Methods and procedures are discussed for more economic and effective use of scandium for alloying aluminum alloys. Specifically, it is recommended to introduce scandium and zirconium into aluminum alloys in a 1 : 1 ratio instead of the usual 3 : 1 with the same overall element content. It is also suggested that microalloying with scandium in an amount of 0.1% instead of 0.22% provides the required level of properties in many cases. The possibility of refining the structure of the Al – 2% Sc master alloy for increasing its adaptability (assimilation by the aluminum melt) due to an increase in crystallization rate during ingot casting and other procedures is considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Scandium is one of the most effective alloying components of aluminum alloys. A small addition of scandium (about 0.2 – 0.3%) facilitates an increase in strength properties of wrought semiproducts of aluminum alloys by 100 – 150 MPa, limited cast grain refinement (up to formation of as non-dendritic structure), a marked improvement in weldability, and an increase in resistance to various forms of corrosion [1,2,3,4,5]. Apparently there is no information about any other components or even about of components introduced in combination that could approach scandium with respect to efficiency of action on aluminum alloy structure and properties. Within Russia work on studying the effect of adding scandium on the structure and properties of aluminum alloys commenced in the 1970s and as a logical completion of this work researchers created industrial aluminum alloys containing scandium and developed industrial technology for manufacturing wrought semiproducts of these alloys [6, 7].
Addition of scandium has a marked effect but differing action on strength of different aluminum alloys. The strongest action on structure and properties is observed on alloying with scandium materials based on the Al – Mg system [6]. On the basis of Al – Mg – Sc a series of industrial alloys has been developed, the most well-known of which are alloys 01570 [8], 1570S [9], and 1545k [10]. Semiproducts of these alloys are manufactured by metallurgical plants for use as objects of aerospace technology. The favorable strong effect of adding scandium on the structure and properties of semiproducts of high-strength alloys based on Al – Zn – Mg – Cu that are used in such high technology branches as aircraft building, atomic engineering, and shipbuilding, makes it possible to improve significantly the engineering indices of objects manufactured by these industries.
Expansion of the scale and field if application of aluminum alloys containing scandium is held back for a number of reasons, including inadequate Al – Sc master alloy. Some ways are suggested in this work of resolving this problem aimed at more economic and effective use of scandium during alloying aluminum materials.
Results and Discussion
Partial replacement of scandium by zirconium
Scandium is added to aluminum alloys together with zirconium, which stabilizes and strengthens the favorable effect of scandium [11]. On the basis of research results and accumulated practical experience there is an opinion that the optimum scandium and zirconium content, at least in alloys of the Al – Mg and Al – Zn – Mg systems, is 0.22 – 0.24% Sc and 0.10 – 0.12% Zr. With this content wrought semiproducts acquire the most favorable set of service and production properties. With an increase in concentration of scandium and zirconium there is formation of primary intermetallic Al3(Sc, Zr), worsening alloy service properties. With a lower content of scandium and zirconium their potential and possibilities are not entirely utilized.
The nature of the favorable effect of scandium on the structure and properties of aluminum alloys is connected with the unique features of Al3Sc intermetallic. Addition of zirconium, together with scandium, dissolves in Al3Sc intermetallic replacing scandium atoms and retaining (even stabilizing and strengthening) all of its unique properties. It has been suggested in [11] that with the aim of saving sand strengthening the effect of adding scandium to change the ratio between scandium and zirconium in favor of zirconium, without changing the overall content of these components. This assumption was based on results of the following experiments.
Ingots 92 mm in diameter of alloys of the system Al – Sc – Zr with a constant overall content of scandium and zirconium (1.5%) and a different ratio of the elements were prepared by continuous casting.
