Abstract
The biological characteristics and state of the gonads of mature individuals of Cantherhines pardalis have been studied. Mature sex products were obtained using double hormonal injections. In the prespawning state, females had low values of the gonadosomatic index (3.2%). The ultrastructure of egg envelopes is described. A detailed illustrative morphological description of the early development of C. pardalis from the end of blastulation to stages close to the transition to the larval state is presented. Fertilized eggs are spherical and about 0.51 (0.50–0.53) mm in diameter and have a narrow perivitelline space and a homogeneous, transparent, green-colored yolk. The yolk contains about 20 almost colorless fat droplets with a size of about 0.01 to 0.13 mm. The duration of the incubation period at a temperature of about 25°C is about 33 hours. Prelarvae during hatching are 1.70–1.75 mm long. The appearance of peristalsis and onset of the mobile state of the jaw apparatus and pectoral fins have been recorded at the beginning of the 4th day (76 h) after hatching.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Honeycomb filefish Cantherhines pardalis (Rüppell, 1837) is widespread in the Indo-Pacific (from the Red Sea to South Africa in the west and to South Japan and southeastern Oceania in the east) and in the Eastern Atlantic (Gulf of Guinea, Annobon Island, and southern coast of Africa) (Froese and Pauly, 2021). C. pardalis is one of the 11 species of the genus from the family Monacanthidae, order Tetraodontiformes. This family is taxonomically diverse, includes 27 genera and 108 species, and is the second largest in the order represented by ten families, 105 genera, and 435 species (Fricke et al., 2021).
A significant number of works have been devoted to studying the features of the spawning behavior of representatives of the family. However, data on early ontogeny have been published only for nine species, which are less than one-third of the genera of the family: Thamnaconus modestus (Kitajima et al., 1964, cited by Kawase and Nakazono, 1994a), Stephanolepis cirrhifer (Fujita, 1955, cited by Kawase and Nakazono, 1994a), Aluterus monoceros (Imura et al., 1986, cited by Kawase and Nakazono, 1994a), Oxymonacanthus longirostris (Barlow, 1987), Paramonacanthus japonicus (Nakazono and Kawase, 1993; Kawase and Nakazono, 1994a), Brachaluteres ulvarum (Akagawa et al., 1995), B. jacksonianus (Kawase, 2005), Rudarius ercodes (Kawase and Nakazono, 1994a), and Eubalichthys bucephalus (Kawase, 2008). In some of these publications, data on the early development are limited to describing the details of the structure of the eggs of the studied species. Given the significant size of the family, the available data are insufficient to form a complete picture of the patterns of the process of the early development of representatives of Monacanthidae.
There are no data on the ultrastructure of the egg envelopes in representatives of Monacanthidae and there is no complete information about the early development of representatives of the genus Cantherhines except a brief textual description of one stage of development of C. pardalis embryos from the recorded eggs collected from natural spawning grounds (Kawase and Nakazono, 1994b).
The purpose of this research is to determine the features of the embryo-larval development and reproductive biology of C. pardalis, assess the state of gonads of adult individuals, and study the ultrastructure of the envelopes of mature oocytes.
MATERIALS AND METHODS
The research was carried out in March 2007 at the Primorskii Branch of the Russian–Vietnamese Tropical Center (city of Nha Trang (Vietnam)). Mature individuals of C. pardalis were bought from local fishermen, who caught them from the Nha Trang Bay (South China Sea). The period from the capture to the delivery of the fish to the laboratory was several hours. Fish were kept in cages with a size of 80 × 80 × 100 cm, established in concrete tanks with a volume of 3 m3. The water in each tank was circulated through a biofilter (4–8 m3/h). The water temperature was 25–27°C and salinity was about 33‰ in the tanks.
All the individuals (20 specimens) were injected with surfagon (LH-RH-a) (a synthetic analog of luliberin, the mammalian hypothalamic releasing hormone) as a hormonal preparation stimulating the maturation of sex products. Surfagon injections combined with the eglonil neuroleptic were administered into the body cavity under the pectoral fin twice with an interval of 17 h. The dose of surfagon was 5 μg/kg and dose of eglonil was 5 mg/kg in the first (preliminary) injection; 20 and 15 mg/kg in the second (challenging) injection, respectively. Ovulated oocytes of four females were obtained after 22 h and ovulated oocytes of other three females after 25 h. Artificial insemination was carried out by mixing oocytes with crushed testes of two males and ejaculate produced from one of them. After a few minutes, the resulting mixture was filled with seawater with a salinity of about 33‰ and a temperature of about 25°C.
