Abstract—
Changes in microbial community composition during formation of an acid mine drainage were studied on a model of two water reservoirs located in the Ozernoye open-cast mine for polymetallic ores in Eastern Siberia. The first reservoir, Bu-18, was filled with groundwater, had a neutral pH and low levels of sulfate and dissolved metal ions. The second reservoir, Bu-16, was an acid mine drainage (pH 2.85) filled with the water from Bu-18, which passed through rocks containing sulfide minerals. The Bu-16 water contained 1405 mg/L of sulfate, 164 mg/L of manganese, 78 mg/L of magnesium, and 26 mg/L of iron. Molecular analysis of the microbial communities of two reservoirs, carried out using high-throughput sequencing of the 16S rRNA gene fragments, showed that formation of the acid mine drainage was accompanied by a decrease in microbial diversity and by selection of several dominant taxonomic and functional groups. Chemolithoautotrophic iron- and sulfur-oxidizing bacteria of the genera Leptospirillum, Acidithiobacillus, Gallionella, Sulfuriferula, and Sulfobacillus constituted most of the prokaryotic community in Bu-16. Heterotrophic bacteria of the genera Ferrimicrobium and Metallibacterium, capable of reducing Fe(III) under anaerobic conditions, were present as minor components. Over 20% of the community were members of the Candidate Phyla Radiation group and of the candidate phylum Dependentiae (TM6), known for their parasitic or symbiotic lifestyle. These groups of bacteria were rarely found in acid mine drainage and only in minor quantities. Potential hosts of the Dependentiae, flagellates of the genus Spumella, were found among eukaryotes in Bu-16.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Drainage waters with a high levels of acidity, often referred to as acid mine drainage, are formed due to oxidation of pyrite (FeS2) and other sulfide minerals in the presence of oxygen and water. The rate of pyrite oxidation increases by orders of magnitude due to the activity of aerobic microorganisms oxidizing reduced iron compounds (Rohwerder et al., 2003; Johnson and Hallberg, 2003). Protons and sulfate are formed during pyrite oxidation by Fe3+ ions (reaction FeS2 + 14Fe3+ + 8H2O > 15Fe2+ + \(2{\text{SO}}_{4}^{{2 - }}\) + 16H+), resulting in rising acidity. In turn, low ambient pH contributes to the dissolution of the metal-containing minerals and metal transition into the solution. These processes result in formation of strongly acidic water (pH 0.5‒4) with a high content of sulfates and metal ions (iron, zinc, copper, manganese, cadmium, etc.) reaching grams per liter. Acidic waters are typical of the sites of disposal of metal recovery wastes, abandoned mines, tailings dams of concentrating factories, open pits, etc.
High acidity and the presence of toxic metal ions in acidic drainage waters create extreme conditions for the development of microorganisms, limiting the diversity of microbial communities (Baker and Banfield, 2003; Tyson et al., 2004; Méndez-García et al., 2015). However, in these ecosystems there are both inorganic electron donors (mainly Fe2+ and reduced sulfur compounds) and oxygen, which creates conditions for the growth of chemolithoautotrophic microorganisms. In anoxic zones, such as sediments of treatment ponds, Fe3+ as well as sulfate and nitrate may act as electron acceptors, while allochthonous organic compounds available from external sources as well as organic matter produced by chemolithoautotrophs may act as electron donors.
Microbial communities of acid mine drainage have been actively studied using both classical microbiological and molecular methods based on analysis of the 16S ribosomal RNA genes (Baker and Banfield, 2003; Méndez-García et al., 2015), as well as by the methods of metagenomics, metatranscriptomics, and metaproteomics (Tyson et al., 2004; Ram et al., 2005; Chen et al., 2015). Among chemolithoautotrophs, which usually predominate in microbial communities of acid mine drainage, members of the genera Acidithiobacillus (phylum Proteobacteria) and Leptospirillum (phylum Nitrospirae) are most often found, while in environments with moderately low pH values (2 to 5), betaproteobacteria of the family Gallionellaceae predominate (Méndez-García et al., 2015). The heterotrophic component of microbial communities is usually more diverse and may include alpha-, beta-, and gammaproteobacteria as well as members of the phyla Firmicutes, Actinobacteria, Acidobacteria, and TM7 (Méndez-García et al., 2015). Bacteria involved in iron and sulfur cycles (representatives of the genera Acidiphilum, Ferrimicrobium, Sulfobacillus, etc.) and those belonging to the groups typical of nonextreme ecosystems (Xanthomonodales, Sphingomonodales, Burkholderiales, etc.) are found among heterotrophs.
