Abstract
Ala River is the main river that drains Akure metropolis in southwestern Nigeria. The river takes its sources from deep-seated fractures in granite and charnockite rock bodies at the North and North-western regions of Akure town, flowing through quartzite and granite gneiss exposures. This study was carried out to assess the geochemical processes influencing the quality of Ala river water. The dendritic river system, covering an area of about 427 km2, was studied and water was sampled at thirty-seven (37) points for in-situ determination of some physicochemical parameters and dissolved concentrations of the major ions. pH values obtained varied between 7.16 and 7.75, electrical conductivity ranged from 123 to 520 µs/cm, total dissolved solids varied between 62 and 260 mg/l-CaCO3 while average temperature value was 26.65 °C. Relative concentration of cations and anions were in the order of Ca2+ > Mg2+ > Na+ > K+ and HCO3− > SO42− > Cl− > NO3−. Mean values of dissolved Na+, K+, Ca2+, Mg2+, Fe2+, Cl−, HCO3−, SO42−, NO3−, and PO42− were 34.028, 8.872, 34.175, 18.289, 0.428, 16.543, 56.41, 12.503, 0.495 and 2.029 mg/l, respectively. Two hydochemical facies namely Calcium-Bicarbonate facies and mixed calcium–magnesium–chloride facies dominated the chemistry of the water. CaHCO3 facies revealed the contribution of geogenic processes while the mixed Ca(Mg)-Cl type reflected domestically induced anthropogenic activities influencing chemistry of the water. Gibbs diagrams identified rock–water interaction as an important geochemical process in the study area. Silicate weathering of rocks and carbonate weathering were identified as the important geochemical processes which influence the water chemistry of the study area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rivers are used as transportation route, source of hydroelectric power generation, fishing and recreational activities all over the world (Ibama et al. 2015). They usually contain large quantity of water but its use in terms of quality is limited due to pollution and contamination. Most opinions attributed the unsafe and poor quality of these waters to anthropogenic activities (industrial effluents, irrigation return flow, mine waste disposals and household waste disposals) (Xian et al. 2007; Raburu and Okeyo-Owuor 2006). Sadly, most of these effluents are discharged into surface water bodies untreated (Venugopal et al. 2009; Kayastha 2015).
Anthropogenic activities are not the sole contributor to the undermining poor quality of surface water (Nitasha and Sanjiv 2015). The underlying geologic materials over which rivers flow through can also be another contributor to the chemistry and hitherto quality of the waters (Feth et al. 1964; Garrels and Mackenzie 1967). Arguments have been that the subsurface water has longer residence time due to its limited in flow, while the surface water has shorter residence time in relation to its free flow (Rasmussen 2011; Boyd 2000). This fact is not disputable, but the weathering activities, nature of the underlying rocks has been the pointer to the pattern of the surface water be it dendritic, trellis and radial (Ritter 2006; Charlton 2008). If the drainage pattern of the surface water can be influenced by the nature of the rock through which it flows, the chemistry and quality of the surface water can be affected as well. The weathering rate of most rocks are also dictated by the geochemistry of rocks (Probst et al. 1992; Louvat and Allègre 1997; Viers et al. 2000; Meybeck 2003; Ollivier et al. 2010; Chorover et al. 2017; Koger et al. 2018). In this light, most studies tends towards establishing the influence of geology on the chemical composition of surface waters (Larson 1983; Gupta and Banerjee 2012; Kematou et al. 2013) (e.g. Hoh and Nisqually rivers; Ngam and Mou Rivers in the Cameroon volcanic line). These studies revealed a strong link between the lithology and mineralization of the rivers (Larson 1983; Kematou et al. 2013). The erosional activities that take place during the flow of rivers pose to be another source of pollution to the surface water (Zhang et al. 2009).