The biological analysis of the injected fish from which we failed to obtain mature sex products included measurements of the standard (SL) and total (TL) lengths, body weight (W), body weight without internal organs (w), and weight of gonads (g). For histological analysis, ovarian fragments were fixed in Bouin’s mixture. Sections were stained according to Mallory; the material was treated and prepared according to standard recommendations (Roskin and Levinson, 1957). For electron microscopy, ovarian fragments and ovulated oocytes were fixed with a mixture of 2.5% glutaraldehyde solution and 2% paraformaldehyde in phosphate buffer at pH 7.4 with the addition of NaCl at a concentration of 2.5%. Postfixation was carried out in 1% osmium tetroxide solution. The further treatment for scanning electron microscopy (SEM) and transmission electron microscopy (TEM) was performed using conventional methods (Weakley, 1975). Objects for SEM were sputtered with gold and palladium alloy and examined in a CamScan S-4 scanning electron microscope (Cambridge Instruments, Great Britain) at an accelerating voltage of 20 kV. Ultrathin sections were viewed using a JEM-1011 transmission electron microscope (Jeol, Japan) at an accelerating voltage of 80 kV. Images were obtained using an ES-500W digital camera with the Digital Micrograph software (Gatan, United States). Oocytes were measured based on their images using the ImageJ software (Wayne Rasband (NIH), United States).
Fertilized eggs were incubated and prelarvae and larvae were maintained in natural sea water with a salinity of about 33‰ and at a temperature of 25 ± 1°C. The studied live material was placed in glass cylindrical vessels with a volume of about 250 mL and a bottom diameter of about 70 mm, which were 50–60% filled with water and half-submerged in a thermostatically controlled container with water during the study period. The stocking density was about 30–50 eggs or prelarvae per vessel. During egg incubation, water was renewed once every 6–8 hours. Prelarvae were transplanted into a vessel with clean water during the first 1–2 h after hatching and the same operation was repeated every subsequent day.
The description of stages of the development of C. pardalis was started 5 h after insemination. Eggs from three females were used in observations of the early development. Material for describing the early ontogeny of C. pardalis was collected by the method of intravital observation. Live embryos and larvae were drawn using a Carl Zeiss drawing tube (Germany). The age of stages is given in minutes and hours. Any moment of development with specific morphological characteristics that distinguish it from the other moments of development is assumed to be a development stage in this research. The results of the study by Lentz and Trinkaus (1967) served as guidelines for determining the stage of blastulation. The moment of directed individual migrations of deep cells, leading to the formation of a germ ring and an embryonic disk, was taken as the onset of gastrulation (Ballard, 1973a, 1973b). The development periods are differentiated according to Rass (1946).
RESULTS
Biological Parameters and State of Gonads of Mature Individuals
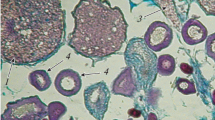
Females from which we failed to obtain ovulated oocytes had ovaries at maturity stages IV–V and low values of the gonadosomatic index (GSI); the other parameters were lower than the respective values for males (Table 1). There were very few oocytes at the maturity stage. Ovaries also contained oocytes of different phases of the pre- and vitellogenic periods. There were lipid droplets in cells that entered the vitellogenic period and had a size of 70–145 μm. They were distributed mainly in the perinuclear cytoplasm in small oocytes, while they were much wider and occupied a larger volume in larger oocytes (Fig. 1a). Oocytes that were in the process of active yolk accumulation had a size of 166–190 µm and oocytes completing the stage of vitellogenesis had a size of 224–289 µm. The diameter of oocytes of the maturity period with first signs of cytoplasm homogenization and fusion of yolk granules and lipid-containing vacuoles was 298–350 μm.
Fragment of an ovary with oocytes in the process of accumulation of lipid inclusions (TL 145 mm, gonad maturity stages IV–V, GSI 3.6%, Mallory stain) (a); ultrastructure of the egg envelopes (chorion surface) of Cantherhines pardalis (b) and surface of an ovulated oocyte (circular folds and formations resembling pores are visible in the micropyle region) (c); cross-section of the egg envelope (g): (1) inner layer of the radiative envelope, (2) outer layer, (3) chorion. Scale, µm: (a) 30 (light microscopy); (b), (c) 3 (scanning electron microscopy); (d) 2 (transmission electron microscopy).
The gonads of four of the five studied males were at maturity stage IV; one male had gonads at maturity stages IV–V. When fragments of testicles at stage IV were placed in water, spermatozoa released from them remained immobile and ejaculate was not formed. The ejaculate of a more mature male contained spermatozoa that were activated by water and retained their mobility for about 30 s.
