Abstract—
Eutrophication of lakes results in the intensification of anaerobic processes, including methanogenesis, and therefore in enhanced emission of methane. A littoral area with its variable oxygen regime is the first to react to eutrophication. The diversity of microbial communities in littoral areas is insufficiently studied, and little data are available concerning the methane cycle microorganisms. In this work, the methanogenesis and methane oxidation were investigated in the littoral site of a freshwater temperate Lake Senezh (Russia). A combination of analytical, microbiological and molecular techniques was used, including physicochemical analyses, high-throughput sequencing, potential activity measurements, and cultivation on selective media. The littoral site was found to be an extremely labile ecological niche, which harbors a diverse community containing aerobic, facultative anaerobic and anaerobic microorganisms, both autotrophs and heterotrophs, which may perform all reactions of the N, S, and CH4 cycles. Methane formation was carried out via hydrogenotrophic, acetoclastic, methylotrophic, and methyl-reducing pathways. Among methanotrophs, type I organisms predominated; type II, nitrate- and nitrite-dependent methanotrophs were also revealed. Comparison of the average rates of methanogenesis and aerobic methane oxidation suggests that all methane, which may potentially be formed in the littoral site of the lake, could simultaneously be oxidized.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Anthropogenic activities and associated global climate change affect aquatic ecosystems, leading to a change in the structure of microbial communities and, as a result, to a change in the microbial processes connected with transformation of the major biogenic elements (carbon, sulfur and nitrogen). Polar and alpine ecosystems are specifically sensitive to climate change (Preston et al., 2016; Dinasquet et al., 2018). Recent studies have dealt with the microbial methane cycle processes occurring in the Arctic freshwater ecosystems due to permafrost thaw and release of stored organic matter (OM) (review by Kallistova et al., 2019 and references therein). At the same time, the effect of climate change on the microbial processes in freshwater lakes located in the temperate zone has attracted considerably less attention. Eutrophication occurring in lakes is the main undesirable result of anthropogenic impact and climate change in temperate latitudes. Increased supply of anthropogenically produced nitrogen and phosphorus results in an enhanced primary production in the water column and therefore in increased accumulation of phototroph-derived OM in the sediments (Diaz and Rosenberg, 2008; Bechtel and Schubert, 2009; Anderson et al., 2014). Excessive supply of OM in such lakes, including various anthropogenic contaminants, together with increased water temperature, result in the formation of an anoxic near-bottom layer, shifting predominant microbial groups from aerobic to anaerobic ones, and intensification of anaerobic biogeochemical processes with release of harmful gases (H2S, СО2, N2O, and CH4) into the atmosphere (Wang et al., 2022). Investigation of microbial profiles in the sediments of different freshwater lakes have revealed that the structure of bacterial communities varies greatly depending on the trophic state of the lake and thus, depends directly on the amount and source of incoming OM. The structure of the archaeal component of the community varies only in the upper (recently formed) sediment layers, while the older sediment layers of oligotrophic, mesotrophic, and eutrophic lakes exhibit no essential difference. Thus, eutrophication has affected primarily the structure of bacterial and archaeal communities in the upper sediment layers (Han et al., 2020).
The metabolic activity of methanogenic and methanotrophic microorganisms plays a central role in the carbon cycle in lakes. In freshwater lakes, methane is mainly formed by the methanogenic archaea inhabiting anoxic sediments and released into the water column via diffusion. Lakes are an important source of this greenhouse gas, responsible for an estimated 23−142 Tg CH4/year, i.e., for ~9% of the global methane emission (Rosentreter et al., 2021). Most methane produced is oxidized by aerobic methanotrophic bacteria inhabiting the oxic water layers (Yang et al., 2019). In the presence of suitable electron acceptors, methane may also be oxidized by the anaerobic methanotrophic bacteria and archaea (Billard et al., 2015). Methanotrophic microorganisms act therefore as a specific biofilter, which reduces emission of biogenic methane from lake sediments. Apart from methane consumption (decreasing its emission), methanotrophs are an important part of the food chain, contributing to the transport of carbon to the higher trophic levels (Reis et al., 2022).
Research on the methane cycle involves different sites across the lake basin, and usually includes a zone with the maximum depth, where hypoxic or anoxic conditions may exist (see, e.g., (Pimenov et al., 2010; Billard et al., 2015; Fuchs et al., 2016; Kallistova et al., 2018; Yang et al., 2019; Han et al., 2020; Tardy et al., 2021)). At the same time, the littoral zone of temperate lakes remains insufficiently explored in respect to microbial communities’ general structure and particularly to methane cycle microorganisms and processes. The littoral (coastal shoal) is the zone from the water’s edge of the lake to the lower boundary of bottom plants allocation (Konstantinov, 1986). Littoral waters are characterized by high dissolved oxygen (DO) concentration due to a shallow depth and active water intermixing (Moschos et al., 2021). In the case of lake eutrophication, the littoral area is the first to be affected: the OM turnover becomes impaired, while DO concentration decreases, which may result in the activation of methanogenesis. The littoral of meso-eutrophic Lake Remoray (France) was shown to have higher content of inorganic carbon compared with deep-water zones and harbors lower microbial diversity with the predomination of members of the taxa Cyanobacteria and Alphaproteobacteria (Tardy et al., 2021). Members of the phyla Proteobacteria, Bacteroidetes, and Chloroflexi were predominant in the bacterial community of the shallow coastal site of the eutrophic Lake Pamvotis (northwestern Greece). Abundant OTUs there were mainly represented by anaerobic or micro-aerobic bacteria, commonly found in the contaminated aquatic ecosystems, digesters, and wastewater (Moschos et al., 2021). The differences were found in the composition of the archaeal communities (including methanogens) between two zones (cyanobacteria- and macrophyte-dominated) of the shallow coastal area of the eutrophic freshwater Lake Taihu (China). The predomination of archaea involved in degradation of complex organic compounds (order Thermoplasmatales) and in sulfur metabolism (Sulfolobales and Desulfurococcales) was observed in sediments of macrophyte- and cyanobacteria-dominated zones, respectively (Fan and Xing, 2016). An object of the present work, the temperate freshwater Lake Senezh (Russia), is a popular destination for family holidays. The increase in water temperature together with highly intensive phytoplankton bloom especially at the littoral area was discovered in summer 2021. We hypothesized that enhanced primary production leads to a decline in DO levels in normally well-aerated shallow coastal waters due to the enrichment of the water and sediments with phototroph-derived OM and this could stimulate methanogenesis. The goal of the present work was to describe the littoral microbial community in regard to methane cycle microorganisms and processes (methanogenesis and aerobic methane oxidation). Lake Senezh has never been studied previously with respect to microbial diversity.
MATERIALS AND METHODS
Research area. Research was carried out by collecting the samples of near-bottom water and upper sediments of the littoral site of freshwater Lake Senezh (Solnechnogorsk region, Moscow province, Russia). This lake is an artificial basin constructed to store fresh water in the mid-19th century by using a dam at the confluence of two rivers, Sestra and Mazikha. Even though the town of Solnechnogorsk is located at the lake coast, the lake is not affected by any industry. The lake area is 8.51 km2, and its drainage area is 69.2 km2. The lake floor is even, and its shape is irregular, with the length of up to 5 km and the width of up to 3.5 km. The maximum and average depths of the lake are 5‒6 and 3 m, respectively. The lake is of the mesotrophic type, and is fed by atmospheric precipitation, springs, and a Sestra River tributary. The sediments are mostly silty or, in the area of the dam and in the northwestern part of the lake, pebble-sandy (Martynov, 1988).
Sampling. The sampling site was located remotely from the river channels, marshlands, the dam and the road. Samples of near-bottom water from the depth of 0.5 m and of the sediments (0‒5-cm layer) were collected 1.5 m from the shore (56°11′44.0′′ N, 36°59′10.3′′ E) on June 11, 2021. The samples were collected manually into plastic containers or bottles without access of the air and stored for 24 h at 15°C prior to analyses.
Analytical techniques. The temperature was measured in situ using a mercury thermometer. DO concentration was determined by titration technique according to the Winkler method using the Aquamerck test kit 1.1107.0001 (Merck, Germany). Methane content was determined using the headspace method (McAuliffe, 1971). Methane concentration in the gas phase was measured using Crystal 2000 gas chromatograph (Chromatec, Russia) equipped with a flame ionization detector and a 3-m column filled with Hayesep N mesh. The temperature of the injector was 100°C, the temperature of the column was 60°C, and the flow rate of the carrier gas (N2) was 30 mL/min. The pH was measured using a SevenCompact pH/Ion S220 meter (Mettler-Toledo AG, Switzerland). Total dissolved solids were determined gravimetrically. The concentrations of the bicarbonate and carbonate ions were determined by titration method. The concentrations of nitrate, nitrite, sulfate, phosphate, and chloride ions were measured by ion chromatography on a Dionex ICS-1100 chromatograph (USA). Total content of Ca, Fe, Mg, Mn, K, Na was determined by atomic emission spectroscopy with inductively coupled plasma on an Agilent 5110 ICP-OES spectrometer (Agilent Technologies, USA). The concentrations of ammonium, Cr(VI) were determined spectrophotometrically on a Hach-Lange DR 3900 laboratory spectrometer (Germany).
