Abstract—The “cooling” method based on the Newton–Richmann law is used to measure specific heat capacity. It is shown that, as the manganese concentration in aluminum—magnesium alloys and temperature increase, the specific heat capacity of the alloys increases; as the scandium concentration increases, the heat capacity slightly decreases. As the temperature increases, the specific enthalpy and entropy of the alloys increase, whereas the specific Gibbs energy decreases. Scandium additions almost do not affect changes in thermodynamic functions of the alloys.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Aluminum—magnesium alloys are widely used in industry. A wide group of industrial alloys, such as of type AMg1, AMg2, AMg3, AMg4, and AMg6 are classified among the Al–Mg alloys. As the magnesium concentration in magnalium alloys increases, the hardness and fatigue strength increase, whereas the plasticity decreases. These alloys are characterized by high plasticity, adequate weldability, and high corrosion resistance.
To improve the properties of magnalium alloys, they are alloyed with various components. It should also be noted that the development of new compositions of Al-based alloys with the given characteristics is possible when data on the thermodynamic properties of each of the components comprising the system are available. The modification of aluminum alloys with metals, particularly rare-earth metals, which are poorly soluble or almost insoluble in solid aluminum and form various chemical compounds with aluminum, is promising [1–7].
The study of heat capacity is one of the main methods used for investigating structural and phase transformations in alloys. The temperature dependence of heat capacity allows one to determine other physical characteristics of a solid, such as the temperature and the kind of phase transformation, Debye temperature, vacancy formation energy, electronic heat capacity coefficient, etc.
THEORY AND EXPERIMENTAL
The Newton-Richmann law of cooling is used to measure the specific heat capacity of alloys over a wide temperature range. Any body that has a temperature higher than that of the environment will cool and the cooling rate will depend on the heat capacity of the body. If we take two metallic samples with the same shape and cool them from the same temperature, the measured cooling curves allow one to determine the heat capacity of one of the samples if the heat capacity of the other sample (standard) is available.
The amount of heat that is lost by a metal volume dV for time dτ is equal to

where  is the specific heat capacity of metal; ρ is the density of metal; and Т is the temperature of the sample (which is taken to be the same for all points of the sample since the linear sizes of the sample are small and its thermal conductivity is high).
is the specific heat capacity of metal; ρ is the density of metal; and Т is the temperature of the sample (which is taken to be the same for all points of the sample since the linear sizes of the sample are small and its thermal conductivity is high).
The value of \(\delta Q\) can be calculated by law:

where dS is the surface area; T0 is the ambient temperature; and α is the heat-transfer coefficient.
Equating Eqs. (1) and (2), we obtain

Assuming that  ρ, and \(\frac{{dT}}{{d\tau }}\) are independent of coordinates of points of the sample volume and α, Т, and Т0 are independent of coordinates of points of the sample surface, we can write
ρ, and \(\frac{{dT}}{{d\tau }}\) are independent of coordinates of points of the sample volume and α, Т, and Т0 are independent of coordinates of points of the sample surface, we can write

or

where V is the volume of the whole of the sample, ρV = m is the mass; and S is the whole surface area of the sample. In assuming that S1 = S2, T1 = T2, and α1 = α2, Eq. (5) for two samples of the same size can be represented as:

where m1= ρ1V1 is the mass of the first sample; m2= ρ2V2 is the mass of the second sample; and \({{\left( {\frac{{dT}}{{d\tau }}} \right)}_{1}},{{\left( {\frac{{dT}}{{d\tau }}} \right)}_{2}}\) are the cooling rates of standard and studied sample at a given temperature.
Therefore, if the masses of samples m1 and m2, the cooling rates of standard and studied sample, and specific heat capacity of standard \(C_{{{{P}_{1}}}}^{0}\) are available, the specific heat capacity  of the studied sample can be calculated using the right side of Eq. (6).
of the studied sample can be calculated using the right side of Eq. (6).
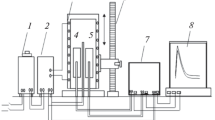
The heat capacity was measured using an installation, schematic diagram of which is shown in Fig. 1. Electric furnace 3 is mounted on a bench and can be moved on it to the right or to the left. Sample 4 (can also be moved) is a cylinder 30 mm in height and 16 mm in diameter and has a channel drilled at one end, in which a thermocouple is inserted. Ends of the thermocouple were attached to a Digital Multimeter UT71B 7 measuring device, which directly records results of measurements in tabulated form with computer 8. The tabulated data were used to plot cooling curves for samples of studied alloys.
Schematic diagram of installation for the determination of heat capacity of solids in regime of cooling: (1) autotransformer; (2) thermocontroller; (3 )electric furnace; (4) studied sample; (5) standard; (6) electric furnace base; (7) multichannel digital thermometer; and (8) recording instrument (computer).
RESULTS AND DISCUSSION
The aluminum—manganese alloys were synthesized under a flux using a SShOL laboratory shaft furnace and A7 grade aluminum (State standard GOST 11069–2001), Mg95 grade manganese (State standard GOST 804–93), and an Al-based master alloy containing 2.0 wt % Sc. The manganese contents in the AMg2, AMg3, and ANg4 alloys were 2, 3, and 4 wt %, respectively. Magnesium was wrapped in an aluminum foil and made a part of aluminum-based melt. The scandium content in the alloys was 0.01, 0.05, 0.1, and 0.5 wt %, respectively.
The composition of the alloy was determined by atomic emission spectroscopy using a DFS-452 diffraction spectrograph equipped with an MORS-9 multichannel optical recording system. An electric arc was used as the excitation source. Figure 2 shows analytical lines, which correspond to magnesium and scandium and indicate the presence of the elements and impurities in the alloys.
Study of the Microstructure of Alloys
Microsections of studied alloys were prepared by grinding, polishing, and etching and were used for metallographic analysis. Samples were cut using a hand saw while preventing substantial heating of samples. The microsection sizes were 1.5–2.0 cm2. The samples were subjected to etching in a 20% NaOH aqueous solution upon heating to 60−70°С. After etching, a microsection was rinsed with water and carefully dried. The structure of alloys was studied with a magnification of ×200 using a NEOPHOT-31 optical microscope.
As an example, Fig. 3 shows the microstructure of the AMg4 alloy with scandium. Our studies allowed us to find that the structure of alloys is of the same type and consists of the aluminum solid solution. Particles of intermetallic phases (particularly Mg2Al3) formed in the course of alloy solidification are also observed. The amount and sizes of the second phase ultimately affect the different properties of the initial alloy. It is seen that scandium additions in the AMg4 alloy refine the structure and it becomes uniform and fine-grained.
Study of the Specific Heat Capacity of Alloys
The temperature dependence of the heat capacity and changes in the thermodynamic properties of the AMg2, AMg3, and AMg4 alloys with scandium were measured in accordance with methods described in [8–15].
The heat capacity of the AMg2, AMg3, and AMg4 alloys with scandium was measured in a cooling regime. The temperature was measured using a multichannel digital thermometer, which allows one to directly record results of measurements in tabular form on a computer. The accuracy of measurements of the temperature was 2°С. The time interval of temperature recording was 10 seconds. The relative error of the temperature measurement in a temperature range of 40 to 800°С was ±1.5%. The error of heat capacity measurements in accordance with the used method does not exceed 1.5% (Tables 1 and 2).
Results of the measurements were processed using MS Excel software. The dependences were plotted using Sigma Plot software. The correlation coefficient was Rcorr > 0.9544; it confirms the accuracy of the used approximating function.
To determine the error of the method, the heat capacity of M00 grade copper was previously measured with respect to A7 grade aluminum. Tables 1 and 2 show the results of three parallel measurements. As is seen, the found errors of the measurements of the heat capacity of M00 grade copper do not exceed 1.5%. Further in the present study, we used M00 copper as the standard, which is a reliable metal since it has a higher melting temperature and is characterized by reliable values of heat capacity determined by many investigators using different methods [15, 16].
The comparison of experimentally obtained values of the heat capacity of copper with the tabulated data [16] indicates their 99% convergence.
Figure 4 shows the measured cooling curves for the AMg2, AMg3, and AMg4 alloys with scandium. The obtained temperature dependences of the alloys (Fig. 4) are described by expression

where a, b, p, and k are constants for the sample and τ is the cooling time.
The differentiation of Eq. (7) with respect to τ allows us to obtain the equation for the determination of the cooling rate of alloys