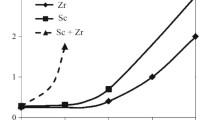
Melting and casting conditions were selected in order to prepare ingots with the minimum amount of intermetallic Al3(Sc, Zr) with a maximum supersaturation of scandium and zirconium solid solution. Adependence is given in Fig. 1 for the electrical conductivity of cast alloys on ratio of the content of scandium and zirconium. The curve has a clearly defined minimum, corresponding to an equal amount of scandium and zirconium in the alloys (0.75% each). Alloys with this or a similar ratio of scandium and zirconium are most inclined towards forming supersaturated solutions. Evaluation of the volume content of excess phase particles of crystallization origin, containing scandium and zirconium, confirm this conclusion. In an ingot of an alloy with equal ratio of scandium and zirconium the volume fraction of intermetallic containing scandium and zirconium is at a minimum and comprises 2.4%. As deviation from the 1 : 1 ratio increases the volume fraction of intermetallic increases to 3.5 – 4.5%. Therefore, a change in the ratio of scandium and zirconium content has a significant effect on the combined metastable solubility within aluminum: minimum solubility is exhibited by binary alloys of the systems Al – Sc and Al – Zr, and the maximum for alloys with an equal content of scandium and zirconium. This is apparently connected with the maximum supercooling of molten alloy with an equal content of scandium and zirconium, and as a consequence an increase in cooling rate during subsequent crystallization, increasing metastable solubility.
Kinetic curves are given in Fig. 2 for the change in electrical conductivity and microhardness of Al – Sc – Zr-alloys with a constant total content of Sc and Zr (1.5%) and different ratios in relation to isothermal exposure at 400°C. As was recalled, the original electrical conductivity of ingots depends on the ratio of scandium and zirconium. However, after prolonged exposure at 400°C for 277 h solid solution decomposition proceeds almost entirely and ingot electrical conductivity approaches that of commercially pure aluminum, with the exception of alloys with an identical content of scandium and zirconium (Fig. 2 a). In this alloy after exposure for 277 h marked residual concentration of scandium and zirconium is retained in aluminum solid solution, due to strong supersaturation in the original (cast) condition.
Kinetics of solid solution decomposition, specifying the change in electrical conductivity, depend on the ratio of scandium and zirconium in alloy. Most rapid decomposition occurs in alloy Al – 1.5% Sc and the slowest rate of decomposition is typical for alloys Al – 1.5% Zr. It should be noted that solid solution decomposition in Al – 1.5% Zr alloy has a longer incubation period, almost comprising days. The rest of the alloys occupy and intermediate position with respect to solid solution decomposition rate.
Of practical interest in analyzing curves for the change in electrical conductivity is the extent of solid solution decomposition, determined as a difference in electrical conductivity values at the end of decomposition (after exposure for 277 h) and at the start of decomposition (in a cast condition). This index specifies the amount of scandium and zirconium precipitated from solid solution in aluminum, or in other words, the volume fraction of precipitated secondary particles. These particles determine alloy stability against recrystallization and the amount of aluminum matrix strengthening. From this point of view the most preferred alloys are those with an identical or similar scandium and zirconium content. In these alloys decomposition proceeds with precipitation of the greatest amount scandium and zirconium from solid solution and correspondingly formation of the maximum amount of fine particles of the Al3(Sc, Zr) type.
Apparently maximum strengthening should be expected in these alloys.
Curves for the change in ingot microhardness during isothermal exposure at 400°C confirm this assumption (Fig. 2 b). The maximum strengthening in the test range of exposure is reached by ingots of alloy with an equal content of scandium and zirconium (0.75% each). As the ratio of content of scandium and zirconium in alloys deviates from the optimum (1 : 1), the overall level of strength and capacity to retain strengthening after prolonged exposure is reduced.
Considering the results obtained, it may be concluded that the optimum properties are exhibited by aluminum alloy containing scandium and zirconium in a ratio of 1 : 1. Alloy is inclined to a maximum to supersaturation of solid solution with scandium and zirconium during ingot casting by a continuous method, good strengthening during decomposition of supersaturated solid solution, and retention of this strengthening after prolonged high-temperature heating, simulating process heating. It is also possible to suggest reliably that semiproducts of this alloy will have good recrystallization resistance.
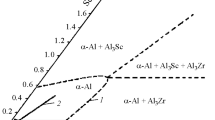
Alloy with a scandium and zirconium ratio of 1 : 1, having the optimum properties, is in the ternary equilibrium diagram Al – Sc – Zr in the phase region α + Al3(Sc1 – x , Zr x ) at the boundary of the ternary region α + Al3(Sc1 – x , Zr x ) + Al3Zr. Particles of Al3(Sc1 – x , Zr x ) phase are saturated to a maximum with zirconium, which exhibits a slower diffusion coefficient in aluminum than scandium. This provides good thermal stability of fine particles (slow coagulation rate). It is also possible that zirconium by dissolving in Al3Sc phase reduces the specific surface energy at the interphase boundary of Al3(Sc1 – x , Zr x ) particles in an aluminum matrix. This also facilitates a reduction in the inclination of particles towards coagulation due to a reduction in the coagulation moving force, i.e., excess surface energy.
