Abstract
The embryonic and larval development of hatchery-reared silver therapon Leiopotherapon plumbeus are described to provide essential information on the early life history of this species. Egg size, larval size at hatching, yolk resorption rate, onset of feeding and development of some morphological characters were examined. Fertilized eggs (430–610 µm in diameter) were spherical, yellowish, demersal and slightly adhesive. First cleavage occurred 6 min post-fertilization and embryos hatched 21–24 h post-fertilization under ambient temperature of 27.5 ± 0.1 °C. Newly hatched larvae [1.79 ± 0.04 mm in total length (TL)] with yolk volume of 0.579 ± 0.126 mm3 had no functional or pigmented eyes, mouth or digestive tract. The eyes became fully pigmented and mouth opened [31 and 36.5 hours post-hatching (hph)] shortly before yolk resorption at 39 hph and when larvae had grown to 2.65 ± 0.14 mm in TL. Some morphological characters such as total length, pre-anal length and eye diameter decreased following yolk resorption, which also coincided with the development of foraging capacities shortly before exogenous feeding was initiated. L. plumbeus larvae initiated exogenous feeding at 54 hph, indicating a short (15 h after yolk resorption) transitional feeding period. Larval growth at the early stages of development (54–72 hph) was rapid and steadily increased from 288 to 720 hph, when larvae, 12.05 ± 4.02 mm in TL, closely resembled the external characteristics of their adult conspecifics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies on the early life history stages of fishes are indispensable (Meijide and Guerrero 2000), considering the nature and influence of several inherent and external factors on survival and growth of larvae (Blaxter and Hempel 1963; Bagarinao 1986). In particular, yolk and oil quantity and resorption rates, mouth development, time of onset of feeding and trophic requirements are of primary interest to ensure larval survival (Hunter 1981). However, smaller fish larvae with less endogenous yolk reserves are generally exposed to possible starvation-induced mortality (Blaxter and Hempel 1963; Bagarinao 1986). Food deprivation, particularly during changeover of nutrient sources, could result in morphological deformity and thus lead to low feeding ability and poor growth and survival (McGurk 1984; Iguchi and Mizuno 1999; Peňa and Dumas 2005; Shan et al. 2009).
The silver therapon Leiopotherapon plumbeus (Perciformes: Terapontidae) is an important freshwater food fish widely caught in the Philippines (Mane 1934; Yapchiongco and Enriquez 1963; Kock et al. 2000; Palma et al. 2002). A native species of Laguna Lake on southern Luzon Island, Philippines, this species can reproduce year round, with peaks during the onset of the rainy season (March to May), and attains sexual maturity even at a total length of less than 15 cm (Mane 1934). It feeds on zooplankton, benthic crustaceans, smaller fish and aquatic insects (Mane 1934; Delmendo 1968; Kock et al. 2000). This species comprised the artisanal and subsistence fishery in lakeside communities. However, intense fishing pressure of wild stocks (Palma et al. 2002) coupled with the introduction of invasive alien species such as clown knife fish (Chitala ornata) in Laguna Lake and adjacent waters (Cagauan 2007) has led to an increased interest in the domestication and conservation of this species. Reviving the production of this species may help increase the local fish biodiversity; secure food fish supply and provide a sustainable source of livelihood and income in rural areas. Although hormone-induced spawning and description on the feeding traits of hatchery-reared larvae of this species have been reported (Aya et al. 2015; Garcia LMB, unpubl. data), aspects of the early life history of this particular species have not been determined.
This study examines the embryonic development, yolk resorption rate, onset of feeding and several morphological characteristics of larval silver therapon L. plumbeus to provide basic early life history information on this important food fish species.