Most studied acid mine drainages are “open” ecosystems in which allochthonous microorganisms may arrive, for example, with surface waters from surrounding terrains. At the same time, in the acid drainage, selection of the microbial community occurs in accordance with the physicochemical conditions.
The goal of the present work was to perform a comparative study of the compositions of microbial communities of two water reservoirs located in an open pit prepared for extraction of polymetallic ores and to identify the groups of microorganisms specific for acid mine drainage. The first reservoir, Bu-18, was filled with rainfall and groundwater and had a near-neutral pH. The second reservoir, Bu-16, was filled with the water flowing from Bu-18 through the rocks, and was characterized by low pH values due to oxidation of the underlying polysulfide ores.
MATERIALS AND METHODS
Sampling and characterization of chemical composition of the water. Water samples from the reservoirs Bu-16 and Bu-18 were collected on August 11, 2016 into sterile plastic bottles. The pH, redox potential (Eh), and temperature were measured immediately after sampling using a HANNA HI 8314F pH meter. Water samples for determination of ion content (30 mL) and element composition (15 mL) were filtered on site using 0.45-µm membrane filters. Chemical composition of the water was analyzed by the Plazma Chemical Analytical Center (Tomsk, Russia) using inductively coupled plasma-mass spectrometry (ICP-MS) and ion chromatography.
Isolation of metagenomic DNA, PCR amplification, and sequencing of 16S rRNA gene fragments. The samples of water (5 L) were filtered through a 0.22 µm membrane filter to collect microbial biomass. Total DNA of the filtered biomass was extracted using MO BIO Power Soil Kit (MO BIO Laboratoties, Carlsbad, United States) according to the manufacturer’s recommendation.
For PCR amplification of the 16S rRNA gene fragments containing variable V3–V6 regions, universal primers U341F (5'-CCT ACG GGR SGC AGC AG-3') and PRK806R (5'-GGA CTA CYV GGG TAT CTA AT-3') were used. The 16S rRNA gene fragments obtained by PCR were sequenced on a GS FLX genome analyzer (Roche, Switzerland) according to the Titanium protocol using the GS FLX Titanium Sequencing Kit XL+. The library was created, amplified, and sequenced on GS FLX according to the relevant Roche protocols.
The sequences of the 16S rRNA gene fragments were deposited in the Sequence Read Archive (SRA) NCBI database under accession nos. SRX5073294 (Bu-16) and SRX5073295 (Bu-18) within the framework of BioProject PRJNA507138.
Analysis of 16S rRNA gene fragments sequencing results. For analysis, from the set of 16S rRNA gene sequences determined by pyrosequencing the reads containing the U341F primer sequence were selected and were trimmed using the Mothur software package (Schloss et al., 2009) to equal length of 250 bp to facilitate cluster analysis. Then, the low-quality sequences were removed and all obtained sequences (for both samples) were grouped into OTUs with nucleotide sequence identity of at least 97% using the Usearch software package (Edgar, 2010). At this stage, the Uchime algorithm removed potential chimerical sequences and singletons (sequences that occurred only once; they mainly represent pyrosequencing errors) (Behnke et al., 2011). Then, the sets of sequences obtained for the Bu-16 and Bu-18 samples were grouped with representative sequences of the OTUs using Uchime algorithm if a sequence was found to exhibit at least 97% homology. Thus, the number of the 16S rRNA gene sequences in each of the two samples assigned to each OTU was determined. The final data set contained 8830 sequences of 16S rRNA genes fragments for sample Bu-16 and 19 104 sequences for sample Bu-18.
Taxonomic identification of OTUs was performed using the SINA online alignment and classification platform and the SILVA version 1.2.11 database with default parameters (Pruesse et al., 2012). Taxonomic identification of OTUs was also verified using the BLASTN search against the database of the 16S rRNA gene sequences in GenBank.
