Abstract
Cisplatin (DDP) is widely used in the chemotherapy of cervical cancer (CC), the fourth most common female malignancy worldwide. However, some patients progress to chemotherapy resistance, which leads to chemotherapy failure, tumor recurrence, and poor prognosis. Therefore, strategies to identify the regulatory mechanisms underlying CC development and increase tumor sensitivity to DDP will help improve patient survival. This research was designed to ascertain the mechanism of EBF1-dependent regulation of FBN1 which promotes chemosensitivity of CC cells. The expression of EBF1 and FBN1 was measured in CC tissues resistant or sensitive to chemotherapy and in DDP-sensitive or -resistant cells (SiHa and SiHa-DDP cells). SiHa-DDP cells were transduced with lentiviruses encoding EBF1 or FBN1 to evaluate the influence of these two proteins on cell viability, expression of MDR1 and MRP1, and cell aggressiveness. Moreover, the interaction between EBF1 and FBN1 was predicted and demonstrated. Finally, to further verify the EBF1/FB1-dependent mechanism of DDP sensitivity regulation in CC cells a xenograft mouse model of CC was established using SiHa-DDP cells transduced with lentiviruses carrying EBF1 gene and shRNA directed to FBN1·EBF1 and FBN1 showed decreased expression in CC tissues and cells, particularly in those resistant to chemotherapy. Transduction of SiHa-DDP cells with lentiviruses encoding EBF1 or FBN1 lead to decreased viability, IC50, proliferation capacity, colony formation ability, aggressiveness, and increased cell apoptosis. We have shown that EBF1 activates FBN1 transcription by binding to FBN1 promoter region. Additionally, it was revealed that FBN1 silencing reversed the promoting effect of EBF1 overexpression on chemosensitivity of CC cells in vivo. EBF1 facilitated chemosensitivity in CC cells by activating FBN1 transcription.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Cervical cancer (CC) is the fourth most common malignancy diagnosed in women globally [1]. About 570 000 cases of CC and 311 000 deaths from the disease were reported 2018 [2]. Risk factors for CC development are human papillomavirus (HPV) infection, venereal diseases, reproductive factors, utilization of long-term oral contraceptives, smoking, obesity, and others [3]. To date, a variety of treatment strategies have been developed for CC, including radiotherapy, chemotherapy, and surgery [4]. Cisplatin (DDP)-based chemotherapy has been used to treat advanced/recurrent CC [5]. This therapy has effectively improved the quality of life and prognosis of the patients. However, 10~20% of patients progress to chemotherapy resistance, leading to chemotherapy failure, tumor recurrence, and worse prognosis [6]. Therefore, the CC treatment will undoubtedly focus on identifying the regulatory mechanisms of CC development and strategies to increase the sensitivity of the tumor to DDP.
Early B cell factor 1 (EBF1) is a transcription factor that regulates the differentiation of B cells, neuronal cells, and adipocytes [7]. Recently, low levels of EBF1 expression were revealed in several cancers, including cholangiocarcinoma (CCA) [8] and gastric cancer [9]. In a previous study using bladder cancer (BC) samples it has been shown that EBF1 is involved in transcriptional regulation of survival-associated hub immune-related genes (IRGs) [10]. EBF1 was also identified as a tumor suppressor in colorectal cancer (CRC) [11]. Notably, a multi-variates analysis suggested that EBF1 deletion was associated with chemoresistance in acute lymphoblastic leukemia [12]. However, there are few studies on the precise role of EBF1 and its downstream targets in chemoresistance of CC cells.
Fibrillin-1 (FBN1) is an essential protein in extracellular matrix that modulates tissue microenvironment [13]. FBN1 could be a recurrence-associated molecule and a prognostic indicator in colon cancer [14]. Additionally, FBN1 was found to be a biomarker associated with early recurrence in patients with ovarian cancer (OC) who were initially sensitive to chemotherapy [15] and strongly correlated with desmoplasia in high-grade serous OC (HGSOC) [16]. In this study, bioinformatic analysis suggested abnormally downregulated EPN1 and FBN1 in cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), as well as the binding of EBF1 to FBN1 promoter. We therefore conducted in vitro and in vivo experiments to investigate the roles of EBF1 and FBN1 in CC and their involvement in the development of chemoresistance. Consequently, EBF1 could promote chemosensitivity in CC cells by activating FBN1 transcription, which may provide a molecular insight for us to understand DDP chemosensitivity in CC cells.
EXPERIMENTAL
Patients and specimens. CC tissue and normal adjacent tissue were surgically collected from 30 patients at First Affiliated Hospital of Gannan Medical University. All cancer tissue specimens were diagnosed as CC. The enrolled patients were stratified into chemosensitivity group (tumor remission after 6 cycles of chemotherapy, N = 12) and chemoresistance group (tumor stabilization or progression after 6 cycles of chemotherapy, N = 18) based on their sensitivity to chemotherapy drugs. This study was approved by the Ethics Committee of First Affiliated Hospital of Gannan Medical University and fully complied with the Declaration of Helsinki. All patients had signed written informed consent.
Cell culture. Human 293T cells, human CC SiHa cells (a DDP-sensitive cell line), and SiHa-DDP cells (a DDP-resistant cell line) were purchased from Hunan Fenghui Biotechnology. All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) and 100 μg/mL penicillin-streptomycin solution (Invitrogen, UK) at 37°C with 5% CO2. The culture medium for SiHa-DDP cells was additionally appended with 600 ng/mL DDP.
