Abstract
A set of 41 stream water samples of the Lo River catchment, Ha Giang province, collected in dry season was analyzed for pH, major cation and anion, trace element concentrations. The stream waters exhibits a midly acidic to alkaline, meanwhile, the Total Dissolved Solids (TDS) values have a wide range of 17.4–284.9 mg L–1. Among major cations and anions, the stream water within the Lo River catchment is characterized by the predominant presence of Ca2+ and \({\text{HCO}}_{3}^{ - }\). This compositional pattern gives rise to the emergence of the Ca–Mg–HCO3 water type as the most dominant species, followed by Na–Cl, Ca–Mg–Cl–SO4 and Na–HCO3–Cl types. However, the distribution of these water types corresponds closely with the geological conditions, with Na–Cl type prevailing in the watershed of granite complex, while watersheds characterized by lithologies such as Quaternary sediments, limestone, marble, shale, schist, and sandstone primarily exhibit the Ca–Mg–HCO3 water type. The dominant reaction in the water system is the dissolution of carbonate minerals, like calcite and dolomite, followed by the contribution of modest rainfall during the dry season, and small-scale processes of mixing and cation exchange. Comparison of major ions and trace element with technical standard reveal the stream waters are generally deemed suitable for the routine activities of the local population in Ha Giang province. However, stream water of a few specific sites may require treatment before these waters can be safely utilized.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Water stands as the most essential constituent of our planet, playing a crucial role in potentially fostering the presence of life on Earth. Water maintains a close and intricate connection with human and social activities across past, present, and future epochs. However, the escalation of population growth and the emergence of other activities, such as urbanization and unchecked forest exploitation, have precipitated unfavorable transformations. These include the depletion and contamination of the aquatic environment, bringing about detrimental changes to water resources. The contamination of water results in the degradation of its quality, posing a threat to the existence of life on Earth and disrupting both economic progress and social well-being. This necessitates comprehensive studies focused on water resources, encompassing their current availability and quality, while also anticipating their responsiveness and viability in the future.
Recently, there has been a growing necessity to establish baselines for evaluating the potential impacts of forthcoming disruptions, whether arising from natural occurrences or human activities. This objective could be achieved through the study of geochemistry of water and its inherent natural progression and also enables the formulation of effective public policies governing the management water resources (Banks et al., 2001; Sharma et al., 2012; Natali et al., 2016; Gassama et al., 2021; Destéfanis et al., 2020).
Amongst the various sources of water, streams and rivers hold a paramount position as the primary reservoirs of freshwater, catering to essential needs such as human consumption as well as agricultural and industrial utilization. Over recent decades, the scientific community has directed its focus towards the management of extensive river basins, with a particular emphasis on characterizing the principal geochemical cycles of the predominant transported elements (Gibbs, 1970; Meybeck, 2003; Berner and Berner, 1996; Gaillardet et al., 1999, 2014; Hartmann et al. 2014). Besides, the attention on mountainous river catchments has been few to date worldwide (Destéfanis et al., 2020). In Vietnam, study on stream and river in mountainous area is almost nonexistence, with a few small-scale studies (e.g., Luyen et al., 2015). Destéfanis et al. (2020) pointed out that headwater catchment, e.g. stream water, have particular characteristics compared to that of large rivers, with differences in scale, temperature, precipitation, reactive capacity, etc…
Ha Giang, situated as the northernmost mountainous border province of Vietnam, occupies a significant strategic position, serving as a pivotal locale for fostering socio-economic and cultural interactions between Vietnam and China (Fig. 1). Beside, Ha Giang province is home to the Dong Van Karst Plateau UNESCO Global Geopark, an esteemed member of the Global Geoparks Network (https://dongvangeopark.com; https://asiapacificgeoparks.org; https://globalgeoparksnetwork.org). This geopark spans across a significant portion of four districts within the province, namely Meo Vac, Dong Van, Yen Minh, and Quan Ba. It serves as a remarkable feature of the region for its extensive limestone expanse, breathtaking mountain vistas, and the cultural wealth and distinctiveness of ethnic minority communities. In Ha Giang province, a harmonious coexistence of 22 distinct ethnic groups prevails. While some communities inhabit the valleys, leading to densely populated villages, others reside in more remote regions or scatter across the slopes of steep mountains. The connectivity between villages faces significant challenges due to the rugged terrain characterized by high mountains and pronounced dissected landscapes. Within this context, the availability of water resources assumes utmost significance. Rainwater and stream water predominantly cater to the essential needs of sustenance and livelihood. Consequently, the comprehensive study of water resources within river basins, including the assessment of the quality of stream water in the upstream areas, emerges as an exigent matter. This matter holds crucial implications not only for daily human activities but also for the formulation of effective public policies governing the management of water resources. This urgency is particularly pronounced in a significant locale like the Dong Van Karst Plateau UNESCO Global Geopark.