Ultrastructure of the Envelopes of Ovulated Oocytes
Scanning electron microscopy. The surface of the chorion of an ovulated oocyte is slightly folded (Fig. 1b). The opening of the micropyle is about 3 μm in diameter; its outer edge is represented by a weakly pronounced circular fold about 1 μm wide. Another similar formation adjoins it outside. The electron diffraction pattern shows that the micropyle canal is noticeably widened in the upper part and narrows towards the middle part to about 2 µm. The micropyle is surrounded by formations with a radius of about 3–5 µm, which resemble pores and are absent in the other parts of the oocyte surface (Fig. 1c).
Transmission electron microscopy. Egg envelopes are represented by a radiative envelope (zona radiata) and chorion. The radiata zone is about 2.5 µm thick and consists of an outer layer (about 0.6 µm thick) and an inner layer (about 1.9 µm thick). On the electron diffraction pattern of the transverse section of the egg envelope (Fig. 1d), the outer layer is shown as much more electron-dense and almost homogeneous, while the inner layer as a layer with a distinct structure in the form of alternating bands (six bands with a high electron density and seven with a lower one).
Chorion is 1.3 µm thick. Its homogeneous layer with a very low electron density has irregular rectangular regions on the inner side, which differ in a significantly higher electron density. They are 0.5–0.6 µm from each other.
Embryonic Period of Development
The eggs of C. pardalis have a spherical shape. Egg envelopes are transparent; the outer envelope (chorion) is sticky and thick, with an unstructured outer surface, and has scattering optical properties. The yolk is transparent, homogeneous, with a well-defined greenish tint, containing about 20 almost colorless (with a barely noticeable yellowish-pink tint) fat droplets of different sizes (three–four large droplets with a diameter up to about 0.13 mm and several smaller ones (with a diameter up to about 0.01 mm). The perivitelline space is narrow. The average diameter of the eggs after termination of the cortical reaction and beginning of the process of plasma aggregation at the animal pole was 0.51 ± 0.0024 mm (0.50–0.53 mm, n = 16). Chorion loses its sticky properties a few hours after fertilization. Fertilized eggs adhere to each other and to the substrate. After separation, chorion retains traces of deformation in the contact points, which affect the optical properties of the egg envelopes, thereby making it difficult to study the fine details of the structure of the embryos.
Age 5 h 20 min (Fig. 2a). Completion of the blastula stage and onset of gastrulation. The base of the blastodisk is even and flat; the differentiated yolk syncytium does not protrude beyond the epithelialized cover layer. During the mechanical destruction of the blastodisk, the deep cells of most of the eggs are easily dispersed, in contrast to the other cells with a much higher mutual adhesiveness.
Embryonic development of Cantherhines pardalis from the completion of blastulation to the beginning of separation of the tail bud: (a) late blastula, onset of transition to gastrulation, age about 5 h 20 min; (b) gastrulation; (c) onset of epiboly; (d) periderm epiboly, about 35–40%; (e) periderm epiboly, about 60%, formation of an axial rudiment; (f) same, top view of the anterior part of the axial rudiment; (g) periderm epiboly, about 75%; (h) completion of epiboly, three–four pairs of somites; (i) same, top view of the middle and posterior part of the developing body of the embryo; (j) five–six pairs of somites, the remainder of the germ ring is preserved; (k) ten–11 pairs of somites, beginning of the isolation of the tail bud, maximum development of Kupffer’s vesicle; (l) 14–15 pairs of somites, beginning of the reduction of Kupffer’s vesicle. Here and in Fig. 3, the scale is 0.5 mm.
Age 5 h 40 min. The base of the germinal disk began to bend inward. The top of this elevation is displaced from the center to the ventral sector, which indicates the beginning of centrifugal and convergent migrations of the hypoblast cell population to the periphery and dorsal sector of the germinal disk. The yolk syncytium layer protrudes beyond the blastodisk in the form of a very narrow strip.
Age 6 h 00 min (Fig. 2b). The elevation of the base of the germinal disk, displaced to the ventral sector, became more pronounced. The band of the part of the yolk syncytium, protruding onto the yolk, became wider.
Age 7 h 20 min (Fig. 2c). The cellular material continues to be redistributed within the germinal disk. A germ ring and a germinal disk were clearly formed. Marginal cells differentiated in the cover layer. Onset of periderm epiboly. Twenty-five–30% of the yolk surface is covered by the germinal disk. The edge of syncytium incrustation completely coincides with the boundary of the distribution of the cover layer.
Age 8 h 00 min (Fig. 2d). Thirty-five–40% of the yolk surface is covered by the cellular material of the embryo. The top of the embryonic shield is approximately in the center of the germinal disk.
Age 9 h 00 min (Fig. 2e, 2f). Organogeneses. Periderm epiboly is about 60%. The cellular material in the zone of formation of the anterior and middle parts of the axial rudiment noticeably pushes the yolk underneath. Two gently sloping longitudinal ridges were formed on the surface of its anterior part, which corresponded to the lateral thickenings of the emerging central nervous system.