PCR amplification and sequencing of eukaryotic 18S rRNA gene fragments of the sample Bu-16. For PCR amplification of the 18S rRNA gene fragments, primers V4_1F (5'-CCA GCA SCY GCG GTA ATW CC) and TAReukREV3 (5'-ACT TTC GTT CTT GAT YRA) as well as metagenomic DNA sample from Bu-16 as a template were used. PCR fragments were sequenced using an Illumina MiSeq sequencer (Illumina, United States) and 52 516 300-bp paired-end reads (2 × 300 bp) were obtained. Overlapping paired-end reads were merged using the FLASH v. 1.2.11 software (Magoc and Salzberg, 2011). The final data set contained 47 284 sequences of 18S rRNA genes. Nucleotide sequences of the 18S rRNA gene fragments of sample Bu-16 were deposited in the NCBI SRA database under accession no. SRX5073293. Sequences homologous to the 18S rRNA gene of Spumella elongata (GenBank AJ236859) with nucleotide sequence identity of at least 94% for at least 90% of their length were searched using BLASTN and 2512 sequences were detected.
RESULTS AND DISCUSSION
Physicochemical characteristics of drainage water. Ozernoye polymetallic deposit located in the Yeravninsky district of Republic of Buryatia (Russia) is one of the ten largest zinc deposits in the world in terms of reserves and ore quality. To date, overburden operations have been carried out to develop the open pit, but exploitation of the deposit has not yet begun. Two small reservoirs located in an open pit were the subjects of investigation (Fig. 1). The first reservoir, Bu-18, has an area of about 2 m2 and depth of no more than 20 cm and at the time of sampling was filled with groundwater from a spring; surface runoff was absent. The second reservoir, Bu-16, was located at a distance of about 15 m from Bu-18 down the pit slope and had an area of about 15 m2 and a depth of no more than 30 cm. The difference in altitude between two reservoirs was ~2 m. Probably, Bu-16 was fed by groundwater from Bu-18, which passed through a layer of underlying rocks containing sulfide minerals.
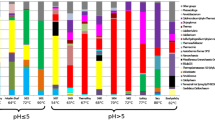
Changes in the composition of microbial communities during the formation of acid mine drainage. (a) Position of the reservoirs Bu-18 and Bu-16. Direction of the water flow is indicated by gray arrows. (b) Diversity of microbial communities Bu-18 and Bu-16. The shares (% of the total number of the 16S rRNA gene sequences) of OTUs, found only in Bu-18 (Bu-18), in both reservoirs (Bu-18 + Bu-16), or only in Bu-16 (Bu-16) in microbial communities Bu-18 (gray bars) and Bu-16 (black bars) are shown. Subgroups of “common” OTUs (Bu-18 + Bu-16): e < 0.5, OTUs with the shares in Bu-16 decreased by more than 2 times compared to Bu-18; 0.5 < e < 2, OTUs with the shares in Bu-18 and Bu-16 differing no more than 2 times; e > 2, OTUs with the shares in Bu-16 increased by more than 2 times compared to Bu-18. The number of OTUs in each category is indicated by the numerals above the bars.
Such a water supply scheme was in agreement with the physicochemical characteristics of the water. The temperature in Bu-18 was 7.9°C, the water was slightly acidic (pH 6.05) and had low content of sulfates and dissolved metal ions (Table 1). Reservoir Bu-16, which temperature was 13.2°C, was an acid mine drainage. It had a low pH (2.85) and higher concentrations of sulfates and metal ions, especially iron and manganese (26 and 164 mg/L, respectively) than Bu-18 (Table 1). Obviously, the differences in the chemical composition of the water of the two reservoirs were caused by oxidation of sulfides during the flow of water from Bu-18 to Bu-16, since the concentrations of chloride and nitrate anions were approximately equal in the both reservoirs. The increased content of silicon and aluminum in Bu-16 also indicated partial hydrolysis of aluminosilicate minerals caused by low pH that is typical of mine drainage.
The composition of microbial communityof acid mine drainage Bu-16 was the result of selection of the community from Bu-18 reservoir. Sequencing of the 16S rRNA gene fragments revealed 487 OTUs (97% sequence identity), of which only 9 belonged to archaea. Alpha diversity indices indicated a lower diversity of the microbial community of acid drainage Bu-16 in comparison with Bu-18, and the relatively small number of predominant OTUs (Table 2).