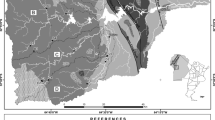
Distribution map of lithological units, water sampling sites and different stream draining of the Lo River catchment. (1) Quaternary sediment; (2) Cretaceous conglomerate, sandstone and siltstone of Ban Hang Formation; (3) Triassic shale and sandstone of Lan Pang formation; (4) Triassic sandstone and shale of Song Hien formation; (5) Triassic shale, siltstone, sandstone of Song Hien formation; (6) Devonian limestone and schist of Mia Le Formation; (7) Devonian Clay shale of Pia Phuong Formation; (8) Devonian biotite granite of Song Chay Complex; (9) Proterozoic dunit and harzburgite of Nam But complex; (10) Cambrian marble, limestone and schist of Ha Giang Formation; (11) Sampling site; (12) Stream and river.
Within Ha Giang province, the Lo River catchment assumes a significant role, standing as the largest and stretching from the northern to the southern extremities of the province (Fig. 1). Consequently, the principal aim of this study revolves around enhancing comprehension regarding the geochemistry of stream water within the Lo River catchment. In addition, the study will establish the base level for the year of 2021 to contribute a pivotal benchmark for future scenarios of changes in regional land use and management policies of water resources in Ha Giang province.
STUDY AREA
Ha Giang is the northernmost mountainous border province of Vietnam, with an expansive area of 7.941 square kilometers. Ha Giang province exhibits a diverse topographical landscape, encompassing valley configurations, low mountainous regions, and high mountainous landscapes, with the latter constituting the predominant feature across the province. Ha Giang province experiences a pronounced cycle of two distinct seasons throughout the year. The rainy season prevails from May to October, while the dry season, known as winter, extends from November to the following April (NAWAPI, 2009).
Ha Giang province lies within four sheets of Geological and mineral resources map of Viet Nam on 1 : 200 000 including Bac Kan, Bao Lac, Bac Quang Ma Quan (Quoc et al., 2000; Tinh et al., 2000; Xuyen et al., 2000a, 2000b) and thus, simplified geological map of Ha Giang province was performed in Fig. 1. Based on the compiled map, all sampling sites in this study were recognized to distribute in five major lithological units, including Quaternary sediment; Granite of Song Chay complex; Shale, sandstone, siltstone of Lan Pang, Song Hien, Pia Phuong formations; Limestone of Mia Le formation; Marble, limestone, and schist of Ha Giang formation.
Ha Giang province is home to several river systems, including the Lo River, Mien River, Gam River, Nho Que River and Chay River (Fig. 1). All rivers in the region share the characteristic of winding and twisting flows, influenced by topography, width, and slope. The flow rate varies with topography and depends mainly on hydro meteorological factors (NAWAPI, 2009). The Lo River, originating from Yunnan China, flows northwest to southeast, passing through Ha Giang town and Dao Duc commune. At the section where it joins the Mien River, the river is 60–100 m wide, with a U-shaped cross-section. Bedrock is continuously exposed along both banks of the river, while small and narrow alluvial grounds can be found along the riverbed. The Mien River originates from the high mountains in the north of the province, flows to Ha Giang town, and joins the Lo River at km 1 of the road from Ha Giang town to Dong Van. The river is 40‒70 m wide with a U-shaped cross-section (NAWAPI, 2009).
The Lo River basin has numerous streams of varying sizes, forming a fishbone shape (Fig. 1). Water flow rates are substantial and vary seasonally, with the average water flow is 144 m3/s (NAWAPI, 2009). These streams share the characteristic of winding flow, tortuous terrain, large bed slope, topographically variable flow rate, and seasonally variable water dynamics and flow into the Lo River, Mien River and Con River of Lo River catchment (Fig. 1). Streams flowing northwest to southeast, including those in the west-northwest of Ha Giang province, originate from high mountains and flow into the Lo River. In contrast, streams flowing northeast, such as those in Kim Linh and Trung Thanh communes in Vi Xuyen district, Quan Ba district, and Bac Quang district, all flow into the Mien River (NAWAPI, 2009).
MATERIAL AND METHOD
In this study, stream water samples of the Lo River catchment were collected from the uppermost reaches, deliberately distancing them from anthropogenic influences. The process of collecting stream water samples adhered to the guidelines specified in both national and international standard methods, including TCVN 6663-6:2018 (2018) (ISO 5667-6:2014) and the protocol outlined by R.B. Baird et al. (2017).