Lentiviral transduction of cells. Lentiviral vectors encoding EBF1 (oe-EBF1), FBN1 (oe-FBN1), shRNA to FBN1 (sh-FBN1), and corresponding negative controls (oe-NC and sh-NC) were purchased from GenePharma (Shanghai, China). Viruses were assembled in 293T packaging cells after transfection with aforementioned vectors, psPAX2 and pMD2.G plasmids (GenePharma, Shanghai, China). Virus-containing media was filtered through a 0.45 μa filter, and virus titers were measured by fluorescence counting method. To infect CC cell lines, cells were incubated with lentiviruses for 48 h (titer of 1 × 108 TU/mL). Short hairpin-RNA (shRNA) sequences we used are listed in Table 1.
Quantitative real-time polymerase chain reaction (qRT-PCR). Total RNA was extracted using RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and reverse-transcribed using a reverse transcription kit in accordance with the kit’s manual (RR047A, TaKaRa, Tokyo, Japan). Target gene fragments were amplified from cDNA templates using SYBR® Premix Ex TaqTM II (Perfect Real Time) kit (DRR081, TaKaRa, Toyko, Japan) and an ABI 7500 instrument (ABI, Foster City, CA, USA). The thermal cycle parameters were as follows: pre-denaturation at 95°C for 30 s, denaturation at 95°C for 5 s, annealing at 60°C for 34 s elongation. The PCR was performed with 40 cycles. Three duplicates were set for each PCR reaction and the Ct values for each well were recorded. Relative expression of EBF1 and FBN1 was calculated by normalizing the expression of target genes to that of β‑actin using the 2–ΔΔCt method: ΔΔCt = experimental group (Ct target gene — Ct housekeeping gene) — control group (Ct target gene—Ct housekeeping gene). Primer sequences used in qPCR reaction were designed by Shanghai Sangon Biotech (see in Table 2).
Western blotting. Tissues or cells were lysed with RIPA lysis buffer (Wuhan Boster Biological Technology Co., Ltd., Wuhan, China). Protein concentration in lysates was measured using BCA kit (Boster, Wuhan, China). Proteins were separated by electrophoresis in 10% SDS-PAAG. Electrophoresis was performed at 80 V for 30 min and continued at 120 V for 90 min after bromophenol blue marker reached the separation gel. Proteins were transferred onto a PVDF membrane in an ice bath at 250 mA for 100 min, after that membranes were rinsed three times 1 ~ 2 min with scrubbing solution. After being blocked with 5% BSA for 2 h, the membranes were incubated with anti-rabbit primary antibodies to β-actin (ab8227, Abcam, Cambridge, UK) diluted 1:2000, EBF1 (#50752, Cell Signaling Technology, MA, USA) diluted 1 : 1000, MDR1 (ab170904, Abcam) diluted 1:2000, MRP1 (ab260038, Abcam) diluted 1 : 1000, and anti-FBN1 antibodies (ab231094, Abcam) diluted 1:1000. Then membranes were washed with TBST solution and then incubated with HRP-labeled goat anti-rabbit secondary antibody IgG (ab205718, 1 : 2000, Abcam) for 1 h. Blots were developed using ECL solution (EMD Millipore company, Billerica, MA, USA). Image Pro Plus 6.0 (Media Cybernetics, San Diego, CA, USA) software was used for quantification of blots using β‑actin signals as an internal reference. The relative protein content was expressed by the ratio of the gray value of the corresponding protein band to the gray value of the β-actin protein band.
Cell counting kit 8 (CCK-8). Cells were seeded into 96-well plates at a density of 1 × 103 cells/well. Cell viability was tested using CCK-8 assay after 0, 24, 48, 72, and 96 h. To estimate cell viability in the presence of DDP, cells were seeded (5 × 103 cells/well) into 96‑well plates, treated with different concentrations of DDP (0, 1, 2, 3, 4, or 5 µg/mL) at 37°C for 24 h, and supplemented with 10 µl of CCK-8 reagent (Beyotime Institute of Biotechnology) and incubated for additional 2 h. The absorbance was measured at 450 nm. IC50 values were calculated as the concentration of DDP resulting in 50% inhibition of cell viability.
Colony forming assay. Transduced SiHa-DDP cells were trypsinized and seeded (1 × 103 cells/well) into 6‑well plates. Subsequently, the cells were cultured for 2 ~ 3 weeks. Visible colonies were fixed in 4% paraformaldehyde for 10 min and stained with 0.1% crystal violet solution for 10 min (Sigma-Aldrich; Merck KGaA). The stained colonies (>30 cells) were counted using a DMM-300D microscope (Caikon, Shanghai, China) at ×40 magnification.
Transwell invasion and migration assays. To determine cell migration rate, SiHa-DDP cells in serum-free medium were seeded at a density of 1 × 105 cells per well into transwell chambers (pore diameter of 8 µm; Corning Inc., NY, USA), and culture media supplemented with 10% FBS were pipetted into basolateral chambers. After 24-h incubation, migratory cells were fixed with 4% paraformaldehyde solution for 30 min and stained with 0.1% crystal violet for 2 h. Stained cells were counted in five randomly selected fields of view using a phase-contrast microscope (Olympus, Tokyo, Japan) (magnification, ×200). To evaluate cell invasion, the transwell chambers were precoated with Matrigel for 30 min and then the aforementioned protocol was performed.
Flow cytometry. Annexin V FITC/propidium iodide (PI) double staining was used to estimate cell apoptosis. 48 h after transfection the concentration of cells was adjusted to 1 × 106 cells/mL. The cells were fixed overnight at 4°C using 70% precooled ethanol solution. After that 100 μL of cell suspensions (no less than 106 cells/mL) were mixed with 200 μL of binding buffer. Subsequently, the cells were stained with 10 μL Annexin V-FITC and 5 μL PI for 15 min in the dark. After the addition of binding buffer (300 μL), cell apoptosis was determined by flow cytometry at the excitation wavelength of 488 nm. The flow cytometer was used to analyze 2 × 104 cells each time. Annexin V was taken as the X axis and PI as the Y axis.