Therefore, the maximum effect of strengthening after prolonged high-temperature exposure applies to alloys with a ratio of scandium to zirconium content of 1 : 1. However, use of this regularity in practice is only possible with presence of a production chain for manufacturing aluminum semiproducts with a high melt crystallization rates providing fixing of scandium and zirconium in supersaturated solid solution. These rates are achieved with use of granulation technology, ingotless rolling, and continuous casting of small ingots.
Consequently, in order to save scarce scandium and improve aluminum alloys properties it is recommended that they are alloyed with a small addition of scandium together with zirconium in equal quantities. In this case the maximum combined solubility of scandium and zirconium in aluminum is achieved during ingot casting, and as a consequence of this maximum strengthening during subsequent solid solution decomposition. In addition, Al3Sc phase is saturated to a maximum with zirconium. Optimum properties are exhibited by Al3(Sc1 – x , Zr x ) phase, within which scandium is replaced by zirconium up to the limit. In this case fine Al3(Sc1 – x , Zr x ) particles have maximum stability and minimum tendency towards coagulation.
In order to check this assumption round ingots ∅ 134 mm were prepared by continuous casting of two alloys based on the Al – Mg – Sc system with the normally adopted ratio of scandium and zirconium content of 3 : 1 and with the proposed ratio of 1 : 1. The overall scandium and zirconium content in both alloys was identical, i.e., 0.40%. The actual alloy chemical compositions are given in Table 1.
Ingots were homogenized at 490°C for 10 h and extruded into strip 3x100 mm in cross section. Mechanical properties of extruded strip in the original (hot extruded) and annealed at 400°C, 1 h (air cooling) conditions2 are given in Table 2. It is seen that the strip properties in both alloys is similar.
2 T. D. Rostova n L. P. Kirillov took part of examining cast alloys.
Extruded strips were tested for creep at 125°C, stress 150 MPa, and test duration 100 h. The amount of residual elongation was: 0.937, 1.075, and 0.961% for alloy 1 and 0.862, 0.946, and 0.211% for alloy 2. Considering the considerable scatter of values obtained during creep tests it may be assumed that these results are similar.
The data provided may serve as a basis for performing future work aimed at creating aluminum alloys economically alloyed with scandium with a ratio of scandium to zirconium content of 1 : 1.
Alloying with small scandium additions
In the previous section the actual possibility was demonstrated of reducing the scandium content in aluminum alloys by partial replacement with zirconium. In this section experimental data will be provided pointing the possible reduction in scandium content in alloys without a marked reduction in its favorable effect.
A temperature dependence is given in Fig. 3 for recrystallization of aluminum and alloy of the Al – Zn – Mg system and scandium concentration. With an increase in scandium content the recrystallization temperature increases significantly and more sharply with addition of the first small amount of scandium. With a subsequent increase in content an increase in recrystallization temperature proceeds with diminution. Other characteristics of aluminum alloys, in particular ultimate strength, yield strength, and hardness, vary in relation to scandium content in a similar way. The strongest effect of the first small scandium additions have the strongest effect. Therefore, a reduction in scandium content by several factors does not lead to such a strong reduction in ultimate strength or any other properties. The reasoning provided below was a basis for performing appropriate experiments.
Ingots 97 mm in diameter of alloys of the Al – Zn – Mg system with addition of transition metals whose chemical composition is given in Table 3 were prepared by continuous casting. The weight of each melt was 30 kg.
Ingots were cast with addition of scandium with a prescribed average content of other alloy components. However, the actual concentration of the main components, i.e., zinc and magnesium, decreased as the amount of scandium added increased, which was a consequence of dilution of the alloy with aluminum on adding Al – 2% Sc master alloy and burn-off and evaporation of these elements during melt heating, carried out after adding a successive portion of master alloy to the furnace. This reduced the accuracy of an experiment.
Ingots were homogenized and extruded into square bar 30 × 30 mm in section and strip 3 × 80 mm in section. Quenching of bars and strip was carried out from 450°C in cold water. After straightening by tension semiproducts were cut into workpieces for specimens and artificially aged by a regime: 100°C, 20 h + 160°C, 10 h.