The pH value was measured in-situ using HANA HI9829-01042. Water samples were filtered through Millipore 0.45 µm membrane filter. Each sample was then acidified to pH < 2 by adding one drop of 16 M HNO3 and preserved in a refrigerator until elemental analysis. After pre-treatment procedure, the major cation (Ca2+, Mg2+, Na+, K+) and group of heavy metals concentrations were determined by using ICP-MS (Model 7900 Agilent, USA) (e.g., Lim et al., 2021, 2022). Determination of \({\text{NO}}_{3}^{ - }\) content was performed utilizing a spectrophotometric method in conjunction with sulfosalicylic acid. Meanwhile, \({\text{HCO}}_{3}^{ - }\) content was assessed through titration of the water sample using 0.1 N HCl acid, employing a 0.1% methyl orange indicator. To determine Cl– content, a titration was conducted on Cl-ions utilizing a standard solution of AgNO3 under either neutral or weakly alkaline conditions, with K2CrO4 acting as the indicator. In addition, \({\text{SO}}_{4}^{{2 - }}\) content was determined by complete precipitation of \({\text{SO}}_{4}^{{2 - }}\) ions as BaSO4 within a hydrochloric acid medium, facilitated by the addition of BaCl2 reagent. The total dissolved solid (TDS) content was determined as sum of major anion and cation, including Fe total in the water samples (Table 1).
The distribution maps for pH, TDS, and the concentration of major cations and anions was constructed through the utilization of the boxplot method (Banks et al., 2001; Reimann et al., 2005). These maps feature values comprising the maximum, minimum, median, first quartile (Q1), third quartile (Q3), and outliers. The symbols depicted on the distribution diagram correspond to or oscillate within the spectrum defined by these values, and illustrated on the geological map showcased in Fig. 1.
RESULTS AND DISCUSSION
Hydrochemical Characteristics of Stream Water of the Lo River Catchment
Table 1 presents the pH, TDS, and concentrations of the major cations and anions in stream water samples of the Lo River catchment in Ha Giang province. The corresponding statistical values, including the minimum, maximum, arithmetic mean, median, and standard deviation parameters, are listed in Table 2.
In accordance with the data presented in Tables 1 and 2, the pH of stream water within the study area displays relatively limited variability, ranging from 6.5 to 8.5, with a mean value of 7.4. Consequently, the stream water exhibits a midly acidic to alkaline, with the majority falling under the classification of alkaline. A comparable investigation conducted in the Ctalamochita river basin, Argentina, reports a pH value of 7.6 ± 0.68, with the prevailing belief that the primary contributing factor is the hydrolysis of silicate rocks (Destéfanis et al., 2020).
Meanwhile, Total Dissolved Solids (TDS) values exhibit considerable fluctuations over a wide range, spanning from 17.4 to 284.9 mg L–1. The statistics in Table 2 indicate that the mean TDS value is 132.4 ± 94.2 mg L–1. This value is relatively similar to the average TDS content found in the waters of the world’s rivers, which is approximately 100 mg L–1 (Berner and Berner, 1996).
On average, the major cations and anions constitute 25.2% and 74.6% of the total dissolved solids (TDS), respectively (Fig. 2). Notably, the concentration of anions is nearly three times higher than that of cations. Among the major cations, Ca2+ emerges as the most dominant ion in the stream water of the Lo River catchment, accounting for 70.3% of the total cations. Following Ca2+, the order of abundance is as follows: Mg2+ (12.9%), Na+ (8.4%), and K+ (8.4%) (Fig. 2). The reported concentrations of Ca2+ span from 1.2 mg L–1 to 60.9 mg L–1, with an average value of 23.4 mg L–1. Subsequently, the average concentrations for Mg2+, Na+, and K+ are 4.3 mg L–1, 2.8 mg L–1, and 2.8 mg L–1, respectively (Table 2).
According to Fig. 2, \({\text{HCO}}_{3}^{ - }\) is the most dominant anion in the stream water of the Lo River catchment, followed by \({\text{SO}}_{4}^{{2 - }}\), Cl– and \({\text{NO}}_{3}^{ - }\). Specifically, on average, the stream waters comprise 89.6% \({\text{HCO}}_{3}^{ - }\), 4% \({\text{SO}}_{4}^{{2 - }}\), 3.7% Cl– and 2.6% \({\text{NO}}_{3}^{ - }\). Concentration ranges of bicarbonate ions vary widely, spanning from 6.1 mg L–1 to 207.4 mg L–1, with average value of 88.5 mg L–1. In addition, the average concentrations of \({\text{SO}}_{4}^{{2 - }}\), Cl– and \({\text{NO}}_{3}^{ - }\) are 4.0 mg L–1, 3.7 mg L–1 and 2.6 mg L–1, respectively (Table 2).
The analysis of major ions in the world’s largest rivers reveals a prevalent occurrence of elevated concentrations of Ca2+ and \({\text{HCO}}_{3}^{ - }\) (Berner and Berner, 1996). In addition, J.D. Hem et al. (1989) pointed out that while Na+ and Cl– dominate in seawater, Ca2+ and \({\text{HCO}}_{3}^{ - }\) typically represent the major ions in freshwater environments. Additionally, it is observed that the concentrations of major cations and anions in most river waters fall within the range of Na+ > K+, Ca2+ > Mg2+, and \({\text{SO}}_{4}^{{2 - }}\) > Cl–. These ions are predominantly sourced from the weathering of exposed bedrock on the continental surface. This observation aligns closely with the mean values of major cations and anions of stream waters of the Lo River catchment mentioned earlier.