Age 10 h 30 min (Fig. 2g). About 75% of the yolk surface is covered by the periderm. Most of the cellular material shifted to the area of embryo body formation. The axial rudiment pushes the yolk throughout its length. A large number of convergently migrating cells are observed around the head region and along the developing embryo body. In the anterior region, they create a narrow envelope that is transformed into a caudally expanding line of cellular material actively migrating towards the axial rudiment. The ventral part of the germ ring became noticeably thinner. The dorsal and ventral relief of the head region of the embryo is determined by the differentiation of three primary parts of the brain: the anterior, middle, and posterior parts.
Age 12 h 10 min (Figs. 2h, 2i). End of the epiboly stage; the entire surface of the yolk is covered by the periderm. The germ ring closed, thereby forming a circular fold of cellular material at the animal pole around the point of contact of cells of the cover layer with a deep funnel-shaped depression in the center. The structures of the axial complex are still differentiated. A chord rudiment was established. There are a posterior primary part of the brain and a spinal cord directly above the visually differentiated region of the chord rudiment. Optical placodes were differentiated. The chord rudiment and parts of the central nervous system directly above the rudiment are covered by aggregates of the lateral mesoderm, which reach the greatest height and differentiation in the proximal regions. Three to four pairs of somites differentiated in the embryo body. Kupffer’s vesicle appeared under the posterior region, slightly cranial to the point of closure of the germ ring.
Age 13 h 40 min (Fig. 2j). Five or six pairs of somites differentiated in the embryo body. The posterior region preserved a remainder of the germ ring material, which had not yet become part of the embryo body. Optic placodes differentiated in the middle part of the medulla oblongata. In some embryos, cavities began to form in these placodes. Olfactory placodes were visually differentiated.
Age 17 h 00 min (Fig. 2k). The embryo body contains ten to 11 pairs of somites. The cellular material of the remainder of the germ ring was completely included in the embryo body. The tail bud began to be isolated. Kupffer’s vesicle reached its maximum size.
Age 19 h 30 min (Fig. 2l). The embryo body contained 14–15 pairs of somites; three to four anterior somites began to acquire a chevron-like shape. The size of Kupffer’s vesicle began to decrease in most of the embryos. Optic placodes began to be formed in optic cups.
Age 21 h 00 min. Onset of the mobile state. Trunk muscles began to make hardly noticeable jerks. Sixteen to 17 muscle segments differentiated in the embryo body. Optic placodes became rounded. Kupffer’s vesicle was completely reduced in all embryos.
Age 23 h 30 min (Fig. 3a). The embryo body contains 19–20 muscle segments. The movements of the body muscles became more complicated; a series of smooth jerks, accompanied by weak lateral deviations of the caudal region, is observed approximately once per minute. Hatching glands appeared on the surface of the head region and anterior part of the trunk region. Each of them looks like a dense cluster of small bubble-like formations. A few melanophores of a very pale gray color appeared on different parts of the body surface and yolk sac. The number of fat droplets decreased, while large ones became significantly larger.
Completion of the embryonic development and beginning of the larval development of Cantherhines pardalis: (a) mobile embryo, 19–20 muscle segments; hatching glands were differentiated; (b) embryo about 3 h before hatching; (c) prelarva with TL 1.70–1.75 mm 2–3 h after hatching; (d) prelarva with TL 2.3–2.4 mm, the appearance of peristalsis, beginning of the mobile state of the jaw apparatus and pectoral fins.
Age 29 h 30 min (Fig. 3b). The intensity of movements of the trunk muscles increased insignificantly; however, their pattern became more complex. Hatching glands became more visible. The pattern of pigmentation did not change. The yolk sac of the vast majority of embryos contains only one large fat droplet (occasionally two droplets, one large droplet and one noticeably smaller droplet).
Age 31 h 35 min. Onset of hatching, first cases. About 30% of embryos hatched at the age of 32 h 00 min and more than 80% at the age of 33 h 00 min.
Larval Development Period (Pre-larval Sub-period)
Age 34 h 30 min (2–3 h after hatching (a.h.)) (Fig. 3c). The length of prelarvae (TL) is 1.70–1.75 mm. There are 24–25 segments in the body: seven–eight trunk segments and 17–18 caudal segments. The yolk sac of the vast majority of prelarvae contains only one fat droplet with a diameter of about 0.186 mm. The moderately developed hydrosinus covers the dorsolateral areas of the posterior part of the head and trunk regions and anterior part of the caudal region. A few dark gray or black melanophores are present on the anterior part of the head region, on the anterior, dorsal, and ventral parts of the yolk sac, and on the dorsal side of the posterior part of the caudal region; there is also a distinctly formed unpaired subcaudal row.