Dual luciferase reporter gene assay. The EBF1 binding sites in FBN1 promoter region were predicted using JASPAR database (https://jaspar.genereg.net/ matrix/MA0154.4/ ). Vectors containing recombinant luciferase reporter gene and wild-type and mutated FBN1 binding sites (FBN1-WT and FBN1-MUT) were designed. To verify the binding of EBF1 to FBN1 promoter region obtained plasmids were used for transfection of SiHa-DDP cells transduced with lentiviruses encoding EBF1 (oe-EBF1). Forty-eight hours after the transfection, the cells were collected and lysed. The luciferase activity was measured using a luciferase detection kit (K801-200, Biovision, USA) and a dual luciferase reporter gene analysis system (Promega, Madison, WI, USA) with Renilla luciferase as the internal reference. The ratio of firefly luciferase to Renilla luciferase was used to evaluate the activation degree of the reporter gene.
Chromatin immunoprecipitation (ChIP) assay. DNA-protein complexes in cells were crosslinked for 10 min with 4% formaldehyde solution, and the cells were ultrasonicated 15 cycles for 10 s each time, with an interval of 10 s, to break chromatin into fragments. Next, samples were incubated with IgG antibody (ab172730, 1 : 1000, Abcam) and anti-EBF1 antibody (#50752, 1 : 100, Cell Signaling Technology, MA, USA) at 4°C overnight. The DNA-protein complexes were precipitated using Protein Agarose/Sepharose. After 5-min centrifugation at 12 000 g, supernatants were discarded. The non-specific complexes were washed off and the bead-DNA-protein complexes were de-crosslinked overnight at 65°C. The DNA fragments were purified and then extracted by phenol/chloroform. The binding of EBF1 to FBN1 promoter was detected by qRT-PCR using FBN1 specific primers.
Xenograft. Female BALB/c nude mice (4 ~ 6 weeks old, weight 18—25 g, n = 24) were provided by Shanghai SLAC Laboratory Animal Co., Ltd. All mice were reared at constant temperature (25—27°C) and humidity. The experiments involving animals were granted by the Experimental Animal Ethics Committee of First Affiliated Hospital of Gannan Medical University and in accordance with the International Convention on the Ethics of Laboratory Animals and national regulations.
Nude mice were divided into oe-NC, oe-FBN1, oe-EBF1 + sh-NC, and oe-EBF1 + sh-FBN1 groups, with six mice in each group. When SiHa-DDP cells reached 80—90% confluency, cells were infected with lentiviruses (MOI =10), in the presence of 10 μg/mL polybrene. Next, the cells were digested treated with trypsin, centrifuged, washed with PBS two to three times, and re-suspended for counting. After that, cell suspension (20 μL, 1 × 107 cells/mL) was inoculated subcutaneously on the back of nude mice to establish a xenograft model of CC. When tumor volume reached 0.1 cm3, DDP (3 mg/kg) was injected weekly for 4 weeks. During the experiment, the length and width of the tumors were measured weekly with a vernier caliper. The tumor volume was calculated as (a × b2)/2 (a—represents the length of the tumor, b—represents the width of the tumor), and thereby tumor growth curve was plotted. Four weeks later, mice were euthanized by intraperitoneal injection of pentobarbital sodium (100 mg/kg). The subcutaneous xenografts were isolated and weighted.
Statistical analysis. Data were processed using SPSS21.0 software (IBM SPSS Statistics, Chicago, IL, USA) and GraphPad Prism 9.0 software (GraphPad Software Inc., San Diego, CA, USA), and expressed as mean ± standard deviation. The normality and homogeneity of variance were evaluated before comparisons. Data with normality and homogeneity of variance between two groups were compared using t-test, and those among multiple groups were compared with one-way analysis of variance (ANOVA) or two-way ANOVA followed by Tukey’s multiple comparisons test. P < 0.05 indicated a statistically significant difference.
RESULTS
EBF1 Was Underexpressed in SiHa-DDP Cells
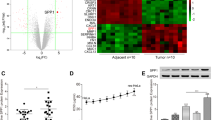
According to the Gene Expression Profiling Interactive Analysis (GEPIA) website (http://gepia.cancer-pku.cn/detail.php?gene=EBF1###) prediction, compared to normal tissues, the EBF1 is downregulated in CESC tissues (Fig. 1a, P < 0.05). This indicates that EBF1 is implicated in progression of CC. By analyzing GSE56363 dataset from GEO database, it was found that EBF1 is expressed at a low level in tissues of patients insensitive to chemotherapy (Fig. 1b, P < 0.05), suggesting that EBF1 activity may be involved in chemosensitivity of CC cells. Along these lines, qRT-PCR and Western blotting showed that EBF1 expression was markedly decreased in CC tissues, especially in those resistant to chemotherapy (Figs. 1c, 1d, P < 0.05).
The expression of EBF1 is low in SiHa-DDP cells. (a) The analysis of EBF1 expression using GEPIA website (http://gepia.cancer-pku.cn/detail.php?gene=EBF1###) showed the protein is downregulated in CESC. (b) Low expression of EBF1 was revealed in tissues of chemo-resistant patients by analyzing GSE56363 dataset from GEO database. (c, d) EBF1 expression in normal (N = 30), chemo-sensitive (N = 12), and chemo-resistant (N = 18) tissues was estimated by qRT-PCR and Western blotting. (e) Cell viability was tested using CCK-8 assay in the presence of different concentrations of DDP. (f, g) EBF1 expression in SiHa and SiHa-DDP cells was assessed using qRT-PCR and Western blotting. Experiments using cell lines were performed in triplicates. Data were expressed as mean ± standard deviation. One-way ANOVA with Tukey’s multiple comparisons test used for post hoc analysis was used to evaluate the statistical significance in c–e and the t-test was used for the statistical evaluation of data in e–g. * indicates P < 0.05.