The analytical results for pH, major anions, and cations are also subjected to a comparison against the National technical regulation on surface water quality QCVN 08-MT:2015/BTNMT (2015) and the Guidelines for drinking-water quality by WHO (2022), as summarized in Table 1. In general, all parameters of the stream waters meet the stipulated criteria set by WHO (2022) and QCVN 08-MT:2015/BTNMT (2015), with the exception of the value for HNO3 at the sample site HG19. The observed value for HNO3 slightly surpasses the QCVN 08-MT:2015/BTNMT (2015) limit (6.2 mg L–1 versus 5 mg L–1). This specific sample is derived from a watershed characterized by shale, siltstone, and sandstone lithology.
Hydrogeochemical Distribution and Facies of Stream Waters of the Lo River Catchment
The pH map of surface water (Fig. 3a) demonstrates a relatively narrow variation within the Lo River catchment, ranging from 6.5 to 8.5. Conversely, the TDS values display a wide range of variation, ranging from 17.7 to 284.9 mg L–1 (Fig. 3b). Generally, both the pH and TDS values exhibit a relatively similar distribution pattern, with lower values predominantly observed in the western part, while higher values are more prevalent in the northern and eastern parts of the study area.
The pH values reveal that the stream water in the Lo River basin has a mildly acidic to alkaline composition, and this characteristic strongly depends on the underlying geological formations. Water with a mildly acidic to neutral composition is primarily distributed in the granite sector of the Song Chay Complex (Fig. 3a). In contrast, the region with high pH values is concentrated in the northern part, where shale, sandstone, siltstone, schist, and limestone of formations Song Hien, Mia Le, and Pia Phuong predominate.
Figure 3b illustrates the distribution of Total Dissolved Solids (TDS) in spring water within the Lo River basin. The data reveals that a considerable portion of the spring water originating from the granite formations of the Song Chay Complex exhibits significantly lower TDS values compared to other areas, with most values below 34.8 mg L–1. Conversely, in the northern part of the study area, spring water exhibits higher TDS values than other regions, with the maximum value reaching 284.9 mg L–1. This spatial variation in TDS content strengthens the influence of geological formations on the chemical composition of stream water within the Lo River catchment.
The spatial distribution of major cations Ca2+, Mg2+, Na+, and K+ in stream water within the Lo River catchment is presented in Fig. 4. It is observed that the concentrations of Ca2+ and Mg2+ follow a similar pattern as that of TDS, with lower levels predominantly found in the granite complex area, and notably higher concentrations in regions characterized by shale, schist, and limestone of the Ha Giang, Pia Phuong, and Mia Le formations.
Contrary to the distribution pattern of Ca2+ and Mg2+, the spatial distribution of Na+ and K+ exhibits a different trend. Stream water with higher concentrations of Na+ and K+ is primarily found in the south and southwest regions, characterized by the presence of granite from the Song Chay complex, and schist, shale and limestone of the Pia Phuong and Mia Le formations. Meanwhile, lower Na+ and K+ concentrations are scatteredly distributed in the western and northern parts of the study area, particularly in areas dominated by the Ha Giang and Song Hien formations.
As mentioned earlier, the \({\text{HCO}}_{3}^{ - }\) ion in the stream water of the Lo River basin exhibits a notably high content, surpassing that of the other major anions. The distribution of \({\text{HCO}}_{3}^{ - }\) ion is illustrated in Fig. 5, displaying a clear resemblance to the TDS values and demonstrating a significant influence of geological conditions. In the northern area, where the main formations consist of shale, sandstone, and limestone, specifically the Pia Phuong, Mia Le, and Song Hien formations, spring water samples present elevated \({\text{HCO}}_{3}^{ - }\) content, with the highest concentration reaching 207.4 mg L–1. In contrast, samples collected from the granite formations of the Song Chay Complex predominantly exhibit considerably lower values, with most concentrations falling below 14.6 mg L–1.
However, the distribution of the Cl– ion in stream water within the Lo River catchment differs from that of the \({\text{HCO}}_{3}^{ - }\) ion and bears resemblance to the distribution patterns of Na+ and K+. Specifically, the concentration of Cl– ions in stream water is higher in the southern part, near the confluence of the Mien River with the Lo River, where shale and limestone formations are prevalent. The highest observed values of Cl– concentration reached 14.2 mg L–1 (Fig. 5). In contrast, lower values are scatteredly distributed as one moves towards the northern regions, primarily concentrated in the granitic rocks of the Song Chay Complex and the Mia Le Formation.
Meanwhile, the \({\text{NO}}_{3}^{ - }\) and \({\text{SO}}_{4}^{{2 - }}\) ions, characterized by considerably lower concentrations in stream water compared to \({\text{HCO}}_{3}^{ - }\) and Cl– ions, did not exhibit a clear influence of geological formations. Instead, their concentrations were observed to be mixed from south to north, with both high and low values scattered throughout the study area. The ranges of concentration for \({\text{NO}}_{3}^{ - }\) and \({\text{SO}}_{4}^{{2 - }}\) are 0.6 to 6.2 mg L–1 and 2.4 to 5.8 mg L–1, respectively.