Design of the experiment. The experiment consisted of three consecutive stages. The first stage included a general description of the in situ microbial community in native samples collected at littoral site of the lake with a focus on methanogenic and methanotrophic populations. For this purpose, native samples without any substrate or medium addition were analyzed by high-throughput sequencing of 16S rRNA gene fragments. The second stage included a determination of potential activities of methanogenesis and aerobic methane oxidation. For this purpose, native samples were amended with different substrates (without any selective media addition) and dynamics of production/consumption of gases during the incubation was followed by using gas chromatography. The third stage included identification of culturable methyl-reducing methanogens and aerobic methanotrophs by applying the selective media. The target methanogenic and methanotrophic microorganisms were identified in enrichment cultures by high-throughput sequencing of 16S rRNA gene fragments after the 10th transfer of cultures into the fresh selective media.
Microbiological techniques. Potential methanogenic activity was determined by incubation of sediments with a total of eight substrates providing the main pathways of methanogenesis: acetoclastic (acetate), hydrogenotrophic (formate), methylotrophic (trimethylamine ((CH3)3N, TMA); methanol; and dimethyl sulfoxide ((CH3)2SO, DMSO)), and methyl-reducing (TMA + H2, methanol + H2, and DMSO + H2). For this purpose, the sediment slurries were prepared by mixing sediments with the near bottom water at 1 : 1 ratio (v/v). Prior to the slurries preparation, the near-bottom water was boiled for the removal of DO and cooled to room temperature under argon flow. The slurries were purged with argon and distributed under argon flow into glass vials resulting in the liquid to gas ratio of 1 : 4 (v/v), then vials were closed with thick rubber stoppers (Bellco Glass, USA) and sealed with aluminum caps. No reducing agents were added to the vials. Series of parallel incubations were prepared with an addition of methanogenic substrates (acetate, formate, methanol, TMA, and DMSO) to a final concentration of 10 mM, each. Mixtures with hydrogen were supplemented with methanol, DMSO, or TMA (10 mM) and 6% H2 (v/v). The substrates were added into closed vials by syringes. After exhaustion of H2 in the gas phase during the incubation, it was added into the vials with a syringe. The control vial contained no substrates and showed the endogenous methanogenesis occurring in the sediments. The vials were incubated statically at 30°C in the dark. Methanogenesis was assayed by methane accumulation into the vial’s headspace. Concentrations of СН4, H2, and СО2 were measured once a day on a Crystal 5000.1 gas chromatograph (Chromatec, Russia) equipped with a flame ionization detector and a 2-m column filled with Hayesep N mesh. The temperature of the chromatograph injector was 50°C, the temperature of the column was 50°C, and the flow rate of the carrier gas (Ar) was 25 mL/min. Excessive pressure in the vials was accounted for during calculation of methane concentrations. The amount of the produced methane was calculated per 1 g of dry weight (DW) of the slurry; DW was determined by drying the slurry to a constant weight at 105°C. The average potential rate of methanogenesis was estimated as the amount of methane produced during 10 days after the start of the experiment. Results are presented as an average of two biological replicates.
The potential aerobic methane-oxidizing activity was also determined in the sediment slurries with near-bottom water (1 : 1 v/v); for these experiments, near-bottom water was not boiled. The slurries were dispensed into the glass vials resulting in the liquid to gas (air) ratio of 1 : 5 (v/v), sealed hermetically, and supplemented with СН4 to the final concentration in the gas phase of 10% (v/v). Vials were incubated statically at 30°C in the dark, and СН4 concentration in the gas phase was measured once a day. Dynamics of aerobic methane oxidation was determined by a decrease in methane concentration in the headspace. The СН4 concentration was measured on a Crystal 5000.1 gas chromatograph as described above. The amount of consumed methane was calculated per 1 g of dry weight (DW) of the slurry. The potential rate of aerobic methane oxidation was estimated as the amount of methane consumed during 10 days after the onset of the experiment. The results are presented as averages for three biological replicates.
After the end of the incubations, the material from the vials was used to inoculate (10% v/v) the selective media for cultivation of methanogenic archaea and aerobic methanotrophic bacteria. Methanogenic archaea were grown in the Pfennig anaerobic medium (Pfennig, 1965) supplemented with the Lippert trace elements solution (Pfennig and Lippert, 1966) and the Wolin vitamins solution (Wolin et al., 1963). The substrates used were DMSO and mixtures of C1-methylated compounds (DMSO, methanol, and TMA) with hydrogen. Hydrogen was injected with syringes into the vials to the final concentration in the gas phase of 18‒20% (v/v). Sterile concentrated solutions of the DMSO, methanol, and TMA were injected into the vials with a sterile Pfennig medium before inoculation to the concentration of 10 mM.
Enrichment cultures of methanotrophic bacteria were obtained on the P mineral medium (Galchenko, 2008) with a decreased nitrate concentration and 10% (v/v) of CH4 in the air gas phase. The medium included (g/L): KNO3—0.5; MgSO4⋅7H2O—0.2; CaCl2—0.02; Na2HPO4⋅5H2O—1.5; KH2PO4—0.7; trace elements solution (mg/L): Na2EDTA—5.0; FeSO4⋅7H2O—2.0; ZnSO4⋅7H2O—0.1; MnCl2⋅ 4H2O—0.03; CoCl2⋅6H2O—0.2; CuSO4⋅5H2O— 0.1; NiCl2⋅6H2O—0.02; Na2MoO4⋅2H2O—0.03. The pH of the medium was in the range of 6.8−7.2.
All cultures were incubated at 30°C, and СН4 concentration in the gas phase was determined at regular intervals. Transfer of cell biomass (10%) into the fresh selective media was carried out after the accumulation of >5% СН4 in the gas phase (methanogenic enrichments) and consumption of 10% СН4 (methanotrophic enrichment).
Molecular techniques. The microbial community composition was determined in 6 samples: 2 native samples (not amended with substrate or medium), 3 methanogenic and one methanotrophic enrichment cultures. The procedures used for DNA isolation, amplification, sequencing, and data analysis of 5 samples (2 native samples and 3 methanogenic enrichments) have been described elsewhere (Savvichev et al., 2021).
DNA from methanotrophic enrichment was isolated using the DNeasy PowerLyzer Microbial Kit (Qiagen, Germany), according to the manufacturer’s recommendations. The quality and concentration of the DNA prepared were assessed spectrophotometrically on NanoDrop™ 8000 (ThermoFischer Scientific, USA). The amplicon libraries of the hypervariable V4 region of the 16S rRNA gene were prepared using the two-stage PCR strategy with the primers 515F (Hugerth et al., 2014) and Pro-mod-805R (Merkel et al., 2019) as was described previously (Toshchakov et al., 2021). PCR of every DNA sample was carried out in duplicate. The libraries were examined using agarose gel and pooled in equimolar amounts. The final pool was purified with the QIAquick PCR Purification Kit (Qiagen, Germany) according to the manufacturer’s recommendations. Sequencing was carried out with the MiSeq™ Personal Sequencing System (Illumina, USA) using the 156-bp paired-end reads.
The reads were tested for agreement with the target amplicon (the presence of the primer sequence), and the primer sequences and the low-quality regions were deleted with CLC Genomic Workbench v. 20.0.4 (Qiagen, Germany). Demultiplexing was carried out using the deML software package (Renaud et al., 2015) with the parameters excluding the mismatches in the index sequences. Approximately 35 000 pairs of high-quality reads were obtained for each sample.
The raw sequences obtained from native samples and methanogenic enrichments were deposited in the Sequence Read Archive (SRA) via the National Center for Biotechnology Information (NCBI) under the accession numbers SRR20855043−SRR20855047. The processed reads obtained from methanotrophic enrichments were uploaded to the NCBI SRA archive with the identifier SRX15219565.
Data analysis. High-quality read pairs were used as input for the DADA2 pipeline (Callahan et al., 2015), which was launched according to the open protocol (https://benjjneb.github.io/dada2/tutorial.html). Taxonomy of the obtained unique amplicon sequence variants (ASVs) was determined using the naive Bayesian classifier using the Silva138 database (Quast et al., 2013). The reads were rarefied and normalized using the phyloseq package in the R environment (McMurdie and Holmes, 2012).
RESULTS AND DISCUSSION
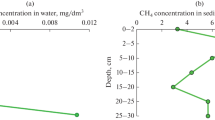
Physicochemical parameters. The data on the physicochemical parameters of the near-bottom water are presented in Table 1. Since the total water hardness determined by the sum of the Са2+ and Mg2+ was <1.5 mM, Lake Senezh littoral water could be considered as very soft in terms of hardness. Total dissolved solids concentration was 188 ± 20 mg/L, which corresponds to a fresh water of the hydrocarbonate type. The pH was weakly alkaline, probably due to the algal bloom at the time of sampling, which shifted pH to higher values. Although, the lake is located within the city limits, low concentrations of nitrogen species (<3 mg/L ammonium and nitrate and <0.001 mg/L nitrite), phosphate (<0.1 mg/L), and iron (<0.05 mg/L) indicated the absence of extraneous contamination. At the same time, sulfate concentration was relatively high (24 mg/L), so that the processes of the sulfur cycle could take place. Methane concentration in the near-bottom water was 8.0 ± 0.2 µg/L (0.5 ± 0.01 µmol/L). Methane content in the upper sediments was 10 times higher than in the water, reaching 79.2 ± 5.3 µg/dm3 (4.95 ± 0.3 µmol/dm3), which indicated the occurrence of methanogenesis in the sediments of the littoral site. DO concentration in the near-bottom water was 4.9 ± 0.25 mg/L, which was comparable to the value of the littoral site of the eutrophic Lake Pamvotis (5.3 mg/L) (Moschos et al., 2021). This oxygen concentration is relatively low and close to the value known for hypoxic conditions (≤2.8 mg/L) (Diaz and Rosenberg, 1995).