The data on the presence of individual OTUs in the two communities and their relative abundance confirmed the assumption that the Bu-16 community was the result of succession of the Bu-18 community accompanied by a decrease in microbial diversity and selection of individual dominant groups. Among all 487 OTUs, 187 OTUs were detected in both communities, while 294 OTUs were unique for Bu-18, and only 6 OTUs were unique for Bu-16 (Fig. 1). The results of analysis of the shares that these OTUs comprised in two communities were even clearer. For example, 6 OTUs specific for Bu-16 comprised 8.1% of 16S rRNA sequences, while other 82.5% of the total sequences in Bu-16 belonged to 46 “common” OTUs which shares in Bu-16 was more than double that in Bu-18 (Fig. 1). The “common” OTUs, with shares varying within +/‒ 2 times, represented only about 4% of 16S rRNA sequences in each community.
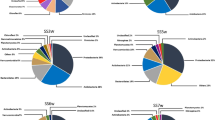
Microorganisms potentially involved in iron and sulfur cycles in the acid mine drainage Bu-16. Microbial community of Bu-18 reservoir included 481 OTUs representing bacterial phyla Acidobacteria, Actinobacteria, Bacteroidetes, Ignavibacteriae, Chlamydiae, Chloroflexi, Cyanobacteria, Deinococcus-Thermus, Dependentiae (TM6), Elusimicrobia, Fibrobacteres, Firmicutes, Gemmatimonadetes, Nitrospirae, Patescibacteria, Planctomycetes, Proteobacteria (alpha-, beta-, gamma-, and deltaproteobacteria), Spirochaetes, Verrucomicrobia, and candidate phylum WPS-2 (Table 3). Archaea comprised ~4.5% of the community and belonged to the class Thermoplasmata of the phylum Euryarchaeota.
In the acid mine drainage Bu-16, the relative abundance of such bacterial groups characteristic of freshwater basins and soils as Acidobacteria, Bacteroidetes, most species of alpha-, gamma-, and deltaproteobacteria, as well as archaea, which together predominated in the community Bu-18, decreased. Relative abundance of only 52 OTUs, which represented over 90% of microorganisms in the Bu-16 community, increased more than twofold compared to Bu-18. Chemolithoautotrophic bacteria capable of oxidizing iron and/or reduced sulfur compounds, members of the genera Leptospirillum (13.25%), Acidithiobacillus (13.06%), and Sulfobacillus (6.22%) as well as betaproteobacteria of the families Gallionellaceae (17.50%) and Sulfuricellaceae (4.22%) were the predominant microorganisms in Bu-16. Bacteria of the genus Leptospirillum are obligate chemolithoautotrophs, which use only Fe2+ as an electron donor, and oxygen as an acceptor. Although they usually predominate in communities developing under extreme acidity (pH below 2) and elevated temperature (25‒40°C), for example, in the Richmond Mine in California (Tyson et al., 2004), they were also found in cold acid mine drainage in Buryatia (Kadnikov et al., 2016a). Acidithiobacillus are more moderate acidophiles (optimal pH 2‒3) while in relation to the optimal growth temperature, they are mainly mesophiles or psychrophiles. Under oxic conditions, these chemolithoautotrophic bacteria oxidize, apart from Fe2+, also reduced sulfur compounds (or only these compounds) and fix CO2 via the Calvin cycle and atmospheric nitrogen with nitrogenase (Liljeqvist et al., 2011). Many Acidithiobacillus strains (for example, A. ferrivorans) were isolated from acid mine drainage with pH values of 2 to 3.5 in areas with a cold climate (Kupka et al., 2007; Hallberg et al., 2010).