To investigate the influence of geological conditions on stream water in the Lo River catchment, water samples were categorized into five groups including Granite; Quaternary sediment; Shale, siltstone, sandstone; Limestone; and Marble, limestone, schist (Fig. 6).
The Piper diagram (Piper, 1944) has proven to be a valuable tool for classifying various types of water, including geothermal water, surface water, and groundwater, as demonstrated in previous studies (e.g., Sharma et al., 2012; Alfarrah et al., 2016; Xu et al., 2019; Sarker et al., 2022). In this study, we applied the Piper diagram to identify the hydrochemical characteristics of stream water in the Lo River catchment. Figure 6 displays the results, revealing the presence of two main types of stream water including Ca–Mg–HCO3 and Na–Cl. Additionally, two other samples exhibited mixed types, characterized as Ca–Mg–Cl–SO4 and Na–HCO3–Cl. The Ca–Mg–HCO3 type emerged as the most dominant species in the stream water, whereas the NaCl type was observed in only a few samples (Fig. 6). This distinction is also evident in the Durov diagram, where the Ca–Mg–HCO3 type exhibits dominance (Fig. 7).
The above-mentioned water types distinctly demonstrate the impact of geological conditions on the chemical composition of stream water within the study area. In regions characterized by the distribution of granite in the Song Chay Complex, stream water primarily exhibits high concentrations of Na+, K+, and Cl– ions. In contrast, in areas with Quaternary sediments, limestone, marble, shale, schist, and sandstone distributions, stream water predominantly falls into the Ca–Mg–HCO3 type (Figs. 6, 7). The high concentration of Ca2+, Mg2+, and \({\text{HCO}}_{3}^{ - }\) in the stream water of the Lo River catchment indicates the occurrence of chemical weathering and mixing processes. These processes likely contribute to the observed abundance of these ions, reflecting the interactions between geological formations and the water bodies within the catchment area.
Hydrogeochemical Processes in Stream Water of the Lo River Catchment
The hydrogeochemistry of stream water within a river catchment is influenced by a multitude of factors, encompassing the chemical composition of rainwater, geological structures, hydrological conditions, mineralogical composition of the watershed, and anthropogenic activities (Berner and Berner, 1996; Sharma et al., 2012; Destéfanis et al., 2020). In the context of the Lo River catchment, stream water samples were collected from the uppermost reaches, deliberately distancing them from anthropogenic influences. This approach was employed to minimize the impact of human activities. Consequently, the hydrogeochemistry of the water samples is primarily governed by natural processes such as evaporation, precipitation and rock weathering.
As previously discussed, the stream waters in the investigated area exhibit four primary types: Ca–Mg–HCO3, Na–Cl, Ca–Mg–Cl–SO4, and Na–HCO3–Cl. Among these, the Ca–Mg–HCO3 type emerges as the dominant classification, with the other types being relatively scarce. Interestingly, the Na–Cl type, for instance, is exclusively associated with the granite complex watershed (Figs. 6, 7). According to NAWAPI (2009), the Song Chay complex encompasses granite formations characterized by minimal weathering and fissuring. This results in a limited capacity to store and transmit groundwater, leading to near depletion of exposed water sources during the dry season. This complex is classified as having a very poor water quality level, with a Ca–HCO3 water type. The Durov diagram (Fig. 7) portrays stream water within this watershed undergoing processes of mixing and cation exchange. These phenomena likely occur in conjunction with sporadic rainfall during the dry season of the study area, giving rise to the Na–Cl water type and a mixed Na–HCO3–Cl water type. Meanwhile, watersheds characterized by lithologies such as Quaternary sediments, limestone, marble, shale, schist, and sandstone primarily exhibit the Ca–Mg–HCO3 water type.
R.J. Gibbs (1970) introduced diagrams designed to ascertain the chemical nature of water by delineating three principal zones: rock weathering, precipitation, and evaporation. These diagrams involve plotting the ratios of TDS vs. Cl–/(Cl– + \({\text{HCO}}_{3}^{ - }\)) for anions and TDS vs. Na+/(Na+ + Ca2+) for cations, with all ion values expressed in meq/l. Using this approach, Gibbs diagrams were constructed for stream water samples collected from the Lo River catchment during the dry season, as presented in Fig. 8. Evidently, all the analyzed stream water samples primarily exhibit characteristics indicative of interactions between rocks and water, as well as precipitation. These findings suggest that the chemical composition of stream water in the study area is predominantly influenced by processes involving the chemical weathering of rock-forming minerals and the occurrence of modest rainfall during the dry season. Notably, while precipitation appears to exert its influence on the catchment area associated with the granite complex, the process of rock weathering is more dominant in regions characterized by other lithological units, where carbonate minerals are abundant.