Overall diversity of littoral microbial community. The microbial community of Lake Senezh littoral site was dominated by bacteria, which contributed 83% of all 16S rRNA gene sequences retrieved from the samples. The relative abundance of archaea did not exceed 6.5% in the sediments (Table S1). Diatoms were the major primary producers (14% according to chloroplast DNA). It should be noted that the relative abundance of algal sequences may be overestimated as diatoms contain numerous or singular chloroplasts and the plastome of an individual may include many copies of the unit-genome (Ren et al., 2020). Detected sequences exhibited high similarity (>98%) with those of the diatoms species Asterionellopsis glacialis (GenBank accession no. KC509520), Amphora ovalis (EU580470), Aulacoseira granulata (FJ002181), and Dickieia ulvacea (FJ002229) (Fig. 1). The relative abundance of cyanobacteria was 1.5%, with the most abundant sequences (0.5%) affiliated with the species Dolichospermum lemmermannii (AJ630424).
Bacterial community contained members of 37 phyla, with 16 of them responsible for more than 1% of total 16S rRNA gene sequences each. The highest relative abundance was revealed for sequences affiliated with the phyla Proteobacteria (18–21%), Chloroflexi (12–16%), Bacteroidota (10–11%), Verrucomicrobiota (5.5–6.5%), Acidobacteriota (5.5%), Actinobacteriota (3–4%), Desulfobacterota (3–4%), Planctomycetota (3%), Myxococcota (2.3–2.5%), Nitrospirota (2%), Bdellovibrionota (1.5–2.3%), etc. (Table S1). The relative abundance of sequences affiliated with members of the phyla Fusobacteriota, Firmicutes, and Spirochaetota was higher in the sediment sample.
The sequences affiliated with the phylum Proteobacteria were highly diverse, with 700 OTUs detected. However, the relative abundance of each OTU was very low: it exceeded 0.1% for 51 OTUs, and among those for only 9 OTUs it was ≥0.5%. Most of the proteobacterial sequences were affiliated with the taxa represented by aerobic, micro-aerobic and facultatively anaerobic organotrophs. Among these, the highest share of the sequences (≥0.5%) belonged to the aerobic chemoorganotrophic members of the family Sinobacteraceae and to the species Leptothrix ginsengisoli (FM886840), the latter is an aerobic organotroph capable of Mn2+ oxidation (Spring et al., 1996). Proteobacteria involved in the nitrogen cycle were detected: nitrogen-fixing (Azovibrio restrictus), ammonium-oxidizing (members of the family Nitrosomonadaceae), denitrifiers (Denitratisoma sp., Thauera sp.), and aerobic chemoheterotrophs able to reduce nitrate to nitrite (Parahaliea mediterranea). Proteobacteria (Ca. “Accumulibacter”) involved in the accumulation of phosphates under cyclic aerobic-anaerobic conditions were identified. Aerobic methanotrophs with predomination of members of the genus Methylobacter were revealed (discussed in detail below), as well as methylotrophs of the family Methylophilaceae. High diversity of proteobacteria involved in the sulfur cycle was found: aerobic and micro-aerobic (some representatives are capable of nitrate reduction under anaerobic conditions), oxidizing hydrogen sulfide, sulfur and thiosulfate uncultured members of the genus Thiobacillus and the family Thiotrichaceae; facultatively anaerobic obligate and facultative autotrophs of the genera Sulfuricella, Sulfuritalea, and Sulfurisoma, oxidizing sulfur and thiosulfate to sulfate and capable of using nitrate as an electron acceptor in the absence of oxygen (Kojima and Fukui, 2010, 2011, 2014). Uncultured anoxygenic phototrophs of the family Chromatiaceae (purple sulfur bacteria oxidizing sulfur and sulfide to sulfate) were predominant (Fig. 1). Apart from proteobacteria, the organisms involved in the sulfur cycle included facultatively anaerobic, chemolithotrophic sulfur oxidizers of the genus Sulfuricurvum (phylum Campylobacterota), which utilize sulfide, elemental sulfur, thiosulfate and hydrogen as the electron donors and nitrate as the electron acceptor under anaerobic conditions (Kodama and Watanabe, 2004). Their relative abundance in the native samples was 0.16‒0.39% of the total number of the 16S rRNA gene sequences.
The second most abundant microbial group comprised the sequences affiliated with the phylum Chloroflexi. Among this phylum, the sequences of uncultured members of the family Anaerolineaceae (Fig. 1) predominated; the family comprises anaerobic filamentous chemoorganotrophs (Yamada et al., 2006).
The sequences affiliated with the phylum Bacteroidota, comprising diverse organotrophs, belonged to uncultured members of the orders Chitinophagales, Cytophagales, Flavobacteriales, Bacteroidales, Sphingobacteriales. Members of candidate phylum Ca. “Kapabacteria” (Bacteroidetes/Chlorobi group), for which aerobic heterotrophic metabolism was assumed (Kantor et al., 2015) were also detected. As in the case of proteobacteria, high diversity of the sequences was found (577 OTUs). However, only 25 OTUs had relative abundance above 0.1%, and among those, the only one OTU accounted for over 0.5% of the total sequences. It exhibited high similarity to uncultured members of the genus Cytophaga (GU269394), which comprises aerobic cellulolytic bacteria (Nakagawa and Yamasato, 1996).
Among members of the phylum Verrucomicrobiota, uncultured organisms with unknown physiological role in the community predominated, as well as aerobic organotrophs of the family Xiphinematobacteriaceae and the genera Limisphaera and Prosthecobacter.
Uncultured organisms were predominant among members of the phylum Acidobacteriota. The sequences affiliated with uncultured members of the family Bryobacteraceae, which comprises moderately acidophilic, mesophilic, aerobic or facultatively anaerobic chemoheterotrophs utilizing various sugars and polysaccharides (Dedysh et al., 2017) were detected, including the sequences with high similarity to Paludibaculum fermentans (NR_134120). Members of this species are capable of fermentation and dissimilatory Fe(III) reduction under anoxic conditions (Kulichevskaya et al., 2014). Saccharolytic members of the genus Ca. “Solibacter” (GQ421112), family Solibacteraceae, and uncultured members of the family Vicinamibacteraceae were also revealed, including the sequences affiliated with aerobic organotrophs of the genus Vicinamibacter (MW274181), which were the most representative acidobacteria in the samples (>0.5%) (Fig. 1).
Predominant members of the phylum Actinobacteriota, which comprises mainly aerobic soil organotrophs, were aerobic saccharolytic organisms: Ilumatobacter fluminis (MT449029) (up to 0.7% of the total number of the 16S rRNA gene sequences), Sporichthya brevicatena (JQ899212) (0.35%), Modestobacter versicolor (JQ899204) (0.4%), Gaiella occulta (NR_118138) (0.14%), and Solirubrobacter (MF439925) (0.2%). The sequences of uncultured members of the family Streptomycetaceae, orders Ca. “Actinomarinales” and Acidimicrobiales, were also detected (>0.1%).
Various sulfate reducers (SRB) were identified among members of the phylum Desulfobacterota, with the sequences affiliated with genera Desulfococcus (MG804074) and Desulfobacca (GU472643) being the most abundant. Sequences affiliated with genus Desulfovibrio were also detected. Over 0.2% of the sequences were affiliated with “cable bacteria” Ca. “Electronema nielsenii” (KP728465), filamentous electroconductive bacteria of the family Desulfo-bulbaceae, which probably employ sulfur-based energy metabolism, including sulfate/sulfite reduction, sulfur/sulfide oxidation, and organosulfonate reduction (Trojan et al., 2016). The sequences with high similarity to syntrophic bacteria of the genera Syntrophus (GQ183340), Syntrophobacter (KX366386), and uncultured members of the order Syntrophobacterales, which oxidize at low H2 partial pressure volatile fatty acids (VFAs) and aromatic acids with production of СО2 and H2, were revealed. The sequences with high similarity to Geobacter psychrophilus (NR_043075), anaerobic organotrophs are not reducing sulfate, but coupling acetate oxidation with Fe(III) reduction (Nevin et al., 2005), were also detected.
Among members of the phylum Planctomycetota, uncultured representatives of the families Phyci-sphaeraceae, Gemmataceae, Isosphaeraceae, Pirellulaceae, Rubinisphaeraceae, and Schlesneriaceae were revealed, as well as soil group WD2101 of the order Tepidisphaerales (microaerobic polysaccharide-degrading planctomycetes). Many planctomycetes thrive under oligotrophic conditions by consuming various high-molecular mass sugars provided by algae (Jeske et al., 2013). Most planctomycetes isolated in pure cultures are aerobic or microaerobic chemoorganotrophs able to degrade complex organic compounds (Storesund et al., 2020). The sequences belonging to uncultured members of the genera Gemmata, Pirellula, and Schlesneria and to the family Rubinisphaeraceae predominated in the littoral site of Lake Senezh (0.1−0.2%). The sequences affiliated with uncultured lineages Pla1, Pla3, and Pla4 were also revealed. Within the phylum Myxococcota, members of two classes, Myxococcia and Polyangia, were detected. Among members of the class Myxococcia, bacteria of the genus Anaeromyxobacter (HE648180) were predominant (>0.6%). Those were chlororespiring facultative anaerobes growing on acetate as an electron donor and a variety of electron acceptors (2‑chlorophenol, 2,6-dichlorophenol, 2,5-dichlorophenol, 2-bromophenol, nitrate, fumarate, or oxygen) at the optimal concentrations <1 mM (Sanford et al., 2002). Among members of the class Polyangia, predominant sequences had a high similarity with aerobic organotrophic members of the genera Racemicystis (MW182266) and Sorangium (MF042664).