Next, the viability of SiHa and SiHa-DDP cells treated with different concentrations of DDP was tested by CCK-8 assay. As a result, SiHa-DDP cells demonstrated better viability and higher IC50 value than SiHa cells, suggesting that SiHa-DDP cells had drug resistance potential (Fig. 1e, P < 0.05). qRT-PCR and Western blotting results showed that EBF1 expression in SiHa-DDP cells was lower than in SiHa cells (Figs. 1f, 1g, P < 0.05), indicating that EBF1 was downregulated in SiHa-DDP cells.
Overexpression of EBF1 Expedited the Sensitivity of CC Cells to DDP
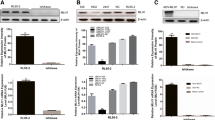
SiHa-DDP cells were transduced with lentiviruses encoding EBF1 (oe-EBF1) or control viruses (oe-NC). Using qRT-PCR and Western blotting it was shown that in oe-EBF1-transduced cells the expression of EBF1 was higher than in cell transduced with the control lentiviruses (Figs. 2a, 2b, P < 0.05). Moreover, the cell viability and IC50 value in oe-EBF1-transduced cells were signally decreased compared to those in control cells (Figs. 2c, P < 0.05). Accumulation of MDR1 and MRP1 proteins in cellular lysates was assessed by Western blotting. Results showed that the expression of MDR1 and MRP1 was decreased in oe-EBF1-transduced cells compared with that in oe-NC-transduced cells (Fig. 2d, P < 0.05). These results indicated that the drug resistance of CC cells with EBF1 overexpression is decreased.
EBF1 overexpression increased the sensitivity of CC cells to DDP. SiHa-DDP cells were transduced with control (oe-NC) and EBF1-encoding lentiviruses (oe-EBF1) (a, b), EBF1 expression was estimated by qRT-PCR and Western blotting. (c) Cell viability was tested by CCK-8 assay after DDP treatment. (d), multidrug resistant proteins (MDR1 and MDR1) were revealed by Western blotting. (e) Cell viability was measured by CCK-8 assay. (f) Cell colony number was analyzed by colony forming assay. (g) Cell migration and invasion were measured by transwell assays (100×, scale bar = 100 μm). (h) Cell apoptosis was assessed by flow cytometry. Experiments using cell lines were performed in triplicates. Data were expressed as mean ± standard deviation. Data in c and e were analyzed by one-way ANOVA with Tukey’s multiple comparisons test. Data in a–d and f–h were compared by t-test. *, P < 0.05.
In addition, CCK-8, colony forming, transwell, and flow cytometry assays were used to test cell aggressiveness. Obtained results revealed that cell viability, colony formation, migration, and invasion were remarkably reduced but cell apoptosis was significantly increased in oe-EBF1-transduced cells when compared to cells transduced with oe-NC (Figs. 2e–2h, P < 0.05). These results suggested that overexpression of EBF1 could increase the sensitivity of CC cells to DDP.
EBF1 Activated the Transcription of Its Target Gene FBN1
By using GEPIA and GEO (GSE56363 dataset) databases, FBN1 was found to be decreased in CESC tissues compared with normal tissues (Fig. 3a, P < 0.05). Analysis of GSE56363 dataset revealed decreased expression of FBN1 in tissues from patients that were insensitive to chemotherapy (Fig. 3b, P < 0.05), suggesting that FBN1 may be also implicated in chemosensitivity of CC cells. By qRT-PCR and Western blotting it was demonstrated that FBN1 expression is decreased in tissues from CC patients in general, but particularly in tissues from patients resistant to chemotherapy (Figs. 3b, 3c, P < 0.05). Correlation analysis showed that EBF1 was positively correlated with FBN1 in CC tissues (Fig. 3d, P < 0.05).
EBF1 binds to FBN1 promoter region. (a) Using GEPIA online resource (http://gepia.cancer-pku.cn/ detail.php?gene=FBN1###) it has been shown that FBN1 is downregulated in CESC. (b, c), FBN1 expression in normal (N = 30), chemo-sensitive (N = 12), and chemo-resistant (N = 18) tissues was measured by qRT-PCR and Western blotting. (d) The correlation analysis between EBF1 and FBN1 mRNA expression was performed. (e) The EBF1 binding site in the promoter region of FBN1 was predicted using JASPAR website (https://jaspar.genereg.net/matrix/MA0154.4/) ([17], Creative Commons Attribution-NonCommercial License). (f, g) The binding of EBP1 to the promotor region of FBN1 was verified by dual luciferase reporter gene and ChIP assays. (h, i), FBN1 expression in SiHa and SiHa-DDP cells was estimated by qRT-PCR and Western blotting. (j, k), FBN1 expression was evaluated in EBF1-overexpressing SiHa-DDP cells by qRT-PCR and Western blotting. Experiments using cell lines were performed in triplicates. Data were expressed as mean ± standard deviation. Data in b, c were analyzed by one-way ANOVA; in f—by two-way ANOVA with Tukey’s multiple comparisons test. Statistical significance was evaluated using t-test (g, k) *, P < 0.05.
Moreover, using JASPAR website, it was predicted that EBF1 can bind FBN1 promoter region (http:// jaspar.genereg.net/) (Fig. 3e). To further study the molecular mechanism and role of EBF1 in drug resistance of CC cells, we verified binding of EBF1 to the promotor region of FBN1 using dual luciferase reporter gene and ChIP assays (Figs. 3f, 3g, P < 0.05). By qRT-PCR and Western blotting we have shown that in SiHa-DDP cells the expression of FBN1 was significantly lower than in SiHa cells (Figs. 3h, 3i, P < 0.05). It was also demonstrated that EBF1 overexpression in SiHa-DDP cells notably promoted the expression of FBN1 (Figs. 3j, 3k, P < 0.05). Taken together, EBF1 activated FBN1 transcription by binding to FBN1 promoter region.