In the digestive tract, a region slightly expanded in the cranial direction is differentiated as a pharynx. Other regions were not visually differentiated. The excretory ducts of the kidneys merge in the poorly differentiated bladder. Prelarvae are inactive, have a weak positive buoyancy, are positioned under the water surface with a fat droplet upwards, and periodically float 3–5 cm straight or in a circle, followed by a long dormant period.
Age 76 h a.h. (Fig. 3d). TL 2.3–2.4 mm. The body consists of 22–23 segments: four–five trunk segments and 17–19 caudal segments. All prelarvae have only one fat droplet with a diameter of about 0.099 mm in the yolk sac. It is surrounded by the remainder of yolk colored in green. Hydrosinus became more pronounced, covering the dorsolateral areas of the head and trunk regions and anterior part of the caudal region. Only black melanophores are involved in pigmentation. The inferolateral rows include six–eight strongly branched cells and the unpaired subcaudal row consists of 12–15 cells and originates on the 4th–6th segment of the caudal region. There are three to five melanophores on the yolk sac near the inferolateral rows and six to nine on its ventral side.
Pharynx is transformed into the esophagus that is directed downward and passes into the stomach. Stomach is represented by a larger section of the digestive tract with an expanded lumen. In the lower part of the intestinal cavity, it bends upward and passes into the short intestine. Weak waves of peristalsis are observed. The jaw apparatus and pectoral fins periodically slightly tremble.
The buoyancy is neutral or weakly positive. Most of the time, prelarvae are in the water column upside down in the suspended state, periodically (every 10–20 s) floating aside or upwards.
DISCUSSION
Many representatives of the family Monacanthidae are characterized by a long spawning period with multiple spawning events, during which females have low GSI values. In particular, the GSI is 2–7% for S. hispida females, which reproduce from May to October (Mancera-Rodrıguez and Castro-Hernandez, 2015) and 8–12% for Meuschenia scaber females with a spawning period from August to December (Visconti et al., 2018). The studied females of C. pardalis had low GSI values and the composition of their sex cells at maturity stages IV–V was close to that described for M. scaber (Visconti et al., 2018). This suggests that C. pardalis also has multiple spawning.
The exceptional capability of spermatozoa in ejaculate for activation was also revealed in other fish species, in particular, from the family Mullidae. In addition, it was shown that spermatozoa isolated from testis fragments were also well activated in representatives of the families Pomacentridae and Acanthuridae (Emel’yanova and Pavlov, 2012; Pavlov and Emel’yanova, 2016). The short period of sperm motility is possibly determined by the fact that the period of natural spawning of sex products in C. pardalis is 2–3 months; after this period, the couple immediately leave the spawning ground (Kawase and Nakazono, 1994b).
At the light level of examination, the surface of C. pardalis eggs looks smooth and unstructured. However, SEM images show the structure of the outer side of the eggs in the form of many shallow folds. The size and depth of the elements of this relief are probably determined by the heterogeneity of the structure in the depth of the chorion layer, reflected in the electron diffraction pattern. This is confirmed by the fact that the size of these structures and space between them roughly correspond to the size characteristics of the folds on the surface.
Ultrastructural studies of the transverse sections of the egg envelope of C. pardalis using TEM showed two clearly defined layers in the radiative envelope: the outer, more electron-dense layer and inner, less dense layer. This separation is most common for bony fish eggs. At the same time, other variants are also known: single-layered structure (Sohn et al., 2016; Kim, 2020), three-layered structure (Mikodina, 1980; Schindler and Vries, 1989), and structure with more layers (Kudo et al., 1988). However, there is no evidence that these characteristics are clearly related to the reproduction characteristics of the species.
The structure of the outer part of the micropyle of C. pardalis and region of the envelope around it does not have clearly defined morphological features, such as those described for other representatives of Tetraodontiformes from the family Ostraciidae (Leis and Moyer, 1985).
The fertilized and developing eggs of C. pardalis have significant similarities with the eggs of other representatives of Monacanthidae and differ from them only in some details. They have a small size (similarly to other representatives of the family) and are the smallest among the so far studied species (Table 2). The existing literature data do not provide sufficiently detailed descriptions of the embryogenesis of species of the family; however, the indirect signs suggest a significant similarity between the ratio of the rates of the process of morphological differentiation of the embryo and process of epiboly of the cover layer within this group. Prelarvae of C. pardalis emerge from the egg envelopes at approximately the same level of morphological development as prelarvae of other species of the family. All the representatives of Monacanthidae during hatching are small, but the studied species is the smallest among them.
As in all other studied species, the yolk of the eggs of C. pardalis contains several fat droplets. However, they differ in their number and size (the minimum and maximum sizes).