Stage II nitrifiers (nitrite-oxidizing bacteria) belonged to the phyla Nitrospinota and Nitrospirota. Within the phylum Nitrospinota, uncultured members of the genus Nitrospina (FN429806) predominated (0.22–0.23%). Among members of the phylum Nitrospirota, uncultured members of the genus Nitrospira predominated; members of the cultured species Nitrospira lenta (NR_148573) were responsible for 0.6% of the total sequences. Apart from nitrite-oxidizers, the sequences closely related to the genus Thermodesulfovibrio (MW274524 and LC070255) were revealed among members of the phylum Nitrospirota (>0.4%). Nitrite-dependent anaerobic methanotrophs (AOM) of the NC10 group (phylum Methylomirabilota) were also involved in the nitrogen and methane cycles in the Lake Senezh littoral site; their relative abundance in the sediments was up to 0.13%.
The predominant sequences within the phylum Bdellovibrionota (comprising aerobic obligate predators of gram-negative bacteria (Li et al., 2021)) belonged to uncultured members of the family Bdellovibrionaceae and to the genera Bacteriovorax (JX493331), and Pigmentibacter (MN791129).
The relative abundance of members of the phyla Fusobacteriota, Firmicutes, and Spirochaetota was higher in the sediments than in the sediment-water slurry. The highest, 17-fold difference (0.09 vs. 1.6%), was observed for the relative abundance of the sequences affiliated with the phylum Fusobacteriota. It was due to the high share (1.45%) of a single sequence identified as an uncultured member of the order Fusobacteriales with 94.4% similarity to members of the genus Streptobacillus (MF195205), which cause infectious diseases in animals (Eisenberg et al., 2015a, 2015b). Members of the phyla Firmicutes and Spirochaetota were two times more abundant in the sediments than in the mixed sample. The share of the sediment sequences affiliated with the phylum Firmicutes was higher due to predominance of various clostridia (anaerobic fermentative bacteria): Clostridium beijerinckii, C. estertheticum subsp. estertheticum, C. paraputrificum, C. hydrogeniformans, C. manihotivorum, and Paeniclostridium ghonii. Other Firmicutes predominated in the mixed sample: Exiguobacterium profundum (MT573192) and Acidaminobacter sp. (FJ391474). These are fermentative bacteria, producing acetate, formate, CO2, and H2, the direct substrates for acetoclastic and hydrogenotrophic methanogenesis. The share of the sequences affiliated with the phylum Spirochaetota, was higher in the sediments due to the generally higher abundance of diverse sequences, among which uncultured members of the genus Spirochaeta (anaerobic organotrophs) predominated.
Archaea were represented in the littoral site of Lake Senezh by the sequences affiliated with the phyla Eu-ryarchaeota (0.13‒0.76% of the total number of sequences); Ca. “Aenigmarchaeota,” Ca. “Altiarchaeota,” Ca. “Micrarchaeota,” and Ca. “Woesearchaeota”—the DPANN group (total relative abundance of 0.85‒1.51%); Ca. “Lokiarchaeota”—the Asgard group (0.02% and only in the sediments); Crenarchaeota and Thaumarchaeota—the TACK group (0.06‒0.29%); and Ca. “Thermoplasmatota” (1.12‒3.85%) (Table S1). The sequences affiliated with members of the phyla Ca. “Woesearchaeota,” Ca. “Thermoplasmatota,” and methanogens of the class Methanomicrobia predominated in the sediments, where they were responsible for 6% of the total number of sequences and 92% of all archaeal sequences. Members of the phylum Ca. “Woesearchaeota” are widespread in various aquatic environments, including freshwater and saline lakes (Ortiz-Alvarez and Casamayor, 2016; Kadnikov et al., 2019; Kallistova et al., 2018, 2020; Savvichev et al., 2020). These archaea are incapable of oxidative metabolism and lack the pathways for synthesis of the major cell components, which may indicate their symbiotic or parasitic lifestyle (Castelle et al., 2015, 2018). Syntrophic interactions between Ca. “Woesearchaeota” and methanogens have been suggested (Liu et al., 2018) as well as contribution of this group to the activity of proteolytic microbial communities (Suominen et al., 2019). Within the phylum Euryarchaeota, in addition to methanogens, sequences belonging to nitrate-dependent AOM of the ANME-2 cluster were detected. Among members of the phylum Ca. “Thermoplasmatota,” the sequences affiliated with methanogens of the order Methanomassiliicoccales constituted 0.16% in the sediments and 0.04% in the mixed sample. The most abundant sequences (>3% in the sediments) were affiliated with uncultured members of the order Thermoplasmatales. The order Thermoplasmatales is mainly represented by facultatively anaerobic thermoacidophilic organisms, autotrophic or heterotrophic (Huber and Stetter, 2006). Among members of the phylum Ca. “Lokiarchaeota,” the sequences were revealed, which exhibited 94% similarity to members of the candidate species Ca. “Prometheoarchaeum syntrophicum” (CP042905), an anaerobic, amino acid-degrading syntroph (Imachi et al., 2020). Within the TACK group, uncultured Crenarchaeota were detected, including a member of the class Thermoprotei (aerobic and anaerobic thermoacidophiles, auto- and organotrophic, capable of sulfur metabolism), and uncultured Thaumarchaeota, including aerobic ammonium-oxidizing archaea of the genus Ca. “Nitrosocosmicus.”
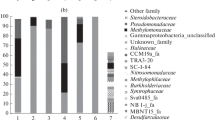
Diversity of the methane cycle microorganisms in the native water and sediment samples. The methanogenic population in the lake sediments was represented by members of the classes Methanobacteria and Methanomicrobia, and the order Methanomassiliicoccales. Acetoclastic (genus Methanothrix) and hydrogenotrophic (genera Methanobacterium, Methanoregula, Methanolinea, Methanosphaerula, Methanospirillum, etc.) methanogens were present in equal proportions (the share of each group was 0.3% of the total number of the 16S rRNA gene sequences). Abundance of methyl-reducing methanogens was twice lower (0.16%). The share of methylotrophic methanogens of the genera Methanosarcina and Methanomethylovorans did not exceed 0.1% (Fig. 2).
Predominant methanogens were represented by uncultured members of the genera Methanothrix and Methanoregula. We have previously reported predominance of members of these genera in freshwater Arctic lakes (Kallistova et al., 2018, 2021). Predominance of methanogens of the genus Methanoregula was also shown for the sediments of the deep perialpine Lake Bourget (France). In this lake, the methanogenic population was mainly regulated by the OM supply, while both the structure and abundance of the methanotrophic population was affected by oxygen availability (Lyautey et al., 2021). Thermophilic methanogens of the genera Methanothermus and Methanocaldococcus were shown to be more abundant in the sediments of the cyanobacteria-dominated zones of the shallow coastal area of the eutrophic freshwater Lake Taihu (China). In the macrophyte-dominated zone, methanogens of the genus Methanomassiliicoccus were significantly more abundant (Fan and Xing, 2016).
Analysis of the 16S rRNA gene sequences revealed the presence of both anaerobic (AOM) and aerobic methanotrophs in Lake Senezh. The sequences affiliated with AOM of the groups NC10 (nitrite-dependent AOM) and ANME-2 (nitrate-dependent AOM) were revealed; their total share in the sediments was 0.2%. Nitrite- and nitrate-dependent AOM are often detected in freshwater basins with a low sulfate concentration (Graf et al., 2018; Mayr et al., 2020; Kallistova et al., 2021; Martin et al., 2021; van Grinsven et al., 2022).
The overall share of aerobic methanotrophs was 1.25% and type I methanotrops were more abundant than type II (1.1 vs. 0.18%, respectively) (Fig. 2). Sequences were affiliated with type I methanotrophs of the genera Methylobacter, Methyloglobulus, Methylococcus, Ca. “Methyloumidiphilus,” Methylosoma, Methylogaea, Methylocaldum, Crenothrix, Methylosarcina, and type II methanotrophs of the genera Methylocapsa, Methylocystis, Methylosinus. Thus, the methanotrophic community of Lake Senezh was diverse in composition, in spite of its low overall relative abundance. Predominant sequences belonged to the genus Methylobacter, which has been routinely detected and often predominated in different freshwater lakes (He et al., 2012; Crevecoeur et al., 2017; Martinez-Cruz et al., 2017; Rissanen et al., 2018; Lyautey et al., 2021; Su et al., 2022). Shallow lakes Dianchi and Erhai (China) are exceptional in this respect. Members of the genus Methyloparacoccus (type Ib), which were predominant in the mesotrophic Lake Erhai, are generally rare in lakes. The eutrophic Lake Dianchi showed high relative abundance of type II methanotrophs, especially in summer, which was probably due to the trophic state of the lake. Lake Dianchi, subject to pronounced eutrophication with periodic algal blooms, was characterized by massive inflow of biogenic elements and organic carbon, as well as by active methanogenesis in the sediments (Yang et al., 2019). Type II methanotrophs were shown to outnumber type I ones in the eutrophic Lake Taihu (China) at the period of low abundance of cyanobacteria. During the cyanobacterial bloom and consequent eutrophication of the lake, the opposite was true (type I methanotrophs predominated over type II ones) (Wang et al., 2022). Type I methanotrophs were shown to predominate along the water column in six temperate freshwater lakes (Canada), while type II methanotrophs played an important role in well-aerated coastal waters (Reis et al., 2020). Investigation of methane oxidation in five freshwater lakes of different trophic states (Central Switzerland) revealed the highest methane flows in eutrophic lakes, while the highest abundance of methanotrophs was observed in the oligotrophic lake. Type I methanotrophs dominated in the oxic and suboxic near-bottom layers, exhibiting strict correlation with abundance of methylotrophic members of the family Methylophilaceae. Unlike the previous work, the abundance of type II methanotrophs increased in the deep anoxic sediment layers (van Grinsven et al., 2022).