Overexpression of FBN1 Facilitated the Sensitivity of CC Cells to DDP
To ascertain the impact of FBN1 on the sensitivity of CC cells to DDP, SiHa-DDP cells were transduced with oe-NC and oe-FBN1. By qRT-PCR it was shown that FBN1 mRNA expression in oe-FBN1-transduced cells was higher than in cells transduced with oe-NC lentivirus (Fig. 4a, P < 0.05). CCK-8 assay results demonstrated that the cell viability and IC50 value were decreased in oe-FBN1-transduced cells compared with those in oe-NC-transduced cells (Fig. 4b, P < 0.05). Additionally, compared with oe-NC group, by Western blotting it was shown that the expression of MDR1 and MRP1 in oe-FBN1 group was significantly decreased (Fig. 4c, P < 0.05). These findings demonstrated that the drug resistance in FBN1-overexpressing cells was suppressed.
Overexpression of FBN1 increased the sensitivity of CC cells to DDP. SiHa-DDP cells were transduced with lentiviruses oe-NC and oe-FBN1. After that, FBN1 expression (a) was estimated by qRT-PCR. (b) Cell viability was tested by CCK-8 assay after DDP treatment. (c) MDR1 and MDR1 levels were analyzed by Western blotting. (d) The viability of transduced cells was estimated by CCK-8 assay. (e) The cell colony number was analyzed by colony forming assay. (f) Cell migration and invasion were analyzed by transwell assays. (g) Cell apoptosis was assessed by flow cytometry. Experiments using cell lines were performed in triplicates. Data were expressed as mean ± standard deviation. Statistical significance (a–c, e–g) was tested using t-test. Data in b and d were analyzed by two-way ANOVA with Tukey’s multiple comparisons test used for post hoc analysis. *, P < 0.05.
Moreover, compared to oe-NC-transduced cells cell proliferation capacity, colony formation, migration, and invasion of oe-FBN1-transduced cells were remarkably inhibited (Figs. 4d–4f, P < 0.05), but cell apoptosis was stimulated (Figs. 4g, P < 0.05). These data indicated that upregulation of FBN1 increased the sensitivity of CC cells to DDP.
EBF1 Promoted the Sensitivity of CC Cells to DDP via Activating FBN1 Transcription
Three shRNA sequences targeting FBN1 were designed. The expression of FBN1 mRNA in cells transduced with lentiviruses encoding corresponding short hairpin RNA molecules was evaluated by qRT-PCR. It has been shown that FBN1 mRNA expression was decreased in transduced cells (Fig. 5a). Among the tested hairpin RNAs the sh-FBN1 #3 demonstrated highest silencing efficiency, and thus was selected for subsequent experiments (referred as sh-FBN1 in the following text) (Fig. 5a, P < 0.05). SiHa-DDP cells were simultaneously transduced with oe-EBF1 and sh-FBN1 to determine whether EBF1 could promote the sensitivity of CC cells to DDP by activating the transcription of FBN1. By CCK-8 assay it was shown that compared to oe-EBF1 + sh-NC-transduced cells cell viability and the DDP IC50 value in oe-EBF1 + sh-FBN1-transduced cells were significantly increased (Fig. 5b, P < 0.05). Consistently, the expression of MDR1 and MRP1 proteins was increased in oe-EBF1 + sh-FBN1-transduced cells (Fig. 5c, P < 0.05).
EBF1 increased the sensitivity of CC cells to DDP by activating FBN1 transcription. (a) FBN1 mRNA expression was estimated by qRT-PCR and sh-FBN1#3 was selected for subsequent experiments among three shRNA sequences targeting FBN1. SiHa-DDP cells were transduced with oe-EBF1 and sh-FBN1 lentiviruses simultaneously. (b) Cell viability was assessed by CCK-8 assay after DDP treatment. (c) MDR1 and MDR1 expression levels were analyzed by Western blotting. (d) Cell viability was measured using CCK-8 assay. (e) The cell colony number was analyzed by colony forming assay. (f) Cell migration and invasion were measured by transwell assays. (g) Cell apoptosis was assessed by flow cytometry. Experiments using cell lines were performed in triplicates. Data were expressed as mean ± standard deviation. Data in b and d were analyzed by two-way ANOVA and (a–c) and (e–g)—by one-way ANOVA, with Tukey’s multiple comparisons test used for post hoc analysis.*, P < 0.05.
As expected, compared to control cells (oe-EBF1 + sh-NC), cell proliferation, colony formation, migration, and invasion of cells transduced with oe-EBF1 + sh-FBN1, were remarkably stimulated (Figs. 5d–5f, P < 0.05) but cell apoptosis level was decreased (Fig. 5g, P < 0.05). Taken together, EBF1 activated FBN1 transcription and increased the sensitivity of CC cells to DDP.
FBN1 Silencing Reversed the Effect of EBF1 Overexpression on Chemosensitivity of CC Cells in vivo
To verify that chemosensitivity of CC cells is dependent on the EBF1 expression levels, a mouse model of CC was established. Compared with oe-NC group the average volume and weight of tumors were decreased in animals from the oe-EBF1 group. However, compared to the oe-EBF1 + sh-NC group the average volume and weight of tumors in mice from the oe-EBF1 + sh-FBN1 group were prominently elevated (Figs. 6a–6c, P < 0.05). Moreover, by qRT-PCR and Western blotting it was shown that the expression of EBF1 and FBN1 was higher in samples from animals of the oe-EBF1 group than of the oe-NC group. Compared with oe-EBF1 + sh-NC group, FBN1 expression in tumors was considerably decreased in samples from the oe-EBF1 + sh-FBN1 group. No significant difference in EBF1 expression was demonstrated in samples from these groups (Figs. 6d, 6e, P < 0.05). In summary, knockdown of FBN1 expression could reverse the effect of EBF1 overexpression on chemosensitivity of CC cells.