The type of structure of extruded bars and strip depends on scandium content. Extruded bars of alloy 1 (Table 3) only containing zirconium (0.07%) without scandium have an unrecrystallized structure and a coarse crystalline rim and thin extruded strip of this alloy has an entirely recrystallized structure. Addition to the alloy of just 0.03% Sc leads to a sharp reduction in rim thickness. Extruded strip has an unrecrystallized structure with presence of a recrystallized rim 1.5 mm thick. Introduction of scandium into the alloy in an amount of 0.07 and 0.12% provides preparation within semiproduct after quenching of a stable unrecrystallized structure (alloys 3, 4).
Mechanical properties of semiproducts of alloys of the Al – Zn – Mg system are given in Table 4. The structure of the gauge length of specimens prepared for extruded bars of all alloys was entirely unrecrystallized. As a result of this the effect of scandium on mechanical properties of semiproducts is insignificant: ultimate strength varies in the range 547 – 563 MPa, yield strength in the range 513 – 525 MPa, and relative elongation 11.3 – 13.3%. Probably property variation is due to variations in chemical composition with respect to the main alloy components, i.e., zinc and magnesium.
In a naturally aged condition there is some tendency towards an increase in strength properties with an increase in scandium content, although there is an effect of content of the main alloying components, which decreases as there is an increase in amount of scandium. This nullifies the strengthening effect of scandium.
Scandium content has a marked effect on mechanical properties of extruded strip due to a change in the type of structure. Strength properties of strip with an entirely recrystallized structure of alloy 1, not containing scandium, are lower by 50 – 100 MPa than for strip pf alloy 2 containing 0.03% Sc, but having an unrecrystallized structure. With an further increase in scandium content from 0.03 to 0.12% the type of structure is unchanged, and the increase in strength properties is small (Table 4).
On the whole, in an artificially aged condition semiproducts of alloy with combined addition of Sc + Zr have ultimate strength of 530 – 540 MPa, yield strength of 480 – 500 MPa, and relative elongation of 10.0 – 13.5%.
Some resource characteristics are provided in Table 5 for extruded semiproducts. Extruded bar exhibits good resistance to crack generation and development, which is indicated by very high values of impact strength and fracture toughness, depending little on scandium content. These values correspond to the best contemporary aluminum alloys of similar strength with increased purity. Low-cycle fatigue resistance is a structurally sensitive property. During LCF tests (σmax = 160 MPa) for alloy 1 (without scandium) having a recrystallized structure, they withstood 75 cycles to failure, but specimens of alloys with addition of 0.03 – 0.12% Sc with an unrecrystallized structure withstood more than 200 cycles. With a change in structure type from recrystallized to unrecrystallized there is a sharp increase in resistance to fatigue loading.
The properties of welded joints of extruded strip after welding and natural ageing for one month are provided in Table 6. The ultimate strength of welded joints of alloys with scandium is very good (460 – 500 MPa). The strength factor for welded joints \( {\sigma}_{\mathrm{f}}^{\mathrm{we}}/{\sigma}_{\mathrm{f}}^{\mathrm{bas}} \)of alloys containing scandium in an amount of 0.07 – 0.12% is greater than 0.9. The strongest effect applies to the first addition of scandium.
On the basis of these research results and taking account of the results of previous work a composition has been selected of a new high-strength aluminum alloy based on the Al – Zn – Mg system, alloyed with scandium. The alloy has been assigned grade 1975.
In spite of the low scandium content (0.11% Sc and 0.11% Zr) alloy 1975 exhibits a good set of properties (Table 7).
Analysis of Table 7 shows that alloys of the Al – Zn – Mg system with a low scandium content (0.11%) may exhibit an excellent set of mechanical properties.
In addition, with a reduction in the calculated scandium content less than 0.2% it is necessary to display care. This method for saving scandium is more suitable for alloys based on the Al – Zn – Mg system and to a lesser extent for Al – Mg alloys, more inclined towards recrystallization.