Potential methanogenic and aerobic methanotrophic activities. Methane formation in the control samples from Lake Senezh littoral site (without addition of methanogenic substrates) was very weak, indicating low activity of endogenous methanogenesis (Fig. 3a, Table 2), which was probably due to the presence of dissolved oxygen in situ. Moreover, the direct methanogenic substrates were probably limited in the pebble-sandy sediment. With substrate addition, lake sediments were characterized by more active hydrogenotrophic methanogenesis (on formate) during the first 4 days of incubation. Hydrogenotrophic methanogens were probably active in situ due to substrate availability, and therefore became active without a significant lag phase. Other methanogens required time to achieve the highest activity upon the substrates’ addition. Similar results have been obtained for shallow freshwater thermokarst lakes (Kallistova et al., 2021). Activation of methylotrophic methanogenesis with methanol and TMA was observed on day 4 of incubation. Acetoclastic methanogenesis and methanogenesis with DMSO addition were activated on day 7 of incubation. It should be noted that methanogens do not use DMSO directly. Its conversion to methane occurs in two stages: first DMSO is converted to dimethyl sulfide (DMS) by SRB, and only then DMS is used as a substrate for methanogenesis (Jonkers et al., 1996). The rate of methanogenesis on DMSO depends directly on the activity of SRB. This both explains the long delay of methanogenesis on DMSO and indicates the presence of active SRB in the community. The highest amount of methane was produced during incubation with methylated compounds in the presence of hydrogen. This probably indicated a potential activity of methyl-reducing methanogens, the sequences of which were revealed in the sediments. It may, however, also result from combined occurrence of endogenous, methylotrophic, and hydrogenotrophic methanogenesis. Decomposition of C1-methylated compounds results in formation of bicarbonate, which, together with H2, is used for methane formation in the course of hydrogenotrophic methanogenesis (Kallistova et al., 2017). Moreover, СО2 may be produced due to the activity of endogenous anaerobic organotrophs present in the samples.
Potential methanogenic (a) and aerobic methanotrophic (b) activities in samples from the littoral site of Lake Senezh amended with following substrates: formate (2); methanol (3); acetate (4); methanol + H2 (5); DMSO (6); TMA (7), DMSO + H2 (8); TMA + H2 (9); CH4 (10). The control curve (1) shows endogenous methanogenesis without substrate addition.
Incubation of Lake Senezh with methane revealed the average potential rate of aerobic methane oxidation of 2.0 µmol/g DW/day (Fig. 3b, Table 2). Low methane-oxidizing potential could probably be due to the stably low in situ methane concentration (0.5 and 5 µmol/L in the water and sediments, respectively). These values were in good agreement with potential rates of methane oxidation in the sediments of the shallow eutrophic Lake Dianchi (1.3–2.2 µmol/g DW/day) and the shallow mesotrophic Lake Erhai (0.8–2.4 µmol/g DW/day) (Yang et al., 2019).
Comparison of the average potential rates of methanogenesis and aerobic methane oxidation suggests that all methane which may potentially be formed in littoral site of the lake could simultaneously be oxidized.
Enrichment cultures of methanogenic archaea. Members of the order Methanomassiliicoccales, which carry out methyl-reducing methanogenesis, were detected in the sediments. While free-living methyl-reducing methanogens are widespread in nature (Cozannet et al., 2021), and their sequences have been detected in freshwater and saline lakes (Kallistova et al., 2020, 2021; Savvichev et al., 2021), this group is difficult to cultivate, and Methanomassiliicoccus luminyensis (Dridi et al., 2012) is the only species that has been isolated so far. Representatives of this order are therefore poorly characterized in terms of physiology and biochemistry (Cozannet et al., 2021). In the present work, methanogens were provided with substrates supporting the growth of methyl-reducers, i.e., on the mixtures of methylated C1-compounds (DMSO, methanol and TMA) with hydrogen. The highest potential methanogenic activity in Lake Senezh sediments was revealed on these substrates. DMSO without hydrogen was used as the control substrate. While the highest potential methanogenic activity was observed on the TMA + H2 mixture, methane formation stopped after the second transfer of enrichment culture, when this mixture was used. Phylogenetic analysis after several transfers of enrichments grown on methanol + H2 and DMSO + H2 revealed that the cultivation conditions were not suitable for enrichment of the target methyl-reducing methanogens. The share of Methanomassiliicoccales on mixtures of C1-compounds with hydrogen did not exceed 0.02% of the total number of the 16S rRNA gene sequences. Hydrogenotrophic methanogens of the species Methanobacterium alcaliphilum (NR_112910.1) and Methanobacterium flexile (KY780618.1) grew well on methanol + H2. The shares of the sequences of these species in the enrichment were 18.5 and 20.6%, respectively (Table 3). A bacterium closely related to Acetoanaerobium sticklandii (NR_102880) was another dominant species (32.7%) in the enrichment culture on methanol + H2. Members of the genus Acetoanaerobium are chemoorganotrophs fermenting carbohydrates and yeast extract with formation of acetate (Sleat et al., 1985). Among the abundant sequences were those related to SRB of the genus Desulfosporosinus (9.3%), and organotrophs of the genera Pseudomonas (4.2%) and Erysipelothrix (2%). A hydrogenotrophic methanogen of the spicies Methanobacterium flexile (KY780618.1) also grew well on DMSO + H2 (24.5% of the total number of sequences). Apart from methanogens, predominant organisms on DMSO + H2 were uncultured members of the order Burkholderiales, SRB of the genus Desulfovibrio (both over 20%), and organotrophs of the genera Exiguobacterium (11%) and Acetoanaerobium (10%) (Table 3). The overall diversity and relative abundance of microorganisms at phylum level in methanogenic enrichment cultures are presented in Table S1.
The СО2 required for hydrogenotrophic methanogenesis was probably formed due to activity of anaerobic organotrophs. Hydrogenotrophic methanogens with a high growth rate and low affinity to hydrogen are probably favored by H2 excess (~20% v/v in the gas phase), and outcompeted methyl-reducing methanogens. The importance of low hydrogen concentrations for methyl-reducing methanogens was evident from the results of analysis of the enrichment culture on DMSO without hydrogen addition. In the course of cultivation, hydrogen was produced in microbial reactions as was detected in trace amounts throughout the experiment. The dominant methanogen in this culture (33.6% of the total number of sequences) exhibited 98.3% similarity with methylotrophic Methanomethylovorans uponensis (NR_133781.1). The sequences affiliated with members of the order Methanomassiliicoccales contributed by 0.74%. Apart from methanogens, the dominant organisms were chemoorganotrophs of the genus Sulfurospirillum (22%), hydrolytic bacteria of the genus Trichococcus (4%), SRB of the genus Desulfoprunum (3.2%), members of the genus Geobacter (2.6%) capable of anaerobic OM oxidation coupled to iron reduction, uncultured members of the family Marinilabiliaceae (5%) and others (Table 3, Table S1). Thus, low hydrogen concentration may be advantageous for methyl-reducing methanotrophs and facilitate their growth (Feldewert et al., 2020).
Enrichment culture of aerobic methanotrophic bacteria. Although type I methanotrophs of the genus Methylobacter predominated in the native samples, laboratory cultivation resulted in enrichment of type II methanotrophs. The sequences retrieved from enrichment culture exhibited 100% similarity to those of the species Methylosinus trichosporium (MK578232.1), Methylosinus sporium (AJ458495.1), and Methylocystis parvus (CP044331.1) (Table 4). Together they were responsible for 14% of the total number of sequences. The share of type I methanotrophs in the enrichment culture was 0.72% and all of them showed 100% similarity to Methylomonas methanica (AF150806.1). The cultivation conditions used in the present work were probably unsuitable for the isolation of Methylobacter. This was possibly due to high oxygen concentration (7.6 mg/L in the gas phase compared with 4.9 mg/L in the near-bottom water). Type I methanotrophs developing in freshwater lakes, including members of the genus Methylobacter, prefer low oxygen concentrations or even hypoxic conditions (Rissanen et al., 2018). The temperature of incubation (30°C) could be a reason for elimination of Methylobacter spp. during the cultivation. Some Methylobacter strains display psychrotolerant and psychrophilic characteristics (Collins et al., 2017), including those detected in freshwater lakes. Nitrogen species in the medium and its concentration probably also had a selective role. Nitrate (300 mg N-NO3/L) was used in the experiments, while nitrate concentration in the lake did not exceed 3 mg/L. Similar results have been obtained previously for enrichment cultures from the chemocline of the alpine oligotrophic freshwater Lake Gek-Gel (Azerbaijan), in which type II methanotrophs of the genus Methylocystis became predominant (Pimenov et al., 2010). Apart from methanotrophs, predominant sequences in the enrichment were affiliated with aerobic chemoorganotrophic members of the genera Flavobacterium (10% of the total number of sequences), Edaphobaculum (12%), Pseudomonas (8%), Terrimonas (7%), Acidovorax (4.4%), Flectobacillus (2.4%). Chitino- and proteolytic bacteria of the genus Lysobacter (3.5%), sulfur-oxidizing obligate autotrophs of the genus Thiobacillus (3.4%), facultative methylotrophs of the genus Hyphomicrobium (2.3%), and aerobic chemoorgano- or chemolithoautotroph of the genus Hydrogenophaga (4%), which use hydrogen as an energy source, were also detected (Table 4). The overall diversity and relative abundance of microorganisms at phylum level in methanotrophic enrichment culture are presented in Table S1.