Betaproteobacteria of the families Gallionelaceae and Sulfuricellaceae were the most numerous group of chemolithoautotrophs. Nine OTUs belonged to the family Gallionelaceae, most of which represented the genus Gallionella (6 OTUs, 15.64% of the community). These autotrophic iron-oxidizing bacteria were found in acid mine drainages with moderate values of pH (2–5) and concentrations of dissolved metals. Bacteria related to Gallionella were among the dominant microbial groups in the acid mine drainages of lead and zinc deposits of the Carnoules region in France (Bruneel et al., 2006), pyrite mines Cae Coch in Wales (Kimura et al., 2011) and Yunfu in China (He et al., 2007), uranium mine Ronneburg in Germany (Fabisch et al., 2013), etc. Bacteria of the genus Gallionella were predominant in microbial community of drainage water from the well ShG14-8 at Sherlovaya Gora polymetallic mine (Chita oblast, Russia), which had pH (2.65) close to that of water from Bu-16 (Kadnikov et al., 2016b). In addition to Gallionella, bacteria of the genera Nitrosospira (0.72%) and Nitrotoga (1.10%) were found; the cultured members of these groups may oxidize ammonium and nitrite, respectively (Schramm et al., 1998; Ishii et al., 2017). Sulfur-oxidizing bacteria of the genus Sulfuriferula, using oxygen or nitrate as an electron acceptor under anoxic conditions (Watanabe et al., 2015), comprised about 4.22% of the Bu-16 community. The presence of bacteria with the energy metabolism associated with oxidation or reduction of nitrogen compounds is consistent with high content of nitrate in the water, the source of which may be technogenic nitrogen compounds originating from the explosive used in the open-cast mining.
Firmicutes of the genus Sulfobacillus (6.22%), as well as a closely related OTU assigned to the same family XVII of Clostridiales (4.93%), were detected among the bacteria allegedly involved in the iron cycle. Representatives of the genus Sulfobacillus can grow either by autotrophic oxidation of iron and sulfur or heterotrophically (Anderson et al., 2012). About 1% of the 16S rRNA gene sequences in Bu-16 sample belonged to actinobacteria Ferrimicrobium acidiphilum. These acidophilic heterotrophs may oxidize Fe2+ under oxic conditions and reduce Fe3+ using organic compounds as electron donors under anoxic conditions (Johnson et al., 2009). Gamma-proteobacteria Metallibacterium scheffleri, the share of which in the community was 0.96%, are also capable of reducing Fe3+ under anoxic conditions (Ziegler et al., 2013).
In general, various groups of bacteria, cultivated members of which are capable of oxidizing iron or reduced sulfur compounds, comprised about half of the total microbial community Bu-16, while in reservoir Bu-18, the share of these OTUs was about 4%.
Microbial community of acid mine drainage Bu-16 contained a large proportion of non-free-living microorganisms. Detection in the studied communities of significant numbers of representatives of two uncultured groups, superphylum Candidate Phyla Radiation (Patescibacteria) and candidate phylum Dependentiae (TM6), was an unexpected result of this work. The Candidate Phyla Radiation is a monophyletic group that includes several dozen lineages of the phylum level (Brown et al., 2015). These bacteria have a small cell size and genomes and lack many key biosynthetic pathways, so they are supposed to grow only in association with other microorganisms as parasites or symbionts (Castelle et al., 2018). In reservoir Bu-18, 40 OTUs representing candidate phyla Parcubacteria (OD1) and Saccharibacteria (TM7) were found, 21 of which were also present in the Bu-16 reservoir. The shares of 5 OTUs in Bu-16 more than doubled compared to Bu-18, and in total, they represented ~13% of microorganisms in Bu-16. Apparently, the increase in the abundances of these OTUs reflects selection of their hosts in the acid mine drainage Bu-16. Representatives of Saccharibacteria were previously detected in various acid mine drainages (Méndez-García et al., 2015), while detection of Parcubacteria was described in a single paper dealing with analysis of such ecosystems on Svalbard Island (García-Moyano et al., 2015). In all the cases described, these groups were the minor components of microbial communities.