Conditions for adding scandium to aluminum alloys
Scandium is classified as a refractory metal. The melting temperature of scandium is 1541°C, and its introduction into readily melting aluminum alloys presents known difficulties. In order to provide assimilation of scandium by molten aluminum it is added in the form of a master alloy Al – 2% Sc. The assimilation process by molten aluminum of scandium is transfer of scandium from master alloy into molten aluminum solution by melting and dissolution of all of the structural components of the Al – 2% Sc master alloy. A considerable part of scandium in master is in the form of primary intermetallic Al3Sc, which exhibits high thermal stability and slow dissolution in molten aluminum. In order to accelerate melting a melt is overheated. Melt overheating is a required but undesirable procedure since during overheating there is rapid melt oxidation and saturation with hydrogen. In addition, loss of scandium is observed due to evaporation, oxidation, or deposition on a hearth surface. For example, during addition of scandium in an amount of 0.22 – 0.23% normally 0.01 – 0.03% of scandium is lost. In view of this the addition of scandium to aluminum alloys used currently cannot be called perfect and economic.
Master alloy Al – 2% Sc is cast in the form of plates with a size of about 20 × 150 × 500 mm weighing about 5 kg [12]. The structure of an Al – 2% Sc master alloy casting is given in Fig. 4. Coarse, apparently primary, particle of Al3Sc are seen, whose volume fraction is 8%. X-ray microanalysis confirms presence of scandium in the particles observed. In the aluminum matrix the concentration of scandium solid solution varies within the limits of 0.41 – 0.75%.
Intermetallic Al3Sc of eutectic origin is not revealed located over grain boundaries.
In order to analyze and best understand the structure of a master alloy the Al – Sc composition should be used. Based on research by Russian and overseas scientists, studying the Al – Sc equilibrium diagram in the region rich in aluminum, it may be stated that scandium reacts with aluminum by a diagram of the eutectic type [13]. The eutectic transformation liquid ? á(Al) ?Al3Sc is 655 – 659°C. The composition of the eutectic point is about 0.6% Sc, and equilibrium solubility of scandium in aluminum at the eutectic temperature horizontal is about 0.35%. It should be considered that formation of a master alloy Al – 2% Sc cast structure occurs under no-equilibrium conditions and for more correct analysis a metastable equilibrium diagram should be used corresponding to the actual rate of cooling during master alloy casting crystallization. This rate in our opinion is approximately 100 K/sec. Drawing attention to results of work in [14], a metastable equilibrium diagram was plotted for a cooling rate of 100 K/sec (Fig. 5). Judging from this diagram the maximum concentration of scandium solid solution in aluminum in Al – 2% Sc master alloy should be about 0.7 – 0.8%.
Al – Sc composition diagram: 1 ) equilibrium diagram; 2, 3 ) metastable equilibrium diagram with cooling rates of 102 and 103 K/sec respectively [4].
Taking account of dendrite liquation the average concentration of scandium solid solution in aluminum will be less, which corresponds to the results obtained by us: 0.41 – 0.75%. On average in solid solution there should be about 0.6% Sc, and the remaining 1.4% should be in the form of Al3Sc intermetallic with a volume fraction of about 8%, which corresponds to results of measuring the volume faction of Al3Sc intermetallic particles.
A quality criterion for aluminum master alloys is ease and completeness of assimilation of alloying component by molten aluminum. On adding Al – 2% Sc master alloy to melt the scandium most readily assimilated will be that in aluminum solid solution. This part of scandium is readily transferred into molten aluminum solution together with molten aluminum grains. Another part of the scandium may in principle be in Al3Sc intermetallic of eutectic origin, and it may also comparatively readily dissolve molten aluminum as a result of the low volume faction and small size of intermetallic particles. Most difficult to assimilate is scandium in the primary intermetallic Al3Sc, due to its considerable dimensions. The process of primary intermetallic dissolution is the weakest link and delays the whole cycle of assimilation of scandium master alloy by molten aluminum. In view of this in order to facilitate assimilation and reduce the duration of the cycle it is expedient to reduce the size and amount of primary Al3Sc intermetallic in master alloy.
The most attractive method for reducing the amount and dimensions of primary intermetallic is an increase in cooling rate during crystallization and master alloy casting. However, with ingotless rolling in water-cooled rolls for strip 10 mm thick the cooling rate in the crystallization temperature range is about 103 K/sec. Judging from the metastable composition diagram, the corresponding cooling rate is 103 K/sec, and with crystallization of Al – 2% Sc master alloy in water-cooled rolls in the form of strip 10 mm thick about 1% of Sc will be fixed in solid solution, and another 1% Sc will be in the form of Al3Sc intermetallic. The size of primary intermetallic particles will be considerably smaller and correspondingly master alloy with this structure will better assimilated by molten aluminum. The method for preparing Al – 2% Sc master alloy by means of ingotless rolling will facilitate an increase in master alloy quality, which will make it possible not only to avoid overheating during melting (or reduce its intensity) but also to increase sharply process productivity. Other production methods during melting and casting Al – Sc mater alloy with the aim of dispersing its structure are possible.