Knockdown of FBN1 reversed the effect of EBF1 overexpression on chemosensitivity of CC cells. (a) Analysis of the tumor growth dynamics. (b) Images of tumors obtained from the animals of different groups. (c) Comparative analysis of the average weight of tumors from the animals of different groups. (d, e) The expression of EBF1 and FBN1 was measured using qRT-PCR and Western blotting. n = 6. Data were expressed as mean ± standard deviation. One-way ANOVA analysis or two-way ANOVA was used for comparisons among multiple groups with Tukey’s multiple comparisons test used for post hoc analysis. * P < 0.05.
DISCUSSION
CC is the one of most common cancer in women worldwide. Different interventions (including behavior change intervention and educational intervention) and the heath belief model offer a useful base for the prevention of this disease [18]. Cisplatin (DDP) is widely used in the chemotherapy of CC [4, 19]. Uncontrolled tumor progression and chemoresistance are the leading causes of poor outcomes in CC [20]. Clarifying the mechanisms involved in CC cell proliferation and chemoresistance is urgently required in order to improve the survival rate of patients with CC. In the present study, we have shown that overexpression of EBF1 and FBN1 decreased aggressiveness and increased apoptosis levels as well as the sensitivity of CC cells to DDP. Moreover, it was revealed that EBF1 may enhance the sensitivity of CC cells to chemotherapeutics through the activation of FBN1 transcription.
Prior studies demonstrated that EBF1 is downregulated in lymphoma cell lines [21] and CCA cells and tissues [8]. Concordantly GEPIA online resource predicted decreased expression of EBF1 in CESC. Our subsequent experiments confirmed low EBF1 expression levels in CC cells and tissues, especially in tissues from patients with chemotherapy resistance. Our in vitro experiments showed that EBF1 overexpression in DDP-resistant CC cells led to a remarkable decrease in the viability and aggressiveness. These findings suggested that EBF1 may inhibit the development of CC. Interestingly, in gastric cancer (GC), overexpression of EBF1 in multiple cell lines dramatically reduced cell proliferation and colony formation capacity [9]. As a transcription factor, EBF1 can activate transcription of downstream target genes [22]. A previous study has been proved that EBF1 directly binds the USP5 promoter and activated its transcription, thus promoting colorectal cancer cell growth and resistance to chemotherapeutics [23]. The available data suggested that EBF1 might regulate transcription of certain genes to influence the resistance of cancer cells to chemotherapeutics. Using GEPIA online resource it was predicted that FBN1 expression is decreased in CESC cells. The correlation analysis revealed that the mRNA expression of EBF1 and FBN1 positively correlated in CC tissues. Functional experiments further verified that EBF1 can bind FBN1 promoter region to activate FBN1 transcription. Moreover, EBF1 promoted the sensitivity of CC cells to DDP by activating FBN1 transcription in vitro and in vivo.
Previously, FBN1 was reported as a new biomarker involved in the development and progression of early-onset CRC [24]. Consistent with our results FBN1 was found to be downregulated in bladder cancer specimens and cell lines, and was identified as a tumor microenvironment-related gene [25]. In this study, decreased expression of FBN1 was demonstrated in CC tissues and cells. Overexpression of FBN1 led to reduced invasion and migration capacity of DDP-resistant CC cells. Moreover, elevated apoptosis, and decreased levels of MDR1 and MRP1 proteins were revealed in cells with high FBN1 expression. All this indicated that overexpression of FBN1 could facilitate the sensitivity of CC cells to DDP. However, the same gene has different functions in different disease context. For example, FBN1 was reported to bind and undergo ubiquitination by the FBXO2. This leads to its degradation and exacerbated proliferation of endometrial carcinoma (EC) due to inactivated cell cycle and inhibition of the autophagy signaling pathways [26]. Another study showed that FBN1 expression level was increased in GC tissues and cells, and its reduction attenuated the progression of the gastric cancer [27]. In addition, OC cell migration and invasion were reported to be attenuated following FBN1 silencing [28]. Furthermore, Wang et al. recently found that knockout of FBN1 combined with treatment with an antiangiogenic drug apatinib improved the DDP-sensitivity of OC cells [29]. Controversial role of FBN1 in different cancers indicates that further studies are necessary to uncover molecular mechanisms that will explain the observed effects.
In summary, our study suggested that EBF1 increases the expression of FBN1 by binding to its promoter, this in turn increases the sensitivity of CC to DDP. Although the present study provides evidence for the impact of EBF1 and FBN1 on the sensitivity of CC to DDP, it is largely undetermined whether these two proteins affect the sensitivity of CC to other chemotherapeutic drugs. Overall, this study may provide promising candidates for the prediction and prevention of chemoresistance in human CC.
REFERENCES
Vu M., Yu J., Awolude O.A., Chuang L. 2018. Cervical cancer worldwide. Curr. Probl. Cancer. 42 (5), 457—465.
Arbyn M., Weiderpass E., Bruni L., de Sanjosé S., Saraiya M., Ferlay J., Bray F. 2020. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health. 8 (2), e191—e203.
Ryan M., Marlow L., Waller J. 2019. Socio-demographic correlates of cervical cancer risk factor knowledge among screening non-participants in Great Britain. Prev. Med. 125, 1–4.
Small W., Jr., Bacon M.A., Bajaj A., Chuang L.T., Fisher B.J., Harkenrider M.M., Jhingran A., Kitchener H.C., Mileshkin L.R., Viswanathan A.N., Gaffney D.K. 2017. Cervical cancer: A global health crisis. Cancer. 123 (13), 2404—2412.