Thus, the studied littoral site of Lake Senezh harbors a diverse microbial community. Aerobes, facultative anaerobes, and obligate anaerobes, autotrophs and heterotrophs, including those involved in the complete cycles of nitrogen, sulfur and methane, are coexisting (Fig. 4). A high diversity of facultative anaerobes indicates that oxygen availability is the main factor controlling the activity of the littoral community. A decrease in the DO concentration, which is in coastal areas often caused by eutrophication, results in the activation of anaerobic processes with release of sulfide and methane since diverse microorganisms responsible for these processes are already present there. Depending on oxygen levels, sulfide may be captured by anoxygenic phototrophs or by aerobic sulfur bacteria; the latter can shift their aerobic metabolism to anaerobic with nitrate as electron acceptor. Produced methane may be oxidized by AOM under anoxic conditions and by aerobic methanotrophs at higher oxygen levels. Comparison of the average potential rates of methanogenesis and aerobic methane oxidation suggests that all methane which may potentially be formed in the studied littoral site could potentially be oxidized by aerobic methanotrophs. The variable oxygen regime will also promote the closing of the nitrogen cycle, since both aerobic nitrifiers and anaerobic denitrifiers and nitrogen-species-dependent AOM are present in the community. The littoral site studied is therefore a case of a highly labile system, where high diversity of microorganisms belonging to different physiological groups provides for stability of the processes under conditions of constantly fluctuating physicochemical parameters.
REFERENCES
Anderson, N.J., Bennion, H., and Lotter, A.F., Lake eutrophication and its implications for organic carbon sequestration in Europe, Glob. Chang. Biol., 2014, vol. 20, pp. 2741–2751.
Bechtel, A. and Schubert, C.J., A biogeochemical study of sediments from the eutrophic Lake Lugano and the oligotrophic Lake Brienz, Switzerland, Org. Geochem., 2009, vol. 40, pp. 1100–1114.
Billard, E., Domaizon, I., Tissot, N., Arnaud, F., and Lyauteyet, E., Multi-scale phylogenetic heterogeneity of archaea, bacteria, methanogens and methanotrophs in lake sediments, Hydrobiologia, 2015, vol. 751, pp. 159–173.
Callahan, B.J., McMurdie, P.J., Rosen, M.J., Han, A.W., Johnson, A.J.A., and Holmes, S.P., DADA2: high-resolution sample inference from Illumina amplicon data, Nat. Methods., 2016, vol. 13, pp. 581–583.
Castelle, C.J., Wrighton, K.C., Thomas, B.C., Hug, L.A., Brown, C.T., Wilkins, M.J., Frischkorn, K.R., Tringe, S.G., Singh, A., Markillie, L.M., Taylor, R.C., Williams, K.H., and Banfield, J.F., Genomic expansion of domain archaea highlights roles for organisms from new phyla in anaerobic carbon cycling, Curr. Biol., 2015, vol. 25, pp. 690–701.
Castelle, C.J., Brown, C.T., Anantharaman, K., Probst, A.J., Huang, R.H., and Banfield, J.F., Biosynthetic capacity, metabolic variety and unusual biology in the CPR and DPANN radiations, Nat. Rev. Microbiol., 2018, vol. 16, pp. 629–645.
Collins, D.A., Akberdin, I.R., and Kalyuzhnaya, M.G., Methylobacter, in Bergey’s Manual of Systematics of Archaea and Bacteria, Whitman, W.B., Rainey, F.A., Kämpfer, P., Trujillo, M., Chun, J., DeVos, P., Hedlund, B.P., and Dedysh, S.N., Eds., New York: Wiley, 2017, pp. 1−12. https://doi.org/10.1002/9781118960608.gbm01179.pub2
Cozannet, M., Borrel, G., Roussel, E., Moalic, Y., Allioux, M., Sanvoisin, A., Toffin, L., and Alain, K., New insights into the ecology and physiology of Methanomassiliicoccales from terrestrial and aquatic environments, Microorganisms, 2021, vol.9, p. 30.
Crevecoeur, S., Vincent, W.F., Comte, J., Matveev, A., and Lovejoy, C., Diversity and potential activity of methanotrophs in high methane-emitting permafrost thaw ponds, PLoS One, 2017, vol. 12, p. e0188223.
Dedysh, S.N., Kulichevskaya, I.S., Huber, K.J., and Overmann, J., Defining the taxonomic status of described subdivision 3 Acidobacteria: proposal of Bryobacteraceae fam. nov., Int. J. Syst. Evol. Microbiol., 2017, vol. 67, pp. 498−501.
Diaz, R.J. and Rosenberg, R., Marine benthic hypoxia: a review of its ecological effects and the behavioural response of benthic macrofauna, in Oceanography and Marine Biology: An Annual Review, Ansell, A.D., Gibson, R.N., Barnes, M., Eds., London: UCL, 1995, pp. 245−303.
Diaz, R.J. and Rosenberg, R., Spreading dead zones and consequences for marine ecosystems, Science, 2008, vol. 321, pp. 926−929.
Dinasquet, J., Ortega-Retuerta, E., Lovejoy, C., and Obernosterer, I., Editorial: microbiology of the rapidly changing polar environments, Front. Mar. Sci., 2018, vol. 5, p. 154.
Dridi, B., Fardeau, M.-L., Ollivier, B., Raoult, D., and Drancourt, M., Methanomassiliicoccus luminyensis gen. nov., sp. nov., a methanogenic archaeon isolated from human faeces, Int. J. Syst. Evol. Microbiol., 2012, vol. 62, pp. 1902–1907.
Eisenberg, T., Glaeser, S.P., Nicklas, W., Mauder, N., Contzen, M., Aledelbi, K., and Kämpfer, P., Streptobacillus felis sp. nov., isolated from a cat with pneumonia, and emended descriptions of the genus Streptobacillus and of Streptobacillus moniliformis, Int. J. Syst. Evol. Microbiol., 2015a, vol. 65, pp. 2172−2178.
Eisenberg, T., Glaeser, S.P., Ewers, C., Semmler, T., Nicklas, W., Rau, J., Mauder, N., Hofmann, N., Imaoka, K., Kimura, M., and Kämpfer, P., Streptobacillus notomytis sp. nov., isolated from a spinifex hopping mouse (Notomys alexis Thomas, 1922), and emended description of Streptobacillus Levaditi et al. 1925, Eisenberg et al. 2015 emend., Int. J. Syst. Evol. Microbiol., 2015b, vol. 65, pp. 4823−4829.
Fan, X. and Xing, P., Differences in the composition of archaeal communities in sediments from contrasting zones of Lake Taihu, Front. Microbiol., 2016, vol. 7, p. 1510.
Feldewert, C., Lang, K., and Brune, A., The hydrogen threshold of obligately methyl-reducing methanogens, FEMS Microbiol. Let., 2020, vol. 367, p. fnaa137.
Fuchs, A., Lyautey, E., Montuelle, B., and Casper P., Effects of increasing temperatures on methane concentrations and methanogenesis during experimental incubation of sediments from oligotrophic and mesotrophic lakes, J. Geophys. Res. Biogeosci., 2016, vol. 121, pp. 1394–1406.
Galchenko, V.F., Methanotrophnye bacterii (Methanotrophic Bacteria), Moscow: GEOS, 2008.
Graf, J.S., Mayr, M.J., Marchant, H.K., Tienken, D., Hach, P.F., Brand, A., Schubert, C.J., Kuypers, M.M.M., and Milucka, J., Bloom of a denitrifying methanotroph, “Candidatus Methylomirabilis limnetica,” in a deep stratified lake, Environ. Microbiol., 2018, vol. 20, pp. 2598–2614.
Han, X., Schubert, C.J., Fiskal, A., Dubois, N., and Lever, M.A., Eutrophication as a driver of microbial community structure in lake sediments, Environ Microbiol., 2020, vol. 22, pp. 3446−3462.
He, R., Wooller, M.J., Pohlman, J.W., Quensen, J., Tiedje, J.M., and Leigh, M.B., Shifts in identity and activity of methanotrophs in arctic lake sediments in response to temperature changes, Appl. Environ. Microbiol., 2012, vol. 78, pp. 4715–4723.
Huber, H. and Stetter, K.O., Thermoplasmatales, in The Prokaryotes, Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., and Stackebrandt, E., Eds., New York: Springer, 2006, pp. 101–112.