Materials and methods
Spawning and egg incubation. Induced spawning of captive broodstock of Leiopotherapon plumbeus was conducted on 11 July 2014 at the hatchery of Binangonan Freshwater Station of Southeast Asian Fisheries Development Center, Aquaculture Department (SEAFDEC/AQD). Two females [total length (TL) (mean ± standard deviation): 105.0 ± 7.1 mm; total body weight (TBW): 22.9 ± 4.7 g)] and four males (TL: 101.5 ± 9.3 mm; TBW: 17.6 ± 4.1 g) were injected intramuscularly once with 50 IU human chorionic gonadotropin (hCG; Ningbo Sansheng Pharmaceutical, Ningbo, China) hormone per gram body weight. After injection, they were transferred to a plastic container filled with tap water to recover, after which broodstock were released in 60-l polyethylene tanks under ambient temperature of 28 °C for spawning at a sex ratio of 1 female:2 males per tank, provided with aeration and covered with a fine-meshed net. Spawning occurred 29 h post-injection at 26.3 ± 0.1 °C. After spawning, the fertilized eggs were incubated and allowed to hatch in three mildly aerated 10-l round plastic basins at an estimated density of 1,287 eggs/l (fertilization rate ca. 89 %). Temperature during egg incubation was 26.1–27.5 °C. Hatching occurred approximately 21–24 h post-fertilization at 27.5 ± 0.1 °C, with a rate of ca. 60 %.
Larval rearing. Newly hatched larvae [4 hours post-hatching (hph); 1.79 ± 0.11 mm TL, n = 10] were reared in three 4 m3 outdoor concrete tanks (4 m length × 1 m width × 1 m depth) for 720 h at an initial density of 0.5 ind/l at ambient water temperature of 25.9–30.9 °C and dissolved oxygen of 4.19–11.85 mg/l. Five days before stocking of larvae, the tanks were filled with freshwater up to 50 cm depth and fertilized and cultured microalgae (Chlorella sp.) were added every two days as a water conditioner and food for rotifers. Tanks were fertilized with ammonium phosphate (16–20–0) at 1 g/m3 and chicken dung at 14 g/m3 every four or five days thereafter to accelerate phytoplankton growth.
The water management and feeding scheme is described in Fig. 1. Starting on day 0 (= the day of hatching), rotifers (Brachionus sp.) were added at increasing densities (0.1–1 ind/ml) in each tank once every 24 h until 240 h. From 240 h, rotifers were gradually replaced by Moina sp. and offered at densities of 2–5 ind/ml once at 24 h interval until 720 h. Water was not changed throughout the rearing period. However, water loss through evaporation was replaced every week to maintain the water level in the tank. Tanks were provided with mild aeration to maintain acceptable levels of water quality. pH and total ammonia nitrogen (TAN) were 9.2–9.4 and 0.01–0.14 mg/l, respectively, during the trial period.
Sample collection. To describe the embryonic development of L. plumbeus, 10 fertilized eggs from each plastic basin were sampled using a glass pipette every 3–5 min to determine the exact time of the first cleavage, then 15–30 min until blastula stage and then 30 min to 1 h until hatching. Developing embryos were observed under a compound microscope with a calibrated ocular micrometer (to the nearest 0.01 mm) and digitally photographed.
Starting from hatching, ten larvae were collected at random from each plastic basin at different intervals and immediately preserved in 5 % buffered formalin for measurements of morphometric characters (in mm): total length (TL), body depth (BD), head length (HL), eye diameter (ED), pre-anal length (PAL) and upper jaw length (UJL). These morphometric characters were measured under a dissecting microscope with a calibrated ocular micrometer (to the nearest 0.01 mm). TL was taken from the tip of the snout to the posterior margin of the caudal fin. Larval mouth size was estimated as the product of the upper jaw length and √2 (Shirota 1970). Additional ten larval samples were collected at 4.5, 31 and 53.5 hph until 96 hph, and paraffin sections were made and stained with eosin and hematoxylin. Larvae samples preserved in 70 % ethanol were first embedded in 1 % agarose and then dehydrated in increasing concentrations of ethanol, then transferred into xylene and finally embedded in paraffin. Serial sections (4 μm thick) were made with a microtome and stained with eosin and hematoxylin. The periods of the following morphological and behavioral events were also documented: (1) time of hatching; (2) eye formation and pigmentation; (3) mouth formation and opening; (4) yolk and oil globule utilization; and (5) onset of feeding.