Pfaendler K.S., Tewari K.S. 2016. Changing paradigms in the systemic treatment of advanced cervical cancer. Am. J. Obstet. Gynecol. 214 (1), 22—30.
Zhu H., Luo H., Zhang W., Shen Z., Hu X., Zhu X. 2016. Molecular mechanisms of cisplatin resistance in cervical cancer. Drug. Des. Devel. Ther. 10, 1885—1895.
Nieminen-Pihala V., Tarkkonen K., Laine J., Rummukainen P., Saastamoinen L., Nagano K., Baron R., Kiviranta R. 2021. Early B-cell Factor1 (Ebf1) promotes early osteoblast differentiation but suppresses osteoblast function. Bone. 146, 115884.
Armartmuntree N., Murata M., Techasen A., Yongvanit P., Loilome W., Namwat N., Pairojkul C., Sakonsinsiri C., Pinlaor S., Thanan R. 2018. Prolonged oxidative stress down-regulates Early B cell factor 1 with inhibition of its tumor suppressive function against cholangiocarcinoma genesis. Redox Biol. 14, 637—644.
Xing M., Ooi W.F., Tan J., Qamra A., Lee P.H., Li Z., Xu C., Padmanabhan N., Lim J.Q., Guo Y.A., Yao X., Amit M., Ng L.M., Sheng T., Wang J., Huang K.K., Anene-Nzelu C.G., Ho S.W.T., Ray M., Ma L., Fazzi G., Lim K.J., Wijaya G.C., Zhang S., Nandi T., Yan T., Chang M.M., Das K., Isa Z.F.A., Wu J., Poon P.S.Y., Lam Y.N., Lin J.S., Tay S.T., Lee M.H., Tan A.L.K., Ong X., White K., Rozen S.G., Beer M., Foo R.S.Y., Grabsch H.I., Skanderup A.J., Li S., Teh B.T., Tan P. 2020. Genomic and epigenomic EBF1 alterations modulate TERT expression in gastric cancer. J. Clin. Invest. 30 (6), 3005—3020.
Zhu J., Wang H., Ma T., He Y., Shen M., Song W., Wang J.J., Shi J.P., Wu M.Y., Liu C., Wang W.J., Huang Y.Q. 2020. Identification of immune-related genes as prognostic factors in bladder cancer. Sci Rep. 10 (1), 19695.
Shen Z., Chen Y., Li L., Liu L., Peng M., Chen X., Wu X., Sferra T.J., Wu M., Lin X., Cheng Y., Chu J., Shen A., Peng J. 2020. Transcription factor EBF1 over-expression suppresses tumor growth in vivo and in vitro via modulation of the PNO1/p53 pathway in colorectal cancer. Front. Oncol. 10, 1035.
Ribera J., Morgades M., Zamora L., Montesinos P., Gómez-Seguí I., Pratcorona M., Sarrà J., Guàrdia R., Nomdedeu J., Tormo M., Martínez-Lopez J., Hernández-Rivas J.M., González-Campos J., Barba P., Escoda L., Genescà E., Solé F., Millá F., Feliu E., Ribera J.M.; Spanish PETHEMA Group and the Spanish Society of Hematology (2015) Prognostic significance of copy number alterations in adolescent and adult patients with precursor B acute lymphoblastic leukemia enrolled in PETHEMA protocols. Cancer. 121 (21), 3809—3817.
Del Cid J.S., Reed N.I., Molnar K., Liu S., Dang B., Jensen S.A., DeGrado W., Handford P.A., Sheppard D., Sundaram A.B. 2019. A disease-associated mutation in fibrillin-1 differentially regulates integrin-mediated cell adhesion. J. Biol. Chem. 294 (48), 18232—18243.
Zhai X., Xue Q., Liu Q., Guo Y., Chen Z. 2017. Colon cancer recurrence-associated genes revealed by WGCNA coexpression network analysis. Mol. Med. Rep. 16 (5), 6499—6505.
Zhang W., Ota T., Shridhar V., Chien J., Wu B., Kuang R. 2013. Network-based survival analysis reveals subnetwork signatures for predicting outcomes of ovarian cancer treatment. PLoS Comput. Biol. 9 (3), e1002975.
Millstein J., Budden T., Goode E.L., Anglesio M.S., Talhouk A., Intermaggio M.P., Leong H.S., Chen S., Elatre W., Gilks B., Nazeran T., Volchek M., Bentley R.C., Wang C., Chiu D.S., Kommoss S., Leung S.C.Y., Senz J., Lum A., Chow V., Sudderuddin H., Mackenzie R., George J.; AOCS Group, Fereday S., Hendley J., Traficante N., Steed H., Koziak J.M., Köbel M., McNeish I.A., Goranova T., Ennis D., Macintyre G., Silva De Silva D., Ramón Y., Cajal T., García-Donas J., Hernando Polo S., Rodriguez G.C., Cushing-Haugen K.L., Harris H.R., Greene C.S., Zelaya R.A., Behrens S., Fortner R.T., Sinn P., Herpel E., Lester J., Lubiński J., Oszurek O., Tołoczko A., Cybulski C., Menkiszak J., Pearce C.L., Pike M.C., Tseng C., Alsop J., Rhenius V., Song H., Jimenez-Linan M., Piskorz A.M., Gentry-Maharaj A., Karpinskyj C., Widschwendter M., Singh N., Kennedy C.J., Sharma R., Harnett P.R., Gao B., Johnatty S.E., Sayer R., Boros J., Winham S.J, Keeney G.L., Kaufmann S.H., Larson M.C., Luk H., Hernandez B.Y., Thompson P.J., Wilkens L.R., Carney M.E., Trabert B., Lissowska J., Brinton L., Sherman M.E., Bodelon C., Hinsley S., Lewsley L.A., Glasspool R., Banerjee S.N., Stronach E.A., Haluska P., Ray-Coquard I., Mahner S., Winterhoff B., Slamon D., Levine D.A., Kelemen L.E., Benitez J., Chang-Claude J., Gronwald J., Wu A.H., Menon U., Goodman M.T., Schildkraut J.M., Wentzensen N., Brown R., Berchuck A., Chenevix-Trench G., deFazio A., Gayther S.A., García M.J., Henderson M.J., Rossing M.A., Beeghly-Fadiel A., Fasching P.A., Orsulic S., Karlan B.Y., Konecny G.E., Huntsman D.G., Bowtell D.D., Brenton J.D., Doherty J.A., Pharoah P.D.P., Ramus S.J. 2020. Prognostic gene expression signature for high-grade serous ovarian cancer. Ann. Oncol. 31 (9), 1240—1250.