Hugerth, L.W., Wefer, H.A., Lundin, S., Jakobsson, H.E., Lindberg, M., Rodin, S., Engstrand, L., and Andersson, A.F., DegePrime, a program for degenerate primer design for broad-taxonomic-range PCR in microbial ecology studies, Appl. Environ. Microbiol., 2014, vol. 80, pp. 5116–5123.
Imachi, H., Nobu, M.K., Nakahara, N., Morono, Y., Ogawara, M., Takaki, Y., Takano, Y., Uematsu, K., Ikuta, T., Ito, M., Matsui, Y., Miyazaki, M., Murata, K., Saito, Y., Sakai, S., et al., Isolation of an archaeon at the prokaryote-eukaryote interface, Nature, 2020, vol. 577, pp. 519−525.
Jeske, O., Jogler, M., Petersen, J., Sikorski, J., and Jogler, C., From genome mining to phenotypic microarrays: Planctomycetes as source for novel bioactive molecules, Antonie van Leeuwenhoek, 2013, vol. 104, pp. 551−567.
Jonkers, H.M., van der Maarel, M.J.E.C., van Gemerden, H., and Hansen, T.A., Dimethylsulfoxide reduction by marine sulfate-reducing bacteria, FEMS Microbiol. Lett., 1996, vol. 136, pp. 283−287.
Kadnikov, V.V., Savvichev, A.S., Mardanov, A.V., Beletsky, A.V., Merkel, A.Y., Ravin, N.V., and Pimenov, N.V., Microbial communities involved in the methane cycle in the near-bottom water layer and sediments of the meromictic subarctic Lake Svetloe, Antonie van Leeuwenhoek, 2019, vol. 112, pp. 1801–1814.
Kallistova, A.Yu., Merkel, A.Yu., Tarnovetskii, I.Yu., and Pimenov, N.V., Methane formation and oxidation by prokaryotes, Microbiology (Moscow), 2017, vol. 86, pp. 671−691.
Kallistova, A., Kadnikov, V., Rusanov, I., Kok-ryatskaya, N., Beletsky, A., Mardanov, A., Savvichev, A., Ravin, N., and Pimenov, N., Microbial communities involved in aerobic and anaerobic methane cycling in a meromictic ferruginous subarctic lake, Aquat. Microb. Ecol., 2018, vol. 82, pp. 1−18.
Kallistova, A.Yu., Savvichev, A.S., Rusanov, I.I., and Pimenov, N.V., Thermokarst lakes, ecosystems with intense microbial processes of the methane cycle, Microbiology (Moscow), 2019, vol. 88, pp. 649−661.
Kallistova, A., Merkel, A., Kanapatskiy, T., Bol-tyanskaya, Y., Tarnovetskii, I., Perevalova, A., Kevbrin, V., Samylina, O., and Pimenov, N., Methanogenesis in the Lake Elton saline aquatic system, Extremophiles, 2020, vol. 24, pp. 657–672.
Kallistova, A.Yu., Kadnikov, V.V., Savvichev, A.S., Rusanov, I.I., Dvornikov, Yu.A., Leibman, M.O., Khomutov, A.V., Ravin, N.V., and Pimenov, N.V., Comparative study of methanogenic pathways in the sediments of thermokarst and polygenetic Yamal lakes, Microbiology (Moscow), 2021, vol. 90, pp. 261–267.
Kantor, R.S., van Zyl, A.W., van Hille, R.P., Thomas, B.C., Harrison, S.T., and Banfield, J.F., Bioreactor microbial ecosystems for thiocyanate and cyanide degradation unraveled with genome-resolved metagenomics, Environ. Microbiol., 2015, vol. 17, pp. 4929−4941.
Kodama, Y. and Watanabe, K., Sulfuricurvum kujiense gen. nov., sp. nov., a facultatively anaerobic, chemolithoautotrophic, sulfur-oxidizing bacterium isolated from an underground crude-oil storage cavity, Int. J. Syst. Evol. Microbiol., 2004, vol. 54, pp. 2297−2300.
Kojima, H. and Fukui, M., Sulfuricella denitrificans gen. nov., sp. nov., a sulfur-oxidizing autotroph isolated from a freshwater lake, Int. J. Syst. Evol. Microbiol., 2010, vol. 60, pp. 2862−2866.
Kojima, H. and Fukui, M., Sulfuritalea hydrogenivorans gen. nov., sp. nov., a facultative autotroph isolated from a freshwater lake, Int. J. Syst. Evol. Microbiol., 2011, vol. 61, pp. 1651−1655.
Kojima, H., and Fukui, M., Sulfurisoma sediminicola gen. nov., sp. nov., a facultative autotroph isolated from a freshwater lake, Int. J. Syst. Evol. Microbiol., 2014, vol. 64, pp. 1587−1592.
Konstantinov, A.S., Obshchaya gidrobiologiya (General Hydrobiology), Moscow: Vysshaya shkola, 1986, 4th ed.
Kulichevskaya, I.S., Suzina, N.E., Rijpstra, W.I.C., Damsté, J.S.S., and Dedysh, S.N., Paludibaculum fermentans gen. nov., sp. nov., a facultative anaerobe capable of dissimilatory iron reduction from subdivision 3 of the Acidobacteria, Int. J. Syst. Evol. Microbiol., 2014, vol. 64, pp. 2857−2864.
Li, Q.M., Zhou, Y.L., Wei, Z.F., and Wang, Y., Phylogenomic insights into distribution and adaptation of Bdellovibrionota in marine waters, Microorganisms, 2021, vol. 9, p. 757.
Liu, X., Li, M., Castelle, C.J., Probst, A.J., Zhou, Z., Pan, J., Liu, Y., Banfield, J.F., and Gu, J.D., Insights into the ecology, evolution, and metabolism of the widespread Woesearchaeotal lineages, Microbiome, 2018, vol. 6, p. 102.
Lyautey, E., Billard, E., Tissot, N., Jacquet, S., and Domaizon, I., Seasonal dynamics of abundance, structure, and diversity of methanogens and methanotrophs in lake sediments, Microb. Ecol., 2021, vol. 82, pp. 559−571.
Martinez-Cruz, K., Leewis, M.C., Herriott, I.C., Sepulveda-Jauregui, A., Anthony, K.W., Thalasso, F., and Leigh, M.B., Anaerobic oxidation of methane by aerobic methanotrophs in sub-Arctic lake sediments, Sci. Total Environ., 2017, vols. 607–608, pp. 23–31.
Martynov, A.A., Spravochnik rybolova-sportsmena Podmoscow’ya (Sports Angler Handbook of the Moscow Region), Moscow: Moskovskaya Pravda, 1988, 2nd ed.
Martin, G., Rissanen, A.J., Garcia, S.L., Mehrshad, M., Buck, M., and Peura, S., Candidatus Methylumidiphilus drives peaks in methanotrophic relative abundance in stratified lakes and ponds across northern landscapes, Front. Microbiol., 2021, vol. 12, p. 669937.
Mayr, M.J., Zimmermann, M., Guggenheim, C., Brand, A., and Bürgmann, H., Niche partitioning of methane-oxidizing bacteria along the oxygen-methane counter gradient of stratified lakes, ISME J., 2020, vol. 14, pp. 274−287.
McAuliffe, C., Gas chromatographic determination of solutes by multiple phase equilibrium, Chem. Technol., 1971, vol. 1, pp. 46–51.
McMurdie, P.J. and Holmes, S., Phyloseq: a bioconductor package for handling and analysis of high-throughput phylogenetic sequence data, Pac. Symp. Biocomput., 2012, pp. 235–246.
Merkel, A.Yu., Tarnovetskii, I.Yu., Podosokorskaya, O.A., and Toshchakov, S.V., Analysis of 16S rRNA primer systems for profiling of thermophilic microbial communities, Microbiology (Moscow), 2019, vol. 88, pp. 671–680.
Moschos, S., Piperagkas, O., and Karayanni, H.A., Vertically and temporally diverse bacterial community in a shallow lake-water sediment site of a eutrophic lake, Inland Waters, 2021, vol. 11, pp. 141−153.
Nakagawa, Y. and Yamasato, K., Emendation of the genus Cytophaga and transfer of Cytophaga agarovorans and Cytophaga salmonicolor to Marinilabilia gen. nov.: phylogenetic analysis of the Flavobacterium-Cytophaga complex. Int. J. Syst. Bacteriol. 1996, vol. 46, pp. 599−603.
Nevin, K.P., Holmes, D.E., Woodard, T.L., Hinlein, E.S., Ostendorf, D.W., and Lovley, D.R., Geobacter bemidjiensis sp. nov. and Geobacter psychrophilus sp. nov., two novel Fe(III)-reducing subsurface isolates, Int. J. Syst. Evol. Microbiol., 2005, vol. 55, pp. 1667−1674.
Ortiz-Alvarez, R. and Casamayor, E.O., High occurrence of Pacearchaeota and Woesearchaeota (Archaea superphylum DPANN) in the surface waters of oligotrophic high-altitude lakes, Environ. Microbiol. Rep., 2016, vol. 8, pp. 210–217.
Pfennig, N., Anreicherungskulturen für rote und grüne Schwefelbakterien, Zbl. Bakt. Hyg., I. Abt. Orig., 1965, Su-ppl. 1, vols. 179−189, pp. 503−504.