To determine yolk resorption rates, newly hatched larvae were stocked in triplicate 10-l capacity glass aquaria at 20 ind/l and yolk and oil globule volume were estimated for larvae (n = 10 for each interval) collected at 1–4 h intervals from hatching until complete resorption of yolk sac occurred. Yolk volume was estimated using the equation for a prolate spheroid (Riley et al. 2009): yolk volume (mm3) = 4/3 π (yolk-sac length) × (yolk-sac depth)2. Oil globule volume was computed using the formula for a sphere: oil globule volume (mm3) = 4/3 π (oil globule diameter/2)3.
For the starvation experiment, newly hatched larvae were stocked at similar density (ca. 80 larvae per aquaria) in triplicate 10-l capacity glass aquaria. Larvae were monitored after transfer to experimental glass aquaria and mortality was recorded every 2–4 h, except for the four sampling periods, until all larvae died.
To examine the onset of feeding and growth of L. plumbeus, seven larvae were sampled daily from three locations in each tank in the morning, one hour after prey were supplied (0900 hours) starting from 24 to 120 hph and then every 48 or 120 h thereafter until larvae became 720 hph. TL and UJL of larvae were measured under a dissecting microscope with a calibrated ocular micrometer (to the nearest 0.01 mm) or a digital caliper, and specimens were digitally photographed. Developmental stages of larvae followed that of Leis and Carson-Ewart (2000). The gut of sampled larvae stored in 5 % buffered formalin solution was dissected on Petri dishes, the entire gut contents were examined with a dissecting microscope and the food items were identified. Feeding incidence (number of larvae with food in the gut) was determined to ascertain the timing of the onset of feeding. Final survival was determined by counting the total number of larvae harvested from each tank at 720 hph.
Results
Embryonic development. The fertilized eggs of Leiopotherapon plumbeus were demersal, spherical, yellowish and slightly adhesive. Egg size ranged from 430 to 610 µm in diameter (Fig. 2a). The embryonic development of L. plumbeus was completed 21–24 hours post-fertilization (hpf) at 27.5 ± 0.1 °C. The first cleavage divides the blastodisc into two blastomeres of nearly equal size 6 min post-fertilization (mpf) (Fig. 2b). The four-cell and eight-cell stages were reached 17 and 28 mpf, respectively (Fig. 2c, d). The sixteen-cell stage was obvious at 41 mpf (Fig. 2e) and the number of cells doubled (32-cell stage) at 58 mpf. The 64-cell stage was attained in the next 14 min (Fig. 2g). The blastula stage was visualized between 1.5 and 4.8 hpf (Fig. 2h, i), while the gastrula stage was completed 8 hpf (Fig. 2j–m). The first somites were observed between 8 and 11 hpf (Fig. 2n). Myomeres were evident and differentiated, and the first heart beat was observed at 12 hpf (Fig. 2o, p). In the 14-somite stage, the head and the tail ends were noticeable, optic vesicles were discernible and the growing embryo occupied the entire perivitelline space (Fig. 2q, r). The newly hatched larvae emerged at 21–24 hpf (Fig. 2s).
Stages of embryonic development of silver therapon Leiopotherapon plumbeus. a Blastodisc; b 2-cell (6 minutes post-fertilization, mpf); c 4-cell (17 mpf); d 8-cell (28 mpf); e 16-cell (41 mpf); f 64-cell (74 mpf); g early blastula (1.5 hours post-fertilization, hpf); h late blastula (2.8 hpf); i epiboly (4.8 hpf); j gastrulation (5 hpf); k 75 % epiboly (6.4 hpf); l 90 % epiboly (7.7 hpf); m segmentation (8.2 hpf); n 3–5 somite (11 hpf); o 10-somite (12 hpf with the heart (ht) beating; p lateral view of 10-somite stage; q 14-somite (18 hpf) showing the head (h) and tail (t); r pre-hatching larvae (20.5 hpf); s newly hatched larvae at 21–24 hpf. Scale bar = 0.1 mm
Yolk resorption and larval development. At the hatching stage, L. plumbeus larvae measured 1.79 ± 0.04 mm in TL (range: 1.66–1.97 mm) with yolk volume of 0.579 ± 0.126 mm3 (range: 0.490–0.668 mm3) and single oil globule of 0.003 ± 0.001 mm3 (range: 0.0020–0.0032 mm3) (Fig. 3a, b). The yolk reserves and oil globule reduced rapidly by 19.5 hours post-hatching (hph) to about 10 % and 7 %, respectively, when larvae grew to 2.54 ± 0.12 mm in TL (range: 2.34–2.68 mm). About 2 % yolk still remained in some larvae having 2.61 ± 0.10 mm TL (range: 2.47–2.70 mm), while yolk reserves disappeared and oil globule remained at 0.1 × 10−3 mm3 (range: 0.0–0.2 × 10−3 mm3) in larvae with 2.65 ± 0.14 mm TL (range: 2.50–2.78 mm) at 39 hph. The oil globule became fully resorbed at 56 hph.