Conclusions
Considering the shortage and high cost of Al – Sc master alloy the article considers some versions making it possible to save scandium during addition to aluminum alloys (alloy development), and to choose a calculated composition during addition of scandium to aluminum alloys.
References
Lowell A. Willey, USA Patent 3.619.181, Aluminum Scandium Alloy, Publ. 9 November 1971.
M. E. Drits, L. S. Toropova, and Yu. G. Bykov, “Effect of REM on mechanical properties of alloy Al – 6.5% Mg,” Metalloved. Term. Obrab. Met., No. 10, 35 – 37 (1980).
V. I. Elagin, V. V. Zakharov, and T. D. Rostova, “Prospects for alloying aluminum alloys with scandium,” Tsvet. Met., No. 12, 96 – 99 (1982).
Yu. A. Filatov, “Industrial alloys based on the system Al – Mg – Sc,” Tekhnol. Legk. Splavov, No. 3, 30 – 35 (1996).
V. S. Sinyavskii, V. D. Val’kov, and E. V. Timkova, “Effect of adding scandium and zirconium on Al – Mg alloy corrosion properties,” Zashch. Met., 34(6), 613 – 619 (1998).
Yu. A. Filatov, “Deformation of alloys based on the Al – Mg – Sc system and prospects for their use in automobile building,” Tsvet. Met., No. 2, 60 – 62 (1997).
V. I. Elagin, V. V. Zakharov, Yu. A. Filatov, and T. D. Rostova, “Development of prospective aluminum alloys containing scandium,” in: Prospective Technology of Light and Special Alloys [in Russian], Fizmatlit, Moscow (2006).
V. I. Elagin V. V. Zakharov, Yu. A. Filatov, et al., RF Patent 2081934, C1, Wrought Thermally Unstrengthened Alloy Based on Aluminum [in Russian], Publ. 13 July 1995.
Yu. A. Filatov, V. G. Davydov, V. I. Elagin, V. V. Zakharov, E. I. Svechkov, L. I. Panasyugina, and R. I. Dobrozhanskaya, RF Patent 2333345, C1. Structural Wrought Thermally Unstrengthened Alloy Based on Aluminum [in Russian], Publ. 27 July 2004.
Yu. A. Filatov, V. I. Elagin, V. V. Zakharov, L. I. Panasyugina, and R. I. Dobrozhanskaya, A. A. Eliseev, G. V. Dodin, A. A. Zvonkov, S. A. Petrovskii, and V. P. Molochev, RF Patent 2233345 C1, Cryogen Wrought Thermally Unstrengthened Alloy Based on Aluminum [in Russian], Publ. 01.10.2009.
V. G. Davydov, V. I. Elagin, V. V. Zakharov, and T. D. Rostova, “Alloying aluminum alloys with scandium and zirconium additions,” Metalloved. Term. Obrab. Met., No. 8, 25 – 30 (1996).
Aluminum-Scandium Master Alloys. Technical Specifications 11-01-01–2001 [in Russian].
Jostein Roset and Nils Ryum, “Scandium in aluminium alloys,” Int. Mater. Rev., 50(1), 19 – 44 (2005).
A. M. Drits, L. S. Toropova, Yu. G. Bykov, et al., “Al – Sc metastable composition diagram in the field rich in aluminum,” Izv. Akad. Nauk SSSR, Metally, No. 1, 179 – 182 (1983).
V. G. Borisov, “Combined casting and rolling in aluminum plants,” in: Proc. Sci.-Tech. Meeting on Theme “Continuous and Combined Methods of Casting and Rolling Nonferrous Metals and Alloys” [in Russian], VAMI, Leningrad (1973).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 5, pp. 15 – 22, May, 2017.
Rights and permissions
About this article
Cite this article
Zakharov, V.V., Fisenko, I.A. Alloying Aluminum Alloys with Scandium. Met Sci Heat Treat 59, 278–284 (2017). https://doi.org/10.1007/s11041-017-0142-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-017-0142-9