The candidate phylum Dependentiae was represented by three OTUs, the total share of which was 0.19% in Bu-18 and increased to 7.83% in Bu-16. Small genome size, reduced cell envelope, the absence of many biosynthetic pathways, the presence of a transport system for ATP import into the cell from the outside, and the presence of proteins enabling interaction with eukaryotic cells are common characteristics of the bacteria of this phylum (Yeoh et al., 2016). All these characteristics indicate that Dependentiae are parasites of eukaryotic protozoans (Yeoh et al., 2016). The Dependentiae detected in Bu-16 were closely related (95–97% 16S rRNA sequence identity) to Candidatus Dependentiae bacterium SeV1 (GenBank CP025544), an intracellular parasite of the protozoan Spumella elongata. Spumella (Stramenopiles; Chrysophyceae; Chromulinales; Chromulinaceae) are heterotrophic flagellates, widespread in soils and freshwater habitats (Boenigk et al., 2005). Sequence analysis of the 18S rRNA gene fragments of eukaryotes from Bu-16 showed that ~5% of them belonged to flagellates of the genus Spumella. It is possible that development of microbial biofilms on the surface of minerals, characteristic of acid mine drainage, creates favorable conditions for the nutrition of Spumella. Despite the fact that various protozoans were repeatedly found in acid mine drainages, the presence of Spumella was not previously reported (Méndez-García et al., 2015).
Thus, our results demonstrated that formation of acid mine drainages results in decreased diversity of the microbial community, which becomes enriched with the microorganisms participating in iron and sulfur cycles, primarily with aerobic chemolithoautotrophic iron- and sulfur-oxidizing bacteria of the genera Acidithiobacillus, Gallionella, Sulfuriferula, and Leptospirillum. The relative abundance of heterotrophic bacteria capable of reducing Fe3+ under anoxic conditions also increased. A specific feature of the studied acid mine drainage Bu-16 was the high content of non-free-living bacteria (memebrs of Candidate Phyla Radiation and Dependentiae), which are symbionts or parasites of other microorganisms. They are rarely found in acid mine drainages and in the previously described cases were minor components of microbial communities. The presence of Dependentiae and their probable hosts, protozoans of the genus Spumella, in the community demonstrated the importance of eukaryotic microorganisms as components of the acid mine drainage ecosystem.
REFERENCES
Anderson, I., Chertkov, O., Chen, A., Saunders, E., Lapidus, A., Nolan, M., Lucas, S., Hammon, N., Deshpande, S., Cheng, J.F., Han, C., Tapia, R., Goodwin, L.A., Pitluck, S., Liolios, K., et al., Complete genome sequence of the moderately thermophilic mineral-sulfide-oxidizing firmicute Sulfobacillus acidophilus type strain (NAL(T)), Stand. Genomic Sci. 2012, vol. 6 , no. 3, pp. 1‒13. https://doi.org/10.4056/sigs.2736042
Baker, B.J. and Banfield, J.F., Microbial communities in acid mine drainage, FEMS Microbiol. Ecol., 2003, vol. 44, pp. 139–152.
Behnke, A., Engel, M., Christen, R., Nebel, M., Klein, R.R., and Stoeck, T., Depicting more accurate pictures of protistan community complexity using pyrosequencing of hypervariable SSU rRNA gene regions, Environ. Microbiol., 2011, vol. 13, pp. 340–349.
Boenigk, J., Pfandl, K., Stadler, P., and Chatzinotas, A., High diversity of the “Spumella-like” flagellates: an investigation based on the SSU rRNA gene sequences of isolates from habitats located in six different geographic regions, Environ. Microbiol., 2005, vol. 7, pp. 685‒697.
Brown, C.T., Hug, L.A., Thomas, B.C., Sharon, I., Castelle, C.J., Singh, A., Wilkins, M.J., Wrighton, K.C., Williams, K.H., and Banfield, J.F., Unusual biology across a group comprising more than 15% of domain Bacteria, Nature, 2015, vol. 523, pp. 208‒211.
Bruneel, O., Duran, R., Casiot, C., Elbaz-Poulichet, F., and Personne, J.C., Diversity of microorganisms in Fe-As-rich acid mine drainage waters of Carnoules, France, Appl. Environ. Microbiol., 2006, vol. 72, pp. 551–556.
Castelle, C.J., Brown, C.T., Anantharaman, K., Probst, A.J., Huang, R.H., and Banfield, J.F., Biosynthetic capacity, metabolic variety and unusual biology in the CPR and DPANN radiations, Nat. Rev. Microbiol., 2018, vol. 16, pp. 629‒645.
Chen, L.X., Hu, M., Huang, L.N., Hua, Z.S., Kuang, J.L., Li, S.J., and Shu, W.S., Comparative metagenomic and metatranscriptomic analyses of microbial communities in acid mine drainage, ISME J., 2015, vol. 9, pp. 1579‒1592.