The values of coefficients a, b, p, k, ab, and pk in Eq. (8) were calculated.
Figure 5 shows curves of the cooling rate of alloys with a regression coefficient of no less than 0.998.
The calculated cooling rates of samples were used to calculate the specific heat capacity of the alloys by Eq. (6). The polynomials describing the temperature dependence of the specific heat capacity of the AMg2, AMg3, and AMg4 alloys with scandium were obtained in the form of total equation:
Figure 6 shows the heat capacity calculated (at a step of 100 K) by Eqs. (6) and (9). To calculate the temperature dependences of the specific enthalpy, entropy, and Gibbs energy changes by Eqs. (10)–(12), the heat capacity integrals determined by Eq. (9) were used:
where T0 = 298.15 К.
Figure 7 shows the temperature dependences of the enthalpy and entropy changes and Gibbs energy for the AMg2 and AMg4 alloys, which were calculated by Eqs. (10)–(12) at a step of 100 K.
CONCLUSIONS
(1) The specific heat capacity of the AMg2, AMg3, and AMg4 alloys with scandium was determined in the cooling regime. Mathematic models describing the temperature dependences of the heat capacity and changes in thermodynamic functions (enthalpy, entropy, and Gibbs energy) of the alloys in a temperature range of 300–800 К were obtained.
(2) It is shown that, as the temperature increases, the specific heat capacity, enthalpy, and entropy of the alloys increase, whereas the value of the specific Gibbs energy decreases.
(3) It was found that scandium additions in a studied concentration range of 0.01–0.5 wt % almost do not affect the values of the specific heat capacity and changes in the thermodynamic functions of the initial AMg2, AMg3, and AMg4 alloys.
REFERENCES
V. M. Beletskii and G. A. Krivov, Aluminum Alloys (Composition, Properties, Technology, Application): A Handbook, Ed. by I. N. Fridlyander (KOMITEKh, Kiev, 2005) [in Russian].
L. I. Kaigorodova, D. Yu. Rasposienko, V. G. Pushin, V. P. Pilyugin, and S. V. Smirnov, “Effect of Annealing on the structure and properties of the aging Al–Li–Cu–Mg–Zr–Sc–Zn alloy subjected to megaplastic strain,” Phys. Met. Metallogr. 120, 157–163 (2019).
S. Nazarov, S. Rossi, P. Bison, I. Ganiev, L. Pezzato, and I. Calliari, “Influence of rare earths addition on the properties of Al–Li alloys,” Phys. Met. Metallogr. 120, 402–409 (2019).
V. V. Krasnoyarskii and N. R. Saidaliev, “Corrosion-electrochemical properties of aluminum alloys with iron in neutral solutions,” Zashch. Korroz. Okruzh. Sredy, No. 3, 14–19 (1991).
V. F. Frolov, S. V. Belyaev, I. Yu. Gubanov, A. I. Bezrukikh, and I. V. Kostin, “The influence of technological factors on the formation of structure defects in large-tonnage ingots from aluminum alloys of the series 1XXX,” Vestn. MGTU im. G. I. Nosova 14, 25–31 (2016).
M. V. Chukin, V. M. Salganik, P. P. Poletskov, A. S. Kuznetsova, G. A. Berezhnaya, and M. S. Gushchina, “The main types and applications of nanostructured high-strength sheet metal,” Vestn. MGTU im. G.I. Nosova, No. 4, 41–44 (2014).
X. G. Chen, “Growth mechanisms of intermetallic phases in DC cast AA1XXX alloys,” in Essential Readings in Light Metals.V. 3.Cast Shop for Aluminum Production (2013), pp. 460–465.
D. A. Grange, “Microstructure control in ingots of aluminum alloys with an emphasis on grain refinement,” in Essential Readings in Light Metals.V. 3.Cast Shop for Aluminum Production (2013), pp. 354–365.
Geoffrey K. Sigworth, “Fundamentals of solidification in aluminum castings,” Int. J. Met. Casting 8 (1), 7–20 (2014).
N. F. Ibrokhimov, I. N. Ganiev, Z. Nizomov, N. I. Ganieva, S. Zh. Ibrokhimov, “Effect of cerium on the thermophysical properties of AMg2 alloy,” Phys. Met. Metallogr. 117, 49–53 (2016).
Kh. Kh. Azimov, I. N. Ganiev, I. T. Amonov, and N. F. Ibrokhimov, “The effect of lithium on the heat capacity and changes in the thermodynamic functions of the aluminum alloy AZh2.18,” Vestn. MGTU im. G.I. Nosova 16, 37–44 (2018).
I. N. Ganiev, A. G. Safarov, F. R. Odinaev, U. Sh. Yakubov, and K. Kabutov, “Temperature dependence of the specific heat and changes in the thermodynamic functions of the alloy AZh4.5 with tin,” Izv. Vyssh. Uchebn. Zaved., Tsvetn. Metall., No. 1, 50–58 (2019).
S. E. Otadzhonov, I. N. Ganiev, M. Makhmudov, and M. M. Sangov, “Temperature dependence of heat capacity and changes in the thermodynamic function of an AK1M2 alloy with calcium,” Izv. Yugo-Zapad. Gos. Un-ta. Ser. Tekh. Tekhnol., No. 3(28), 105–115 (2018).
U. Sh. Yakubov, I. N. Ganiev, M. M. Makhmadizoda, A. G. Safarov, and N. I. Ganieva, “The effect of strontium on the temperature dependence of specific heat and changes in the thermodynamic functions of the alloy AZh5K10,” Vestn. Sankt-Peterburg. Gos. Univ. Tekhnol. Dizain. Ser. Estestv. Nauk, No. 3, 61–67 (2018).
Dzh. Kh. Dzhailoev, I. N. Ganiev, A. Kh. Khakimov, N. F. Ibrokhimov, and Kh. Kh. Azimov, “The effect of barium on the temperature dependence of specific heat and changes in the thermodynamic functions of the alloy AZh2.18,” Vestn. TNU. Ser. Estestv. Nauk, No. 4, 240–248 (2018).
V. E. Zinov’ev, Thermophysical Properties of Metals at High Temperatures (Metallurgiya, Moscow, 1989) [in Russian].
Thermodynamic Properties of Individual Substances. A Handbook, Ed. by V. P. Glushkov (Nauka, Moscow, 1982) [in Russian].
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by N. Kolchugina
Rights and permissions
About this article
Cite this article
Ganiev, I.N., Norova, M.T., Eshov, B.B. et al. Effect of Scandium Additions on the Temperature Dependences of the Heat Capacity and Thermodynamic Functions of Aluminum–Manganese Alloys. Phys. Metals Metallogr. 121, 21–27 (2020). https://doi.org/10.1134/S0031918X20010068
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0031918X20010068