Kawase and Nakazono (1994b) studied the developing eggs of C. pardalis at the age of about 25 h. The number and size of fat droplets recorded by them at this stage corresponded to that observed in our study at approximately the same age. During the embryogenesis of C. pardalis, the fat droplets gradually merged and their average size increased and number decreased; by the onset of hatching, the vast majority of embryos had only one fat droplet in the yolk sac. A similar process was also observed in the development of other Monacanthidae species: O. longirostris (Barlow, 1987), B. ulvarum (Akagawa et al., 1995), R. ercodes, and P. japonicus (Kawase and Nakazono, 1994b). Kawase and Nakazono (1994b) did not record the green color of the yolk of eggs of C. pardalis; this may be due to the fact that they studied the object after preliminary fixation with a formaldehyde solution. The size of C. pardalis eggs according to the study by Kawase and Nakazono (0.53 mm) was slightly larger than our measurements (0.51 mm). Among the studied representatives of the family, the green color of the yolk was recorded only in O. longirostris (Barlow, 1987).
The embryonic period in C. pardalis is one of the shortest periods among representatives of Monacanthidae. Its duration is only slightly longer than that in P. japonicus (Kawase and Nakazono, 1994a), almost the same as that determined for R. ercodes (Nakamura, 1942, cited by Akagawa et al., 1995), and slightly shorter than that described for T. modestus (Kitajima et al., 1964, cited by Kawase and Nakazono, 1994a). The value of this parameter is approximately 1.5 times higher for the other species; it is more than 3 times higher in B. ulvarum (Akagawa et al., 1995).
The prelarvae of C. pardalis are pelagic, which is quite consistent with the prevailing viewpoint that presumably all representatives of Balistoidei have demersal eggs and pelagic larvae (Aboussouan and Leis, 1984). Prelarvae of C. pardalis acquire embryonic features soon after hatching, which is usually more typical for pelagophils. They retain a rather large yolk sac, their digestive system is in the embryonic state, their circulatory system does not function, and they are inactive.
Representatives of Tetraodontiformes have the smallest number of vertebrae among all fishes (Brainerd and Patek, 1998); in particular, representatives of Monacanthidae most often have 19 vertebrae (usually 7 + 12, sometimes 6 + 13), and, less frequently, a larger number (up to 30: (7–8) + (13–23)) (Hutchins, 1977; Tyler, 1980). Data on the vertebral formula for C. pardalis are absent in the literature; however, according to the studies of a significant number of other species of this genus (Hutchins and Randall, 1982), this indicator is 19–20 (7 + (12–13)). With account of these data, it is of interest that the body of C. pardalis prelarvae during hatching has 24–25 ((7–8) + (17–18) muscle segments and their number increases to 22–23 ((4–5) + (17–19) three days after hatching. In most of the studied species, the individual initially has the maximum number of trunk segments after hatching, which gradually decreases and becomes close to the definitive number. In the observed case, there are fewer trunk segments in prelarvae than the number of vertebrae in this region in adult individuals. The body of newly hatched prelarvae of P. japonicas and R. ercodes (Kawase and Nakazono, 1994a) has 23 segments; only five of them are trunk segments, which suggests a different pattern of change in the segmental formula in the early ontogeny of Monacanthidae.
Melanophores with the beginning of the formation of melanin were first recorded at the late stages of embryonic development. The pigmentation of the prelarvae of C. pardalis is nonintensive; the pattern of distribution of pigment cells differs from all variants described for Monacanthidae in the literature; it significantly varies in representatives of different genera. The reproductive biology of C. pardalis and other species of the family has certain features. Two main reproductive types prevail among representatives of the fauna of coral reefs: demersal spawning with parental care and pelagic spawning without parental care (Thresher, 1984). However, Barlow (1987) believes that demersal eggs are much less common for these ecosystems. All representatives of Balistoidei, include the families Monacanthidae and Balistidae (Nelson et al., 2016), are most likely demersal (Aboussouan and Leis, 1984); many or most of them show care for their progeny (Kawase, 2002). The eggs of demersal species are usually larger and contain more nutrients; the larvae of most of these species hatch in a morphologically more advanced state and are larger than those of fish species with pelagic spawning, while it is usually the opposite in pelagophilic individuals (Thresher, 1984; Barlow, 1987; Leis et al., 2013). Contrary to expectations, C. pardalis, like the other studied representatives of Monacanthidae, has eggs with a size similar to that in the smallest of the mass pelagophilic species (Ahlstrom and Moser, 1980), and its larvae are small and underdeveloped during hatching.
