Lampreys. Class Chondrichthyes—Cartilaginous Fishes. Class Holocephali—Chimaeras. Class Osteichthyes—Bony Fishes, in Catalog of vertebrates of Kamchatka and adjacent waters, Petropavlovsk-Kamchatsky: Kamchatskiy Pechatniy Dvor, 2000, pp. 7–69.
Shelekhov, V.A. and Panchenko, V.V., Age and growth of threaded sculpin Gymnocanthus pistilliger (Cottidae) in the southern Primorye (Sea of Japan), J. Ichthyol., 2007, vol. 47, no. 2, pp. 175–183. https://doi.org/10.1134/S0032945207020051
Stasko, A.D., Swanson, H., Majewski, A., et al., Influences of depth and pelagic subsidies on the size–based trophic structure of Beaufort Sea fish communities, Mar. Ecol. Prog. Ser., 2016, vol. 549, pp. 153–166. https://doi.org/10.3354/meps11709
Tokranov, A.M., Reproduction of sculpins of genus Gymnocanthus (Cottidae) in coastal waters of Kamchatka, Vopr. Ikhtiol., 1987, vol. 27, pp. 1026–1030.
Tokranov, A.M., Sexual dimorphism of mass species of sculpins (Cottidae) in waters off Kamchatka, Bull. Mosc. Natur. Soc. Section Biol., 1993, vol. 98, pp. 19–26.
Tokranov, A.M., Sexual dimorphism in cottid fishes (Cottidae, Pisces) in waters off Kamchatka, Tr. mezhdunar. konf. “Sovremennyye problemy ekologii i evolyutsii” (Proc. Internat. Conf. “Modern problems of Ecology and Evolution”), Ulyanovsk: Ulyanovsk Gos. Pedagog. Univ., 2016, pp. 124–131.
Tokranov, A.M., Cottid fishes of the genus Gymnocanthus (Cottidae) of waters off Kamchatka and the problems of their resources use, Mater. VIII Vseross. nauchno-prakt. konf. “Prirodnyye resursy, ikh sovremennoye sostoyaniye, okhrana, rybolovstvo i tekhnicheskoye ispol’zovaniye”. Ch. 1 (Mater. VIII All–Russia Sci.-Pract. Conf. “Natural Resources, Their Contemporary State, Conservation, Fisheries, and Technical Use.” Pt. 1), Petropavlovsk-Kamchatsky: Kamchatka Gos. Tekhn. Univ., 2017, pp. 176–180.
Tokranov, A.M., Trophic groups of the sculpins (Cottidae) in the waters near Kamchatka, Principy Ecologii, 2019, vol. 8, pp. 123–132. https://doi.org/10.15393/j1.art.2019.9662
Tokranov, A.M. and Orlov, A.M., Some biological features of rare and poorly studied sculpins (fam. Cottidae, Hemitripteridae, Psychrolutidae) in the Pacific waters off the northern Kuril Islands and southeastern Kamchatka, Raffles Bull. Zool., 2007, Suppl. 14, pp. 171–182.
Tokranov, A.M. and Orlov, A.M., Specific features of distribution and ecology of two species of armorhead sculpins of the genus Gymnocanthus (Cottidae) in Pacific waters of the northern Kuril Islands and Southeastern Kamchatka, J. Ichthyol., 2012, vol. 52, pp. 599–612. https://doi.org/10.1134/S0032945212060094
Tuponogov, V.N. and Kodolov, L.S., Polevoi opredelitel’ promyslovykh i massovykh vidov ryb. dal’nevostochnykh morei Rossii (Handbook for Identification of the Commercial and Mass Species of Fishes of Far Eastern Seas of Russia), Vladivostok: Russkiy Ostrov, 2014.
Walkusz, W., Williams, W.J., Harwood, L.A., et al., Composition, biomass and energetic content of biota in the vicinity of feeding bowhead whales (Balaena mysticetus) in the Cape Bathurst upwelling region (south eastern Beaufort Sea), Deep Sea Res. I, 2012, vol. 69, pp. 25–35. https://doi.org/10.1016/j.dsr.2012.05.016
Wiedmann, M.A., Aschan, M., Certain, G., et al., Functional diversity of the Barents Sea fish community, Mar. Ecol. Prog. Ser., 2014, vol. 495, pp. 205–218. https://doi.org/10.3354/meps10558
Wienerroither, R., Johannesen, E., Dolgov, A., et al., Atlas of the Barents Sea fishes, IMR/PINRO Joint Rept. Ser., 2011, vol. 1, pp. 1–274.
Wilson, D.E., Revision of the cottid genus Gymnocanthus, with a description of their osteology, M.Sci. Thesis, Vancouver: University of British Columbia, 1973.
Yamazaki, A., Markevich, A., and Munehara, H., Molecular phylogeny and zoogeography of marine sculpins in the genus Gymnocanthus (Teleostei; Cottidae) based on mitochondrial DNA sequences, Mar. Biol., 2013, vol. 160, pp. 2581–2589. https://doi.org/10.1007/s00227-013-2250-4
Yamazaki, A., Ogino, A., and Munehara, H., Dispersion and settlement of two sympatric sculpins of the genus Gymnocanthus, J. Fish Biol., 2020, vol. 96, pp. 1004–1013. https://doi.org/10.1111/jfb.14291
ACKNOWLEDGMENTS
The authors are grateful to their colleagues from VNIRO, PINRO, and TINRO for their help with sample collection at sea during sampling the materials used in this paper. Special thanks to Olga Romanenko (Arctic Science and Policy, Ocean Conservancy, Seattle, USA) and Thomas Van Pelt (University of Washington, Joint Institute for the Study of Atmosphere and Ocean, Seattle, USA) for the editorial assistance with the English language.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
A.M. Tokranov, P.O. Emelin and A.M. Orlov equally contributed to study design, drafting, and revision of the manuscript, and final approval of its published version.
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Tokranov, A.M., Emelin, P.O. & Orlov, A.M. Small but Abundant: Distribution and Biology of Arctic Staghorn Sculpin Gymnocanthus tricuspis (Cottidae) in the Kara Sea. J. Ichthyol. 62, 885–899 (2022). https://doi.org/10.1134/S0032945222050216
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0032945222050216


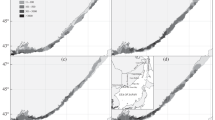





 ) Arctic staghorn sculpin Gymnocanthus tricuspis in the Kara Sea, September 2019.
) Arctic staghorn sculpin Gymnocanthus tricuspis in the Kara Sea, September 2019.
 ) and female (⚪) Arctic staghorn sculpin Gymnocanthus tricuspis.
) and female (⚪) Arctic staghorn sculpin Gymnocanthus tricuspis.
 ) Arctic staghorn sculpin Gymnocanthus tricuspis in different age classes in the Kara Sea, September, 2019; (−) median line. (I) interquartile range, (◻) mean, (◆) outliers.
) Arctic staghorn sculpin Gymnocanthus tricuspis in different age classes in the Kara Sea, September, 2019; (−) median line. (I) interquartile range, (◻) mean, (◆) outliers.