F. Albarède (2009) elucidates that chemical weathering can be understood as a series of dissolution reactions. Among the ions with higher solubility, such as Na+, K+, Ca2+, and Mg2+, are liberated into runoff and river water following the interaction of silicate minerals with precipitation. According to T. Xuyen et al. (2000a), the Song Chay complex is comprised of granite biotite, primarily consisting of potassium feldspar, quartz, plagioclase, and biotite minerals. The weathering of these silicate minerals, particularly through processes like dissolution and hydrolysis, occurs at a relatively slow rate. As a result, its immediate impact on water quality is not as pronounced as carbonate dissolution (Gaillardet et al., 1999; Appelo and Postma, 2005; Sharma et al., 2012). Consequently, the TDS values observed in the area of granite complex are much lower than those of other lithological units (Fig. 3). In addition, the temperature-dependent nature of dissolution rates holds significant importance, with warm climates encouraging chemical erosion (Albarède, 2009). However, as a mountainous region in northern Vietnam, the Lo River catchment experiences relatively cold weather during the dry season, with minimum temperatures reaching as low as 0.9°C. This prevailing cold climate has the potential to diminish the rate of rock weathering within the study area.
Figure 9 exhibits scatter plots depicting the concentrations of major ions in the stream waters of the Lo River catchment. A noteworthy observation is the alignment of the plots for (Ca2+ + Mg2+)/\({\text{HCO}}_{3}^{ - }\), (Ca2+ + Mg2+)/(\({\text{HCO}}_{3}^{ - }\) + \({\text{SO}}_{4}^{{2 - }}\)) along the 1 : 1 line. The similarity between these two plots arises from the substantially higher concentrations of \({\text{HCO}}_{3}^{ - }\) compared to \({\text{SO}}_{4}^{{2 - }}\). As pointed out by A. Sharma et al. (2012), when the dominant reaction in a system is the dissolution of calcite and dolomite, the (Ca2+ + Mg2+)/(\({\text{HCO}}_{3}^{ - }\) + \({\text{SO}}_{4}^{{2 - }}\)) plot closely resembles a 1 : 1 line. This phenomenon appears to apply to the stream water of the Lo River catchment, where silicate weathering rates are notably low, and the dissolution of carbonates such as calcite and dolomite holds a significant role.
The (Ca2+ + Mg2+)/total cation plot aligns closely with a 1 : 1 line, indicating the predominant influence of Ca2+ and Mg2+ over Na+ and K+ cations. This observation is reinforced by the (Ca2+ + Mg2+)/(Na+ + K+) plot. With the exception of certain water samples originating from the granite complex catchment, the majority of samples exhibit high (Ca2+ + Mg2+)/(Na+ + K+) ratios, reaching a maximum value of 56.5. A similar trend is evident in the (Na+ + K+)/total cation plot, with a remarkably high (TZ+)/(Na+ + K+) ratio. Conversely, the (Na+ + K+)/Cl– plot maintains a close proximity to the 1 : 1 line, indicative of limited silicate weathering contributing Na+ and K+ to the stream water. These findings affirm the dominance of carbonate dissolution as the principal reaction in the stream waters of the Lo River watershed, constituting the primary source of major anions and cations within the system.
Trace Elements of Stream Waters of the Lo River Catchment
The concentrations of fourteen trace elements in the stream waters of the Lo River catchment are presented in Table 3 and categorized based on the primary lithology of their respective watersheds (Fig. 10). These analytical results are compared against the National technical regulation on surface water quality QCVN 08-MT:2015/BTNMT (2015) and the Guidelines for drinking-water quality by WHO (2022). The elements subjected to comparison include As, Ba, Cd, Cu, Ni, Pb, Sb, U, and Zn.
Table 3 reveals that the elements Ba, Cd, Cu, Ni, Sb, U, and Zn exhibit concentrations significantly lower than the allowable limits of both WHO (2022) and QCVN 08-MT:2015/BTNMT (2015). Meanwhile, for As, all water samples adhere to the requirements of QCVN 08-MT:2015/BTNMT (2015), but two samples, HG12 and HG41, surpass the allowable limit specified by WHO (2022). These two samples originate from watersheds characterized by shale, siltstone, sandstone, marble, limestone, and schist lithologies (Fig. 10). Additionally, samples HG18 and HG20 display concentrations of Pb that exceed the allowable limits outlined by both WHO (2022) and QCVN 08-MT:2015/BTNMT (2015). These specific samples are associated with the watershed of shale, siltstone, and sandstone (Fig. 10).
Consequently, stream water from these above-mentioned sites necessitates treatment before utilization for drinking purposes and other human activities. In contrast, the comparison indicates that stream waters within the limestone and granite watersheds meet the criteria for safe drinking water and are suitable for the daily activities of local inhabitants in Ha Giang province, affirming their relatively clean nature.