The results of the study showed that there were differences in certain details of the embryo-larval development of C. pardalis against the background of a significant general similarity with other studied representatives of Monacanthidae. The resulting data supplemented the knowledge of the early period of life of Monacanthidae; however, there is currently too little information on the development of representatives of Monacanthidae for a deeper comparative analysis of the features of the early development within this rather large family.
REFERENCES
Aboussouan, A. and Leis, J.M., Balistoidei: development, in Ontogeny and Systematics of Fishes, Lawrence: Allen Press, 1984, pp. 450–459.
Ahlstrom, E.H. and Moser, H.G., Characters useful in identification of pelagic marine fish eggs, Calif. Coop. Oceanic Fish. Invest. Rep., 1980, vol. 21, pp. 121–131.
Akagawa, I., Tsukamoto, Y., and Okiyama, M., Sexual dimorphism and pair spawning into a sponge by the filefish, Brachaluteres ulvarum, with a description of the eggs and larvae, Jpn. J. Ichthyol., 1995, vol. 41, no. 4, pp. 397–407. https://doi.org/10.11369/jji1950.41.397
Ballard, W.W., A new fate map for Salmo gairdneri, J. Exp. Zool., 1973a, vol. 184, no. 1, pp. 49–73. https://doi.org/10.1002/jez.1401840105
Ballard, W.W., morphogenetic movements in Salmo gairdneri Richardson, J. Exp. Zool., 1973b, vol. 184, no. 1, pp. 27–48. https://doi.org/10.1002/jez.1401840104
Barlow, G.W., Spawning, eggs and larvae of the longnose file fish Oxymonacanthus longirostris, a monogamous coralivore, Env. Biol. Fishes, 1987, vol. 20, no. 3, pp. 183–194. https://doi.org/10.1007/BF00004953
Brainerd, E.L. and Patek, S.N., Vertebral column morphology, C-start curvature, and the evolution of mechanical defenses in tetraodontiform fishes, Copeia, 1998, vol. 1998, no. 4, pp. 971–984. https://doi.org/10.2307/1447344
Emel’yanova, N.G. and Pavlov, D.A., From Oocyte to Larva: Hormonal Induction of Oocyte Maturation and Initial Development of Coral Reef Fishes, Moscow: KMK Sci. Press, 2012.
Eschmeyer’s Catalog of Fishes: Genera, Species, References, Version 02/2021, Fricke, R., Eschmeyer, W.N., and van der Laan, R., Eds., 2021. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp.
FishBase. World Wide Web Electronic Publication Version 02/2021, Froese, R. and Pauly, D., Eds., 2021. http://www.fishbase.org.
Hutchins, J.B., Descriptions of three new genera and eight new species of monacanthid fishes from Australia, Rec. West. Aust. Mus., 1977, vol. 5, no. 1, pp. 3–58.
Hutchins, J.B. and Randall, J.E., Cantherines longicaudus, a new filefish from Oceania, with a review of the species of the C. fronticinctus complex, Pac. Sci., 1982, vol. 36, no. 2, pp. 175–185.
Kawase, H., Simplicity and diversity in the reproductive ecology of triggerfish (balistidae) and filefish (Monacanthidae), Fish. Sci., 2002, vol. 68, no. Sup1., pp. 119–122. https://doi.org/10.2331/fishsci.68.sup1_119
Kawase, H., Spawning behaviour of the pygmy leatherjacket Brachaluteres jacksonianus (Monacanthidae) in south-eastern Australia, Ichthyol. Res., 2005, vol. 52, no. 2, pp. 194–197. https://doi.org/10.1007/s10228-005-0272-8
Kawase, H., Reproductive ecology of the black reef leatherjacket, Eubalichthys bucephalus (Monacanthidae) in temperate Australia, Ichthyol. Res., 2008, vol. 55, no. 3, pp. 294–298. https://doi.org/10.1007/s10228-007-0028-8
Kawase, H. and Nakazono, A., Embryonic and pre-larval development and otolith increments in two filefishes, Rudarius ercodes and Paramonacanthus japonicus (Monacanthidae), Jpn. J. Ichthyol., 1994a, vol. 41, no. 1, pp. 57–63. https://doi.org/10.11369/jji1950.41.57
Kawase, H. and Nakazono, A., Reproductive behavior of the honeycomb leatherjacket, Cantherhines pardalis (Monacanthidae), at Kashiwajima, Japan, Jpn. J. Ichthyol., 1994b, vol. 41, no. 1, pp. 80–83. https://doi.org/10.11369/jji1950.41.80