Castro-Mondragon J.A., Riudavets-Puig R., Rauluseviciute I., Lemma R.B., Turchi L., Blanc-Mathieu R., Lucas J. Boddie P., Khan A., Manosalva Pérez N., Fornes O., Leung T.Y., Aguirre A., Hammal F., Schmelter D., Baranasic D., Ballester B., Sandelin A., Lenhard B., Vandepoele K., Wasserman W.W., Parcy F., Mathelier A. 2022. JASPAR 2022: The 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2022, D165—D173.
Saei Ghare Naz M., Kariman N., Ebadi A., Ozgoli G., Ghasemi V., Rashidi Fakari F. 2018. Educational interventions for cervical cancer screening behavior of women: A systematic review. Asian Pac. J. Cancer Prev. 19 (4), 875—884.
Webb K., Prakash V., Kirresh O., Stewart A. 2018. A case of aortitis during cisplatin-based chemotherapy for cervical cancer. BJR Case Rep. 5 (1), 20180054.
Li R., Song Y., Chen X., Chu M., Wang Z.-W., Zhu X. 2021. METTL3 increases cisplatin chemosensitivity of cervical cancer cells via downregulation of the activity of RAGE. Mol. Ther. Oncolytics. 22, 245—255.
Bullerwell C.E., Robichaud P.P., Deprez P.M.L., Joy A.P., Wajnberg G., D’Souza D., Chacko S., Fournier S., Crapoulet N., Barnett D.A., Lewis S.M., Ouellette R.J. 2021. EBF1 drives hallmark B cell gene expression by enabling the interaction of PAX5 with the MLL H3K4 methyltransferase complex. Sci. Rep. 11 (1), 1537.
Harder L., Eschenburg G., Zech A., Kriebitzsch N., Otto B., Streichert T., Behlich A.S., Dierck K., Klingler B., Hansen A., Stanulla M., Zimmermann M., Kremmer E., Stocking C., Horstmann M.A. 2013. Aberrant ZNF423 impedes B cell differentiation and is linked to adverse outcome of ETV6-RUNX1 negative B precursor acute lymphoblastic leukemia. J. Exp. Med. 210 (11), 2289—2304.
Xu X., Huang A., Cui X., Han K., Hou X., Wang Q., Cui L., Yang Y. 2019. Ubiquitin specific peptidase 5 regulates colorectal cancer cell growth by stabilizing Tu translation elongation factor. Theranostics. 9 (14), 4208—4220.
Mo X., Su Z., Yang B., Zeng Z., Lei S., Qiao H. 2020. Identification of key genes involved in the development and progression of early-onset colorectal cancer by co-expression network analysis. Oncol. Lett. 19 (1), 177—186.
Wang Z., Tu L., Chen M., Tong S. 2021. Identification of a tumor microenvironment-related seven-gene signature for predicting prognosis in bladder cancer. BMC Cancer. 21 (1), 692.
Che X., Jian F., Wang Y., Zhang J., Shen J., Cheng Q., Wang X., Jia N., Feng W. 2020. FBXO2 Promotes proliferation of endometrial cancer by ubiquitin-mediated degradation of FBN1 in the regulation of the cell cycle and the autophagy pathway. Front. Cell Dev. Biol. 8, 843.
Yang D., Zhao D., Chen X. 2017. MiR-133b inhibits proliferation and invasion of gastric cancer cells by up-regulating FBN1 expression. Cancer Biomark. 19 (4), 425—436.
Wang Z., Liu Y., Lu L., Yang L., Yin S., Wang Y., Qi Z., Meng J., Zang R., Yang G. 2015. Fibrillin-1, induced by Aurora-A but inhibited by BRCA2, promotes ovarian cancer metastasis. Oncotarget. 6 (9), 6670—6683.
Wang Z., Chen W., Zuo L., Xu M., Wu Y., Huang J., Zhang X., Li Y., Wang J., Chen J., Wang H., Sun H. 2022. The Fibrillin-1/VEGFR2/STAT2 signaling axis promotes chemoresistance via modulating glycolysis and angiogenesis in ovarian cancer organoids and cells. Cancer Commun. (London). 42 (3), 245—265.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ADDITIONAL INFORMATION
The text was submitted by the author(s) in English.
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement of compliance with standards of research involving humans as subjects. This study was approved by the Ethics Committee of First Affiliated Hospital of Gannan Medical University and fully complied with the Declaration of Helsinki. All patients had signed written informed consent.
Rights and permissions
About this article
Cite this article
Shen, N.N., Lin, J.H. & Liu, P.P. EBF1 Promotes the Sensitivity of Cervical Cancer Cells to Cisplatin via Activating FBN1 Transcription. Mol Biol 57, 491–501 (2023). https://doi.org/10.1134/S0026893323030093
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893323030093