CONCLUSIONS
In the Lo River catchment, the pH of stream water exhibits a midly acidic to alkaline, with the majority falling under the classification of alkaline. Meanwhile, the TDS values exhibit a wide range, spanning from 17.4 to 284.9 mg L–1, with mean TDS value is 132.4 ± 94.2 mg L–1. However, there is a distinct distribution of TDS in stream water, with very low values observed in the catchment of granite complex and higher values in other lithological units.
The major cations and anions constitute 25.2% and 74.6% of the total dissolved solids (TDS) on average, respectively. Among the major cations, Ca2+ emerges as the most dominant ion in the stream water of the Lo River catchment, accounting for 70.3% of the total cations, followed by Mg2+ (12.9%), Na+ (8.4%), and K+ (8.4%). For anion, \({\text{HCO}}_{3}^{ - }\) is the most dominant anion in the stream water of the Lo River catchment, followed by \({\text{SO}}_{4}^{{2 - }}\), Cl– and \({\text{NO}}_{3}^{ - }\). Specifically, on average, the stream waters comprise 89.6% \({\text{HCO}}_{3}^{ - }\), 4% \({\text{SO}}_{4}^{{2 - }}\), 3.7% Cl– and 2.6% \({\text{NO}}_{3}^{ - }\).
Using the Piper diagram, Durov diagram and scatter plots depicting the concentrations of major ions in the stream waters of the Lo River catchment, the water types in stream water of Lo River catchment were identified with the dominance of Ca–Mg–HCO3 type, followed by Na–Cl, Na–HCO3–Cl, and Ca–Mg–Cl–SO4 types. The distribution of these water types corresponds closely with the geological conditions, with Na–Cl, Na–HCO3–Cl types prevailing in the watershed of granite complex, while watersheds characterized by lithologies such as Quaternary sediments, limestone, marble, shale, schist, and sandstone primarily exhibit the Ca–Mg–HCO3 water type. The dominant reaction in the water system is the dissolution of carbonate minerals, like calcite and dolomite, followed by the contribution of modest rainfall during the dry season, and small-scale processes of mixing and cation exchange.
The analytical results for pH, major anions, cations and trace elements are also subjected to a comparison against the National technical regulation on surface water quality QCVN 08-MT:2015/BTNMT (2015) and the Guidelines for drinking-water quality by WHO (2022). The compared results showed that the stream waters are generally suitable for the routine activities of the local population in Ha Giang province with a few specific sites that stream water required treatment before these waters can be safely utilized.
REFERENCES
Albarède, F., Geochemistry—An Introduction, Cambridge: Univ. Press, 2009.
Alfarrah, N., Hweesh, A., Camp, M.V., and Walraevens, K., Groundwater flow and chemistry of the oases of Al Wahat, NE Libya, Environm. Earth Sci., 2016, vol. 75, no. 12, p. 985.
Appelo, C.A.J. and Postma, D.E., Geochemistry, Groundwater and Pollution, London: CRC Press, 2005.
Baird, R.B., Eaton, A.D., and Rice, E.W., Standard Methods for the Examination of Water and Wastewater, Am. Publ. Health Ass., 2017.
Banks, D., Sæthèr, O.M., Ryghaug, P., and Reimann, C., Hydrochemical distribution patterns in stream waters, Trøndelag, central Norway, Sci. Total Environ., 2001, vol. 267, pp. 1–21.
Berner, E.K. and Berner, R.A., Global Environment: Water, Air and Geochemical Cycles. United States, 1996.
Destéfanis, G., Martínez, J.O., Ribeiro, G., and Gaiero, D.M., Geochemistry of surface water and weathering effects in the upper catchment of the Ctalamochita River, Cordoba’s Sierras Pampeanas (Central Argentina), Environm. Earth Sci., 2020, vol. 79, no. 19, p. 451.
Gaillardet, J., Dupré, B., Louvat, P., and Allegre, C.J., Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers, Chem. Geol., 1999, vol. 159, nos. 1–4, pp. 3–30.
Gaillardet, J., Viers, J., and Dupré, B., Trace elements in river waters, Treatise on Geochemistry, Hoboken, N.J., Ed., Elsevier, 2014, pp. 195–235.
Gassama, N., Curie, F., Vanhooydonck, P., Bourrain, X., and Widory, D., Determining the regional geochemical background for dissolved trace metals and metalloids in stream waters: Protocol, results and limitations - The upper Loire River basin (France), Water, 2021, vol. 13, no. 13, p. 1845.
Gibbs, R.J., Mechanisms controlling world water chemistry, Science, 1970, vol. 170, no. 3962, pp. 1088–1090.
Hartmann, J., Lauerwald, R., and Moosdorf, N., A brief overview of the global river chemistry database, GLORICH, Proc. Earth Planet. Sci., 2014, vol. 10, pp. 23–27.
Hem, J.D., Study and interpretation of the chemical characteristics of natural water, USGS Water-Supply Pap. 2254, Washington: US Govt. Print. Off., 1989.