Edgar, R.C., Search and clustering orders of magnitude faster than BLAST, Bioinformatics, 2010, vol. 26, pp. 2460–2461.
Fabisch, M., Beulig, F., Akob, D.M., and Kusel, K., Surprising abundance of Gallionella-related iron oxidizers in creek sediments at pH 4.4 or at high heavy metal concentrations, Front. Microbiol., 2013, vol. 4, p. 390.
García-Moyano, A., Austnes, A.E., Lanzén, A., González-Toril, E., Aguilera, Á., and Øvreås, L., Novel and unexpected microbial diversity in acid mine drainage in Svalbard (78° N), revealed by culture-independent approaches, Microorganisms, 2015, vol. 3, pp. 667‒694.
Hallberg, K.B., González-Toril, E., and Johnson, D.B., Acidithiobacillus ferrivorans, sp. nov.; facultatively anaerobic, psychrotolerant iron-, and sulfur-oxidizing acidophiles isolated from metal mine-impacted environments, Extremophiles, 2010, vol. 14, pp. 9‒19.
He, Z., Xiao, S., Xie, X., Zhong, H., Hu, Y., Li, Q., Gao, F., Li, G., Liu, J., and Qiu, G., Molecular diversity of microbial community in acid mine drainages of Yunfu sulfide mine, Extremophiles, 2007, vol. 11, pp. 305–314.
Ishii, K., Fujitani, H., Soh, K., Nakagawa, T., Takahashi, R., and Tsuneda, S., Enrichment and physiological characterization of a cold-adapted nitrite-oxidizing Nitrotoga sp. from an eelgrass sediment, Appl. Environ. Microbiol., 2017, vol. 83. pii: e00549-17.
Johnson, B.D. and Hallberg, K.B. The microbiology of acidic mine waters, Res. Microbiol., 2003, vol. 154, pp. 466‒473.
Johnson, D.B., Bacelar-Nicolau, P., Okibe, N., Thomas, A., and Hallberg, K.B., Ferrimicrobium acidiphilum gen. nov., sp. nov. and Ferrithrix thermotolerans gen. nov., sp. nov.: heterotrophic, iron-oxidizing, extremely acidophilic actinobacteria, Int. J. Syst. Evol. Microbiol., 2009, vol. 59, pp. 1082‒1089.
Kadnikov, V.V., Ivasenko, D.A., Beletsky, A.V., Mardanov, A.V., Danilova, E.V., Pimenov, N.V., Karnachuk, O.V., and Ravin, N.V., Effect of metal concentration on the microbial community in acid mine drainage of a polysulfide ore deposit, Microbiology (Moscow), 2016a, vol. 85, pp. 745–751. https://doi.org/10.1134/S0026261716060126
Kadnikov, V.V., Ivasenko, D.A., Beletsky, A.V., Mardanov, A.V., Danilova, E.V., Pimenov, N.V., Karnachuk, O.V., and Ravin, N.V., A novel uncultured bacterium of the family Gallionellaceae: description and genome reconstruction based on metagenomic analysis of microbial community in acid mine drainage, Microbiology (Moscow), 2016b, vol. 85, no. 4, pp. 449–461. https://doi.org/10.1134/S002626171604010X
Kimura, S., Bryan, C.G., Hallberg, K.B., and Johnson, D.B., Biodiversity and geochemistry of an extremely acidic, low-temperature subterranean environment sustained by chemolithotrophy, Environ. Microbiol., 2011, vol. 13, pp. 2092–2104.
Kupka, D., Rzhepishevska, O.I., Dopson, M., Lindström, E.B., Karnachuk, O.V., and Tuovinen, O.H., Bacterial oxidation of ferrous sulfate at low temperatures, Biotechnol. Bioeng., 2007, vol. 97, pp. 1470‒1478.
Liljeqvist, M., Valdes, J., Holmes, D.S., and Dopson, M., Draft genome of the psychrotolerant acidophile Acidithiobacillus ferrivorans SS3, J. Bacteriol., 2011, vol. 193, pp. 4304–4305.