TL initially increased reaching 2.68 ± 0.09 mm (range: 2.50–2.81 mm) at the time of yolk resorption, and growth leveled off thereafter (Fig. 4a). ED and PAL of unfed larvae increased initially, followed by a gradual decrease (Fig. 4b, c). HL and BD both increased gradually, reaching 0.517 ± 0.009 mm (range: 0.494–0.520 mm) at 84 hph and 0.294 ± 0.021 mm (range: 0.260–0.312 mm) at 81 hph (Fig. 4d, e). Larvae started to die at 53.5 hph, with 40 % mortality at 81 hph, and all died at 102 hph (Fig. 4f).
Newly hatched larvae remained passive in the water column showing regular upside down and jerky movement. The yolk sac of the newly hatched larva is located at the ventral region attached to the head and had primordial anus already visible between the yolk and tail region; orbit pigmentation started to develop at 6 hph (Figs. 5a, b, 6a). Head development was noted at 9.5 hph, otic capsules were defined and anus opened (Fig. 5b). At the time when the yolk was rapidly consumed by 19.5 hph, eye pigmentation and pectoral fin buds were first observed (Fig. 5c). At the time of full eye pigmentation (31 hph), some larvae already changed their swimming activity (from vertical to horizontal direction) concurrently with mouth and digestive tract formation (Figs. 5d, 6b, c).
Early development of larval silver therapon Leiopotherapon plumbeus. a Newly hatched larvae at 4.5 hours post-hatching (hph); 1.79 mm TL; PA primordial anus; YS yolk sac; OG oil globule); b 9.5 hph (2.19 mm TL; OP orbit pigmentation; CD cranial development); c 29 hph (2.37 mm TL; PF pectoral fin development; EP eye pigmentation); d 31 hph (2.54 mm TL; MF start of mouth formation; DTF digestive tract formation; FP full orbit pigmentation); e 36.5 hph (2.57 mm TL; MO mouth opening; GF gut formation; MY myomeres; ME melanophores); f 53.5 hph (2.61 mm TL; FM functional mouth parts; WA well-developed anus; HTB head–thoracic boundary; SB swim bladder); g 83 hph (2.63 mm TL; HME higher occurrence of melanophores; WMY well-defined myomeres). Scale bar = 0.1 mm
Mouth opening was observed at 36.5 hph, and gut, myomeres and melanophores were relatively noticeable (Fig. 5e). At 53.5 hph, functional upper and lower jaws were observed, anus was fully opened, brain lobes and swim bladder were visible and head–thoracic boundary was well defined (Fig. 5f). At this stage, histological sections of the larvae confirmed that yolk reserves were already resorbed and intestine was coiled, but liver and pancreas were not well developed yet (Fig. 6d). The mouth gape of unfed larvae was 202 ± 78 µm or about 8 % of TL. At this period when the upper and lower jaws were functional, some larvae demonstrated jerking motion, indicating search for available food. At 83 hph, myomeres were well defined, while melanophores occurred in higher numbers (Fig. 5g) and the kidney was prominent.