Lim, D.T., Huong, N.T.L., Hue, N.T., Quan, D.T., Thuy, N.T.H., Thuy, T.T., Trang, T.T.M., Nhiem, D.N., Bac, N.Q., and Tien, M.V., Preliminary assessment of marine debris pollution and coastal water quality on some beaches in Thanh Hoa province, Vietnam, Vietn. J. Mar. Sci. Techn., 2021, vol. 21, no. 3, pp. 329–340.
Lim, T.D., Bac, Q.N., Cham, D.D., Nhiem, N.D., Huong, L.T.N., Thuy, H.T.N., Chi, H.T.N., Dien, C.T., and Ngo, N.P., Heavy metals in surface sediments of the intertidal Thai Binh Coast, Gulf of Tonkin, East Sea, Vietnam: Distribution, accumulation and contamination assessment, Environm. Sci. Poll. Res., 2022, vol. 29, no. 27, pp. 41261–41271.
Luyen, N.V., Savichev, O.G., Tin, Q.D., Nguyen, D.D., and Huyen, D.T.N., The distribution and dispersion of chemical components in natural water of Cho Don district, Bac Kan province, Vietnam, Ho Chi Minh City Univ. Educ, J. Sci., 2015, vol. 9, no. 75, pp. 130–139.
Meybeck, M., Global occurrence of major elements in rivers, Treatise Geochem., 2003, vol. 5, pp. 207–223.
Natali, C., Bianchini, G., Marchina, C., and Knöller, K., Geochemistry of the Adige River water from the Eastern Alps to the Adriatic Sea (Italy): evidences for distinct hydrological components and water-rock interactions, Environm. Sci. Pollut. Res., 2016, vol. 23, no. 12, pp. 11 677–11 694.
NAWAPI, Report on Investigation and Assessment of Groundwater Sources in the Midland and Mountainous Areas of Northern Vietnam, Ha Giang Province, Nat. Center Water Res. Plann. Invest., Min. Nat. Res. Environm., 2009.
Piper, A.M., A graphic procedure in the geochemical interpretation of water analyses, Eos, Trans. Am. Geoph. Union, 1944, vol. 25, no. 6, pp. 914–928.
QCVN 08-MT:2015/BTNMT: National Technical Regulation on Surface Water Quality, Hanoi, 2015 (in Vietnamese).
Quoc, N.K., (Ed.) et al., Geological and Mineral Resources Map of Vietnam on 1 : 200 000, Bac Kan: Dept. Geol. Miner. Vietn., Hanoi, 2000.
Reimann, C., Filzmoser, P., and Garrett, R.G., Background and threshold: critical comparison of methods of determination, Sci. Total Environm., 2005, vol. 346, nos. 1–3, pp. 1–16.
Sarker, Md.M., Hermans, T., Camp, M.V., Hossain, D., Islam, M., Ahmed, N., Bhuiyan, Md.A.Q., Karim, Md.M., and Walraevens, K., Identifying the major hydrogeochemical factors governing groundwater chemistry in the coastal aquifers of southwest Bangladesh using statistical analysis, Hydrology, 2022, vol. 9, no. 2, p. 20.
Sharma, A., Singh, A.K., and Kumar, K., Environmental geochemistry and quality assessment of surface and subsurface water of Mahi River basin, western India, Environm. Earth Sci., 2012, vol. 65, no. 4, pp. 1231–1250.
TCVN 6663-6:2018 (ISO 5667-6:2014). Water quality – Sampling, Vietnam Standard, 2018, Part 6: Guidance on sampling of rivers and streams.
Tinh, H.X. (Ed.) et al., Geological and Mineral Resources Map of Vietnam on 1 : 200 000, Bao Lac: Dept. Geol. Miner. Vietnam, Hanoi, 2000.
WHO: Guidelines for Drinking-Water Quality, World Health Organization, 2022.
Xu, P., Li, M., Qian, H., Zhang, Q., Liu, F., and Hou, K., Hydrochemistry and geothermometry of geothermal water in the Guanzhong Basin, China: a case study in Xi’an, Environm. Earth Sci., 2019, vol. 78, no. 3, p. 87.
Xuyen, T. (Ed.) et al., Geological and Mineral resources map of Vietnam on 1 : 200 000, Bac Quang: Dept. Geol. Miner. Vietnam, Hanoi, 2000a.
Xuyen, T. (Ed.) et al., Geological and Mineral Resources Map of Vietnam on 1 : 200 000, Ma Quan, Dept. Geol. Miner. Vietnam, Hanoi, 2000b.
Funding
This study was financially supported by Vietnam Academy of Science and Technology (VAST) under the project coded TĐĐHQG.01/21-23 and TĐĐHQG.02/21-23. The research was carried out as part of Russian governmental program 124022100078-7.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tran, A., Le, L.D., Shakirov, R.B. et al. Geochemistry of Stream Waters of the Lo River Catchment, Ha Giang Province (Northern Vietnam). Lithol Miner Resour 59, 340–356 (2024). https://doi.org/10.1134/S0024490224700494
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0024490224700494