Kim, D.H., Ultrastructure of the fertilized egg envelopes in Ancistrus cirrhosus, Loricariidae, Teleostei, Appl. Microsc., 2020, vol. 50, Article 13. https://doi.org/10.1186/s42649-020-00034-7
Kudo, S., Sato, A., and Inoue, M., Chorionic peroxidase activity in the eggs of the fish Tribolodon hakonensis, J. Exp. Zool., 1988, vol. 245, no. 1, pp. 63–70. https://doi.org/10.1002/jez.1402450110
Leis, J.M. and Moyer, J.T., Development of eggs, larvae and pelagic juveniles of three Indo-Pacific ostraciid fishes (Tetraodontiformes): Ostracion meleagris, Lactoria fornasini and L. diaphana, Jpn. J. Ichthyol., 1985, vol. 32, no. 2, pp. 189–202. https://doi.org/10.11369/jji1950.32.189
Leis, J.M., Caselle, J.E., Bradbury, I.R., et al., Does fish larval dispersal differ between high and low latitudes?, Proc. R. Soc. B, 2013, vol. 280, no. 1759, Article 20130327. https://doi.org/10.1098/rspb.2013.0327
Lentz, T.L. and Trinkaus, J.P., A fine structural study of cytodifferentiation during cleavage, blastula, and gastrula stages of Fundulus heteroclitus, J. Cell Biol., 1967, vol. 32, no. 1, pp. 121–138. https://doi.org/10.1083/jcb.32.1.121
Mancera-Rodriguez, N.J. and Castro-Hernandez, J.J., Reproductive biology of the planehead filefish Stephanolepis hispidus (Pisces: Monacanthidae), in the Canary Islands area, 2015, Ichthyol. Res., vol. 62, no. 3, pp. 258–267. https://doi.org/10.1007/s10228-014-0435-6
Mikodina, E.V., Structure of the egg membranes of mature eggs of some bony fish, Ontogenez, 1980, no. 1, pp. 101–106.
Nakazono, A. and Kawase, H., Spawning and biparental egg-care in a temperate filefish, Paramonacanthus japonicus (Monacanthidae), Environ. Biol. Fishes, 1993, vol. 37, no. 3, pp. 245–256. https://doi.org/10.1007/BF00004632
Nelson, J.S., Grande, T.C., and Wilson, M.V.H., Fishes of the World, Hoboken: John Wiley and Sons, 2016. https://doi.org/10.1002/9781119174844
Pavlov, D.A. and Emel’yanova N.G., Reproductive features of Upeneus margarethae (Mullidae), a species recorded in the coastal zone of Vietnam for the first time, J. Ichthyol., 2016, vol. 56, no. 4, pp. 600–612. https://doi.org/10.1134/S0032945216040093
Rass, T.S., Stages of ontogeny of bony fishes, Zool. Zh., 1946, vol. 46, no. 2, pp. 137–149.
Roskin, G.I. and Levinson, L.B., Mikroskopicheskaya tekhnika (Microscopic Equipment), Moscow: Sov. Nauka, 1957.
Schindler, J.F. and de Vries, U., Polarized distribution of binding sites for concanavalin A and wheat-germ agglutinin in the zona pellucida of goodeid oocytes (teleostei), Histochemistry, 1989, vol. 91, no. 5, pp. 413–417. https://doi.org/10.1007/BF00493828
Sohn, J.H., Kwon, O., and Kim, D.H., Ultrastructure of the fertilized egg envelopes from Pseudobagrus fulvidraco, Bagridae, Teleostei, Appl. Microsc., 2016, vol. 46, no. 3, pp. 150–154. https://doi.org/10.9729/AM.2016.46.3.150
Thresher, R.E., Reproduction in Reef Fishes, Neptune City: T.F.H. Pubns., 1984.
Tyler, J.C., Osteology, phylogeny, and higher classification of the fishes of the order Plectognathi (Tetraodontiformes), in NOAA Tech. Rept. NMFS Circ., vol. 434, Washington, DC: NOAA, 1980.
Weekley, B., Elektronnaya mikroskopiya dlya nachinayushchikh (Electron Microscopy for Beginners), Moscow: Mir, 1975. [Russian translation].
Visconti, V., Trip, E.D.L., Griffiths, M.H., and Clements, K.D., Reproductive biology of the leatherjacket, Meuschenia scaber (Monacanthidae) (Forster 1801) in the Hauraki Gulf, New Zealand, N. Z. J. Mar. Freshwat Res., 2018, vol. 52, no. 1, pp. 82–99. https://doi.org/10.1080/00288330.2017.1331919
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Zabolotny
Rights and permissions
About this article
Cite this article
Shadrin, A.M., Emel’yanova, N.G. Embryo-larval Development and Some Data on the Reproductive Biology of Cantherhines pardalis (Monacanthidae) from the South China Sea (Central Vietnam). J. Ichthyol. 62, 932–942 (2022). https://doi.org/10.1134/S0032945222050150
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0032945222050150