Exogenous feeding and early growth. Larvae started to feed exogenously at 54 hph. About 57–71 % of larvae had food items in the gut at 54 hph (2.83 ± 0.11 mm in TL) and this fraction increased to 100 % by 168 hph. However, a more irregular trend in feeding incidence was observed thereafter (Fig. 7a). In addition to cultured prey (Brachionus sp. and Moina sp.) which were the main food resource for L. plumbeus larvae, wild zooplankton prey were also ingested and observed in the gut. Rotifers ranged from 68 to 132 µm in lorica length, which corresponds to 19–45 % of mouth gape size. The amount of rotifers ingested by the larvae increased almost tenfold from the onset of feeding (2.1 ± 1.9 ind/larvae) up to 168 hph (18.7 ± 11.4 ind/larvae). As the larvae grew, rotifer abundance in the gut decreased at 288 hph, where larger prey such as cladocerans, which were offered 240 hph, became more frequent in the gut (3.3–4.6 ind/larvae). Likewise, insect larvae (Chironomid sp.) in the gut were recorded for the first time at 288 hph (Fig. 7b). The amount of insect larvae in the gut increased more rapidly thereafter to reach an average of 27.4 ind/larvae by 552 hph and remained high until 720 hph. The larvae started ingesting adult copepods at 552 hph.
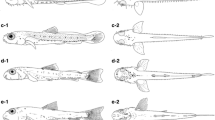
Mean (± standard deviation) TL of L. plumbeus larvae during the rearing period ranged from 2.42 (± 0.17) mm to 12.05 (± 4.02) mm (Figs. 7c, 8). The mouth size of L. plumbeus larvae at 54 hph was estimated at 8 % of TL and increased to 13 % of TL at 720 hph (Fig. 7d). Larval growth was rapid at the early stages of development (54–72 hph). At the time of initial feeding on Moina sp. at 288 hph, larvae measured 5.11 ± 0.50 mm in TL and growth steadily increased from 552 up to 720 hph when larvae, 8.66 ± 0.93 mm in TL, preyed intensely on insect larvae. Some larvae began transforming to early juvenile stage with full complementation of fin rays and scales (Fig. 8k). Survival rates varied from 12.0 to 26.5 % and production of 0.08–0.19 fish/l among rearing tanks. The morphological development and associated behavioral events are summarized in Fig. 9.
Hatchery-reared larval and juvenile silver therapon Leiopotherapon plumbeus from 24 to 720 hours post-hatching (hph). a Yolk sac larva, 24 hph (2.42 mm TL); b preflexion larva, 54 hph (2.83 mm TL); c preflexion larva, 72 hph (3.31 mm TL); d preflexion larva, 96 hph (3.50 mm TL); e preflexion larva, 120 hph (3.87 mm TL); f preflexion larva, 168 hph (3.97 mm TL); g flexion larva, 288 hph (5.11 mm TL); h flexion larva, 360 hph (6.07 mm TL); i postflexion larva, 432 hph (7.42 mm TL); j postflexion larva, 552 hph (9.57 mm TL); k juvenile, 720 hph (12.05 mm TL). Scale bar = 0.5 mm (a–e); 1 mm (f–k)
Discussion
The embryonic development of Leiopotherapon plumbeus was rapid (Fig. 2) and followed a meroblastic pattern similar to that of other described freshwater species (Arockiaraj et al. 2003; Osman et al. 2008). The latency and incubation periods of L. plumbeus eggs [29 h and 21–24 h post-fertilization (hpf), respectively] were shorter compared to those of Australian silver perch Bidyanus bidyanus (35.7–41 hpf; Rowland 1984; Levavi-Sivan et al. 2004) and other percid species (Zarski et al. 2011), due to higher temperature (26.3 °C, this study vs. 21–24 °C in other studies). However, eggs of L. plumbeus developing at 28 °C require 24 h incubation and 18 h to hatch (Aya FA, unpubl. data), indicating that development is temperature dependent. In terms of fecundity, L. plumbeus produced between 5,000 and 7,500 eggs/female in one spawning event. The eggs of L. plumbeus were small (430–610 µm) compared with other freshwater and marine fish species reared in the laboratory (Table 1). There is a direct correlation between the size of eggs and the length of larvae at hatching (Blaxter and Hempel 1963; Bagarinao 1986), except for Laotian carp Hypsibarbus malcolmi (see Ogata et al. 2010).
The size of newly hatched L. plumbeus larvae is similar to rabbitfish Siganus guttatus (see Avila and Juario 1987; Juario et al. 1985), climbing perch Anabas testudineus (see Morioka et al. 2009), and Laotian carp Hypsibarbus malcolmi (see Ogata et al. 2010), but smaller than percid (Zarski et al. 2011) and other cyprinids (Yi et al. 2006) (Table 1). The small size of L. plumbeus larvae may require less metabolic reserves in terms of amount of yolk present. Moreover, newly hatched L. plumbeus larvae have poorly developed structures such as non-functional or pigmented eyes, mouth or digestive tract (Fig. 2), which are critical features for first feeding and may therefore affect larval survival (Kiørboe et al. 1985; Bagarinao 1986; Mookerji and Rao 1999).