Magoč, T. and Salzberg, S.L., FLASH: fast length adjustment of short reads to improve genome assemblies, Bioinformatics, 2011, vol. 27, pp. 2957‒2963.
Méndez-García, C., Peláez, A.I., Mesa, V., Sánchez, J., Golyshina, O.V., and Ferrer, M., Microbial diversity and metabolic networks in acid mine drainage habitats, Front. Microbiol., 2015, vol. 29, no. 6, p. 475.
Pruesse, E., Peplies, J., and Glöckner, F.O., SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes, Bioinformatics, 2012, vol. 28, pp. 1823–1829.
Ram, R.J., Verberkmoes, N.C., Thelen, M.P., Tyson, G.W., Baker, B.J., Blake, R.C. 2nd, Shah, M., Hettich, R.L., and Banfield, J.F., Community proteomics of a natural microbial biofilm, Science, 2005, vol. 308, pp. 1915‒1920.
Rohwerder, T., Gehrke, T., Kinzler, K., and Sand, W., Bioleaching review part A: progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation, Appl. Microbiol. Biotechnol., 2003, vol. 63, pp. 239‒248.
Schramm, A., De Beer, D., Wagner, M., and Amann, R., Identification and activities in situ of Nitrosospira and Nitrospira spp. as dominant populations in a nitrifying fluidized bed reactor, Appl. Environ. Microbiol., 1998, vol. 64, pp. 3480‒3485.
Schloss, P.D., Westcott, S.L., Ryabin, T., Hall, J.R., Hartmann, M., Hollister, E.B., Lesniewski, R.A., Oakley, B.B., Parks, D.H., Robinson, C.J., Sahl, J.W., Stres, B., Thallinger, G.G., Van Horn, D.J., and Weber, C.F., Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities, Appl. Environ. Microbiol., 2009, vol. 75, pp. 7537–7541.
Tyson, G.W., Chapman, J., Hugenholtz, P., Allen, E.E., Ram, R.J., Richardson, P.M., Solovyev, V.V., Rubin, E.M., Rokhsar, D.S., and Banfield, J.F., Community structure and metabolism through reconstruction of microbial genomes from the environment, Nature, 2004, vol. 428, no. 6978, pp. 37–43.
Watanabe, T., Kojima, H., and Fukui, M., Sulfuriferula multivorans gen. nov., sp. nov., isolated from a freshwater lake, reclassification of “Thiobacillus plumbophilus” as Sulfuriferula plumbophilus sp. nov., and description of Sulfuricellaceae fam. nov. and Sulfuricellales ord. nov., Int. J. Syst. Evol. Microbiol., 2015, vol. 65, pp. 1504‒1508.
Yeoh, Y.K., Sekiguchi, Y., Parks, D.H., and Hugenholtz, P., Comparative genomics of candidate phylum TM6 suggests that parasitism is widespread and ancestral in this lineage, Mol. Biol. Evol., 2016, vol. 33, pp. 915‒927.
Ziegler, S., Waidner, B., Itoh, T., Schumann, P., Spring, S., and Gescher, J., Metallibacterium scheffleri gen. nov., sp. nov., an alkalinizing gammaproteobacterium isolated from an acidic biofilm, Int. J. Syst. Evol. Microbiol., 2013, vol. 63, pp. 1499‒1504.
ACKNOWLEDGMENTS
The work was performed using the scientific equipment of the Core Research Facility “Bioengineering.”
Funding
The work was supported by the Russian Science Foundation (project no. 14-14-01016, analysis of prokaryotes) and by the Russian Foundation for Basic Research (project no. 18-34-00356, analysis of eukaryotes). Sampling and physicochemical analysis of acid mine drainage samples were supported by the Russian Foundation for Basic Research, project no. 16-54-150011.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement of the welfare of animals. This article does not contain any research using animals as objects.
Conflict of interest. The authors declare that there is no conflict of interest.
Additional information
Translated by A. Bulaev
Rights and permissions
About this article
Cite this article
Kadnikov, V.V., Gruzdev, E.V., Ivasenko, D.A. et al. Selection of a Microbial Community in the Course of Formation of Acid Mine Drainage. Microbiology 88, 292–299 (2019). https://doi.org/10.1134/S0026261719030056
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261719030056