Pfennig, N. and Lippert, K.D., Über das Vitamin B12-Bedürfnis phototropher Schwefelbakterien, Archiv. Mikrobiol., 1966, vol. 55, pp. 245–256.
Pimenov, N.V., Kallistova, A.Yu., Rusanov, I.I., Yusupov, S.K., Montonen, L., Jurgens, G., Münster, U., Nozhevnikova, A.N., and Ivanov, M.V., Methane formation and oxidation in the meromictic oligotrophic Lake Gek-Gel (Azerbaijan), Microbiology (Moscow), 2010, vol. 79, pp. 247−252.
Preston, D.L., Caine, N., McKnight, D.M., Williams, M.W., Hell, K., Miller, M.P., Hart, S.J., and Johnson, P.T.J., Climate regulates alpine lake ice cover phenology and aquatic ecosystem structure, Geophys. Res. Lett., 2016, vol. 43, pp. 5353–5360.
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., Peplies, J., and Glöckner, F.O., The SILVA ribosomal RNA gene database project: improved data processing and web-based tools, Nucleic Acids Res., 2013, vol. 41, pp. D590−D596.
Reis, P.C.J., Thottathil, S.D., Ruiz-González, C., and Prairie, Y.T., Niche separation within aerobic methanotrophic bacteria across lakes and its link to methane oxidation rates, Environ. Microbiol., 2020, vol. 22, pp. 738−751.
Reis, P.C.J., Thottathil, S.D., and Prairie, Y.T., The role of methanotrophy in the microbial carbon metabolism of temperate lakes, Nat. Commun., 2022, vol. 13, p. 43.
Ren, Y., Yu, M., Low, W.Y., Ruhlman, T.A., Hajrah, N.H., El Omri, A., Alghamdi, M.K., Sabir, M.J., Alhebshi, A.M., Kamli, M.R., Sabir, J.S.M., Theriot, E.C., Jansen, R.K., and Rather, I.A., Nucleotide substitution rates of diatom plastid encoded protein genes are positively correlated with genome architecture, Sci. Rep., 2020, vol. 10, p. 14358.
Renaud, G., Stenzel, U., Maricic, T., Wiebe, V., and Kelso, J., deML: robust demultiplexing of Illumina sequences using a likelihood-based approach, Bioinformatics, 2015, vol. 31, pp. 770–772.
Rissanen, A.J., Saarenheimo, J., Tiirola, M., Peura, S., Aalto, S.L., Karvinen, A., and Nykänen, H., Gammaproteobacterial methanotrophs dominate methanotrophy in aerobic and anaerobic layers of boreal lake waters, Aquat. Microb. Ecol., 2018, vol. 81, pp. 257–276.
Rosentreter, J.A., Borges, A.V., Deemer, B.R., Holger-son, M.A., Liu, S., Song, C., Melack, J., Raymond, P.A., Duarte, C.M., Allen, G.H., Olefeldt, D., Poulter, B., Battin, T.I., and Eyre, B.D., Half of global methane emissions come from highly variable aquatic ecosystem sources, Nat. Geosci., 2021, vol. 14, pp. 225–230.
Sanford, R.A., Cole, J.R., and Tiedje, J.M., Characterization and description of Anaeromyxobacter dehalogenans gen. nov., sp. nov., an aryl-halorespiring facultative anaerobic myxobacterium, Appl. Environ. Microbiol., 2002, vol. 68, pp. 893−900.
Savvichev, A.S., Kadnikov, V.V., Rusanov, I.I., Belet-sky, A.V., Krasnova, E.D., Voronov, D.A., Kallistova, A.Yu., Veslopolova, E.F., Zakharova, E.E., Kokryatskaya, N.M., Losyuk, G.N., Demidenko, N.A., Belyaev, N.A., Sigalevich, P.A., Mardanov, A.V., et al., Microbial processes and microbial communities in the water column of the Polar meromictic Lake Bol’shie Khruslomeny at the White sea coast, Front. Microbiol., 2020, vol. 11, p. 1945.
Savvichev, A., Rusanov, I., Dvornikov, Y., Kadnikov, V., Kallistova, A., Veslopolova, E., Chetverova, A., Leibman, M., Sigalevich, P., Pimenov, N., Ravin, N., and Khomutov, A., The water column of the Yamal tundra lakes as a microbial filter preventing methane emission, Biogeosciences, 2021, vol. 18, pp. 2791–2807.
Sleat, R., Mah, R.A., and Robinson, R., Acetoanaerobium noterae gen. nov., sp. nov.: an anaerobic bacterium that forms acetate from H2 and CO2, Int. J. Syst. Bacteriol., 1985, vol. 35, pp. 10−15.
Spring, S., Kampfer, P., Ludwig, W., and Schleifer, K.-H., Polyphasic characterization of the genus Leptothrix: new descriptions of Leptothrix mobilis sp. nov. and Leptothrix discophora sp. nov. nom. rev. and emended description of Leptothrix cholodnii emend., System. Appl. Microbiol., 1996, vol. 19, pp. 634−643.
Storesund, J.E., Lanzèn, A., Nordmann, E.-L., Armo, H.R., Lage, O.M., and Øvreås, L., Planctomycetes as a vital constituent of the microbial communities inhabiting different layers of the meromictic Lake Sælenvannet (Norway), Microorganisms, 2020, vol. 8, p. 1150.
Su, G., Zopfi, J., Niemann, H., and Lehmann, M.F., Multiple groups of methanotrophic bacteria mediate methane oxidation in anoxic lake sediments, Front. Microbiol., 2022, vol. 13, p. 864630.
Suominen, S., Dombrowski, N., Sinninghe Damsté, J.S., and Villanueva, L., A diverse uncultivated microbial community is responsible for organic matter degradation in the Black Sea sulfidic zone, Environ. Microbiol., 2021, vol. 23, pp. 2709−2728.
Tardy, V., Etienne, D., Masclaux, H., Essert, V., Millet, L., Verneaux, V., and Lyautey, E., Spatial distribution of sediment archaeal and bacterial communities relates to the source of organic matter and hypoxia—a biogeographical study on Lake Remoray (France), FEMS Microbiol. Ecol., 2021, vol. 97, p. fiab126.
Toshchakov, S.V., Izotova, A.O., Vinogradova, E.N., Kachmazov, G.S., Tuaeva, A.Y., Abaev, V.T., Evteeva, M.A., Gunitseva, N.M., Korzhenkov, A.A., Elcheninov, A.G., Patrushev, M.V., and Kublanov, I.V., Culture-independent survey of thermophilic microbial communities of the North Caucasus, Biology, 2021, vol. 10, p. 1352.
Trojan, D., Schreiber, L., Bjerg, J.T., Bøggild, A., Yang, T., Kjeldsen, K.U., and Schramm, A.A., Taxonomic framework for cable bacteria and proposal of the candidate genera Electrothrix and Electronema, Syst. Appl. Microbiol., 2016, vol. 39, pp. 297−306.
van Grinsven, S., Meier, D.V., Michel, A., Han, X., Schubert, C.J., and Lever, M.A., Redox zone and trophic state as drivers of methane-oxidizing bacterial abundance and community structure in lake sediments, Front. Environ. Sci., 2022, vol. 10, p. 857358.
Wang, J., Wei, Z.P., Chu, Y.X., Tian, G., and He, R., Eu-trophic levels and algae growth increase emissions of methane and volatile sulfur compounds from lakes, Environ. Pollut., 2022, vol. 306, p. 119435.
Wolin, E.A., Wolin, M.J., and Wolfe, R.S., Formation of methane by bacterial extracts, J. Biol. Chem., 1963, vol. 238, pp. 2882−2886.
Yamada, T., Sekiguchi, Y., Hanada, S., Imachi, H., Ohashi, A., Harada, H., and Kamagata, Y., Anaerolinea thermolimosa sp. nov., Levilinea saccharolytica gen. nov., sp. nov. and Leptolinea tardivitalis gen. nov., sp. nov., novel filamentous anaerobes, and description of the new classes Anaerolineae classis nov. and Caldilineae classis nov. in the bacterial phylum Chloroflexi, Int. J. Syst. Evol. Microbiol., 2006, vol. 56, pp. 1331−1340.
Yang, Y., Chen, J., Tong, T., Li, B., He, T., Liu, Y., and Xie, S., Eutrophication influences methanotrophic activity, abundance and community structure in freshwater lakes, Sci. Total Environ., 2019, vol. 662, pp. 863−872.
ACKNOWLEDGMENTS
The authors are thankful to LLC “MGULAB” (https://www.msulab.ru/) for the chemical analysis of water samples and to Prof. N.V. Ravin (Research Centre of Biotechnology RAS) for critical reading of the manuscript and useful recommendations.
Funding
This research was partially funded by Russian Science Foundation, grant no. 22-14-00038 (activity measurements, enrichments isolation, sequencing of native samples and methanogenic enrichments). Sequencing of the methanotrophic enrichment and bioinformatic analyses were funded by Russian Foundation for Basic Research according to the research project no. 20-04-60190. Field work was supported by State Assignment for the Laboratory of relict microbial communities, Research Centre of Biotechnology RAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Information
Rights and permissions
About this article
Cite this article
Kallistova, A.Y., Koval, D.D., Kadnikov, V.V. et al. Methane Cycle in a Littoral Site of a Temperate Freshwater Lake. Microbiology 92, 153–170 (2023). https://doi.org/10.1134/S0026261722602901
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261722602901