Yolk resorption time (39 hph) is earlier in L. plumbeus compared to other tropical freshwater and marine fish species (Fig. 3; Table 1). Yolk resorption rate may be influenced by the level of activity or motor performance of the larvae (Kiørboe et al. 1985; Mookerji and Rao 1999). Indeed, larvae of L. plumbeus (31 hph) showed increased swimming ability suggesting early attempts at foraging behavior. During development, L. plumbeus showed a rapid increase in TL concomitant with fast yolk utilization rates like in other marine fish larvae (Lim et al. 1985; Mihelakakis et al. 2001; Moteki et al. 2001; Williams et al. 2004). However, this observation was followed by some degree of tissue resorption (Williams et al. 2004), as indicated by a reduction in TL, PAL and ED, a few hours after the exhaustion of yolk reserves (Fig. 4). While the decrease in growth may indicate some nutritional deficiency (Mookerji and Rao 1999; Moteki et al. 2001) due to a short (15 h after yolk resorption) transitional phase to exogenous feeding, it appears that energy is channeled to the continued development of other body parts such as head and jaws, and increase in body depth, aside from increased motor activity. Yolk resorption also coincided with the development of foraging capacities (i.e., digestive tract, mouth and eyes) shortly before exogenous feeding was initiated (Figs. 5, 6).
Leiopotherapon plumbeus larvae begin to feed exogenously at 54 hph, shortly after yolk resorption, full eye pigmentation and appearance of functional mouth parts (Figs. 7, 9). Active or increased swimming and foraging behavior may also explain the earlier initiation of active visual feeding in the present and in other studies as well (Dou et al. 2005; Peňa and Dumas 2005). However, the absence of a preparatory or mixed feeding period in L. plumbeus larvae may critically influence their survival at first feeding. Indeed, following Kamler’s (1992) estimation of the R ratio (R = T y/T f, T y is the time from yolk exhaustion to first feeding, and T f is the time from hatching to first feeding), L. plumbeus larvae have a very short mixed feeding period (R = 0.28). This suggests that L. plumbeus larvae were sensitive to starvation at the onset of exogenous feeding. Moreover, the small size of L. plumbeus larvae is a disadvantage under conditions of patchy distribution of food, and therefore gives little time for the larvae to search food and escape starvation-induced mortality at first feeding (Kiørboe et al. 1985).
Leiopotherapon plumbeus larvae are difficult to rear during the early larval stages due to its small mouth size requiring suitable food items such as small-sized rotifers. In this study, rearing success was confirmed by the mean survival of 18 % at 720 hph, better than the previous estimates for the same species (Aya et al. 2015). While copepod nauplii are the preferred food items in a previous work (Aya et al. 2015), they are very difficult to maintain and mass produce in the hatchery. Thus, the production of small-sized food items such as rotifers must be considered an essential part of larval feeding techniques (Riley et al. 2009). Likewise, the provision of rotifers must be done as early as 48 h before first feeding to meet the trophic requirements of the fish larvae and to obtain good growth during culture. Indeed, growth was rapid during the initial periods of active feeding due in part to the abundance of cultured and wild prey inside the rearing tank. However, L. plumbeus had poor ability for initial feeding as evidenced by low abundance of ingested rotifers in the guts (Fig. 7b). The shift in feed preference from rotifers to cladocerans beginning at 288 hph resulted further in increased growth rate of L. plumbeus larvae. At this stage, the feeding success rate was higher for cladocerans than for rotifers due to its high protein and energy content (Evangelista et al. 2005) and increased mouth gape size of L. plumbeus larvae. However, the increase in TL at 720 hph suggests that the fish larvae may have just started to enter the exponential growth phase.
This study provides basic early-life history information on L. plumbeus useful in further advancing larval rearing techniques for this species. Larvae are characterized by a small mouth gape and body size, poor endogenous reserves, fast yolk resorption rates and poor ability at first feeding, which would make them sensitive to food deprivation. Further studies on the screening and testing of suitable live food for early stage L. plumbeus larvae to improve larval rearing success are warranted by the significant results of this study.
References
Arockiaraj A, Haniffa M, Seetharaman S, Singh S (2003) Early development of a threatened freshwater catfish Mystus montanus (Jerdon). Acta Zool Taiwan 14:23–32
Avila EM, Juario JV (1987) Yolk and oil globule utilization and developmental morphology of the digestive tract epithelium in larval rabbitfish (Siganus guttatus B.). Aquaculture 65:319–331
Aya FA, Corpuz MN, Garcia LMB (2015) Diet composition, feed preferences and mouth morphology of early stage silver therapon (Leiopotherapon plumbeus, Kner 1864) larvae reared in outdoor tanks. J Appl Ichthyol 31:77–82
Bagarinao T (1986) Yolk resorption, onset of feeding and survival potential of larvae of three tropical marine fish species reared in the hatchery. Mar Biol 91:449–459
Blaxter JHS, Hempel G (1963) The influence of egg size on herring larvae (Clupea harengus L.). J Cons Int Eplor Mer 28:211–244
Cagauan AG (2007) Exotic aquatic species introduction in the Philippines for aquaculture – a threat to biodiversity or a boom to the economy? J Environ Sci Manage 10:48–62
Delmendo MN (1968) Food and feeding habits of the economic species of fish in Laguna de Bay. Proc Indo-Pacific Fish Counc 13:143–161
Dou S, Masuda R, Tanaka M, Tsukamoto K (2005) Effects of temperature and delayed initial feeding on the survival and growth of Japanese flounder larvae. J Fish Biol 66:362–377
Evangelista AD, Fortes NR, Santiago CB (2005) Comparison of some live organisms and artificial diet as feed for Asian catfish Clarias macrocephalus (Günther) larvae. J Appl Ichthyol 21:437–443
Hunter JR (1981) Feeding ecology and predation of marine fish larvae. In: Lasker R (ed) Marine fish larvae. Washington Sea Grant Program, Seattle, pp 34–77
Iguchi K, Mizuno N (1999) Early starvation limits survival in amphidromous fishes. J Fish Biol 54:705–712
Juario JV, Duray MN, Duray VM, Nacario JF, Almendras JME (1985) Breeding and larval rearing of the rabbitfish, Siganus guttatus (Bloch). Aquaculture 44:91–101
Kamler E (1992) Early life history of fish: an energetics approach. Chapman & Hall, London
Kiørboe T, Munk P, Støttrup JS (1985) First feeding by larval herring Clupea harengus L. Dana 5:95–107
Kock M, Focken U, Richter H, Becker K, Santiago CB (2000) Feeding ecology of silver perch, Terapon plumbeus Kner, and the impact of fish-pens in Laguna de Bay, Philippines. J Appl Ichthyol 16:240–246
Leis JM, Carson-Ewart BM (2000) The Larvae of Indo-Pacific Coastal Fishes: An identification guide to marine fish larvae. Fauna Malesiana Vol 2. Leiden, Boston; Koln: Brill
Levavi-Sivan B, Vaiman R, Sachs O, Tzchori I (2004) Spawning induction and hormonal levels during final oocyte maturation in the silver perch (Bidyanus bidyanus). Aquaculture 229:419–431
Lim LC, Cheong L, Lee HB, Heng HH (1985) Induced breeding studies of the John’s snapper, Lutjanus johni (Bloch), in Singapore. Singap J Prim Ind 13:70–83
Mane AM (1934) Spawning and feeding habits of Mesopristes plumbea (Kner), a common theraponid in Laguna de Bay. Phil Agric 6:502–515
McGurk MD (1984) Effects of delayed feeding and temperature on the age of irreversible starvation and on the rates of growth and mortality of Pacific herring larvae. Mar Biol 84:13–26
Meijide FJ, Guerrero GA (2000) Embryonic and larval development of a substrate-brooding cichlid Cichlasoma dimerus (Heckel, 1840) under laboratory conditions. J Zool 252:481–493
Mihelakakis A, Yoshimatsu T, Tsolkas C (2001) Spawning in captivity and early life history of cultured red porgy, Pagrus pagrus. Aquaculture 199:333–352
Mookerji N, Rao TR (1999) Rates of yolk utilization and effects of delayed initial feeding in the larvae of the freshwater fishes rohu and singhi. Aqua Int 7:45–56
Morioka S, Ito S, Kitamura S, Vongvichith B (2009) Growth and morphological development of laboratory-reared larval and juvenile climbing perch Anabas testudineus. Ichthyol Res 56:162–171
Moteki S, Yoseda K, Sahin T, Üstündağ C, Kohno H (2001) Transition from endogenous to exogenous nutritional resources in larval Black Sea turbot Psetta maxima. Fish Sci 67:571–578
Ogata Y, Morioka S, Sano K (2010) Growth and morphological development of laboratory-reared larval and juveniles of the Laotian indigenous cyprinid Hypsibarbus malcolmi. Ichthyol Res 57:389–397
Ordonio-Aguilar R, Kohno H, Ohno A, Moteki M, Taki Y (1995) Development of grouper, Epinephelus coioides, larvae during changeover of energy sources. J Tokyo Univ Fish 82:103–108
Osman AGM, Wiertz S, Mekkawy IA,Verreth J, Kirschbaum F (2008) Early development of the African catfish Clarias gariepinus focusing on the ontogeny of selected organs. J Appl Ichthyol 24:187–195
Palma AL, Diamante AS, Pol RM (2002) An assessment of fishery resources of Laguna de Bay. Aquat Ecosyst Health 5:121–128
Peňa R, Dumas S (2005) Effect of delayed first feeding on development and feeding ability of Paralabrax maculatofasciatus larvae. J Fish Biol 67:640–651
Riley KL, Weirich CR, Cerino D (2009) Development and growth of hatchery-reared larval Florida pompano (Trachinotus carolinus). Fish Bull 107:318–328
Rowland SJ (1984) The hormone-induced spawning of silver perch Bidyanus bidyanus (Mitchell) (Teraponidae). Aquaculture 42:83–86
Shan XJ, Cao L, Huang W, Dou SZ (2009) Feeding, morphological changes and allometric growth during starvation in miiuy croaker larvae. Environ Biol Fish 86:121–130
Shirota A (1970) Studies on the mouth size of fish larvae. Bull Jpn Soc Sci Fish 36:353–368
Williams K, Papanikos N, Phelps RP, Shardo JD (2004) Development, growth, and yolk utilization of hatchery-reared red snapper Lutjanus campechanus larvae. Mar Ecol Prog Ser 275:231–239
Yapchiongco JV, Enriquez GL (1963) Notes on certain aspects in the biology of Therapon plumbeus (Kner). Phil Jour Sci 92:265–289
Yi B, Liang Z, Yu Z, Lin R, He M (2006) A study of the early development of grass carp, black carp, silver carp and bighead carp in the Yangtze River, China. In: Chapman DC (ed) Early development of four cyprinids native to Yangtze River. US Geological Survey, Reston, pp 15–51
Żarski D, Palinska K, Targońska K (2011) Oocyte quality indicators in Eurasian perch, Perca fluviatilis L., during reproduction under controlled conditions. Aquaculture 313:84–91
Acknowledgements
The study was funded by the SEAFDEC Aquaculture Department (Study Codes Nr-04-F2010B and Nr-01-F2013B) and UP Natural Sciences Research Institute (Project Codes BIO-09-2-06 and BIO-13-2-04). Part of this work was conducted at the Faculty of Fisheries Sciences, Hokkaido University, Hakodate, Japan, with support from the Japan Society for the Promotion of Science through its “Invitation for East Asian Young Researchers” Program. Comments of the four anonymous reviewers improved an earlier version of this manuscript. The authors are grateful to MN Santos, MN Corpuz and NB Olorvida for assistance during the experiment.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Aya, F.A., Nillasca, V.S.N., Garcia, L.M.B. et al. Embryonic and larval development of hatchery-reared silver therapon Leiopotherapon plumbeus (Perciformes: Terapontidae). Ichthyol Res 63, 121–131 (2016). https://doi.org/10.1007/s10228-015-0481-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-015-0481-8