The geochemical studies of surface waters have provided information not only limited to the quality of the waters but also establish the link to the chemical weathering of the underlying rocks and imprints of anthropogenic activities as well (Han and Liu 2004; Huang et al. 2010). Geochemical studies were found very necessary to identify and understand different geochemical processes influencing the chemistry of an urban River, Ala River. The river drains the metropolitan city of Akure, located in the south-western part of Nigeria with a population of 556,300 and growth rate of 4.64% per year (City Population 2016). This river with a total length of about 22 km along the longitudinal flow direction within the city and a total length of 57 km downstream (Oluwaniyi 2018). It took its source from a spring in north-western part of Akure town and flows toward the south-eastern part of the town. The upstream of the river lies within the township where there is increased urban activities while the downstream section drains the rural suburbs like Ilado, Ehinala, Imafon, and Araromi quarters. The river water is being exploited at the downstream areas for irrigation along with some domestic applications. This is recurrent mostly in the rainy season. However, most houses are built along this river and this river also recharge nearby wells thereby serving as an additional supply of water. It is with this concern that this study is embarked upon to identify the extent to which lithology controls the water chemistry of Ala River.
Area of investigation
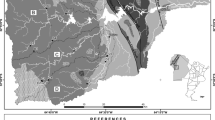
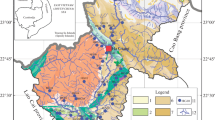
Ala River is the main river that drains Akure, south-western Nigeria. Akure is the capital city of Ondo State Nigeria and it is located in the central senatorial district of the state (Federal Office of Statistics 1992). Akure is bounded in the North by both Akure North and Ifedore Local Government Area, and in the West by Ile-Oluji/Oke-Igbo Local Government Area, East by Owo Local Government Area, in the South by Idanre local government area. Geographically, the study area is located within latitudes 07° 10′ N and 07° 18′ N and longitudes 005° 10′ E and 005° 19′ E (Fig. 1).The river took its source from North-Western part of Akure town and flow towards the South-Eastern part of the town and drains into Ogbese River in the eastern part of the metropolis.
The area falls within the tropical rain forest of South-western Nigeria, where there exist two major seasons; wet and dry seasons (Olabode et al 2020). The study area experiences frequent rainfall between April and July with a short break in August and continues between September and November, while the dry season is usually between November and March. The annual average temperature is 26.7° C while the average annual rainfall is 1334 mm (Ondo State Ministry of Economic Planning and Budget 2010).
Generally, the study area is of tropical rainforest vegetation belt, but some portions of the area is of re-growth rainforest type which consists of grasses and scattered trees which in many parts been modified with human activity such as buildings, roads land cultivation, etc.
Akure and its environs is characterised by two distinctive geomorphological expressions which described the topography of the study area; a gentle or low-lying rolling relief in the south and southwest and an inselberg landscape of high altitude rising up to 442 m in the north and northwest.
Geology of the study area
The Akure metropolis is underlain by four of the six petrological units of the Basement Complex of Southwestern Nigeria as identified by Rahaman (1988) and described by Olarewaju (1988). Granite-gneiss accounts for almost 60% of the total outcrops in the area (Fig. 2). Its mineralogy includes quartz, feldspar, hornblende, biotite and muscovite. Quartzite is a metasediment and forms not more than 5% of the outcrops in the area. It tends to form prominent topographical features which rise up above the surrounding terrains and consists of about 90% quartz with minor amounts of muscovite, sillimanite, staurolite, hematite, tourmaline and zircon. The Older Granite accounts for about 10–15% of the rock units present in the area. Its texture varies from coarse to porphyritic, while the mineralogical composition includes quartz, feldspar and biotite. Charnockite occurs in the northern part of the study area along the margins of Older Granite bodies. The mineral composition of the rock includes quartz, feldspar, orthopyroxene, clinopyroxene, hornblende, biotite and apatite.
Methodology
General reconnaissance field appraisal of the study area was carried out with the topographical map of Akure as base map. In the course of the field work, prevailing hydrological and geological conditions as well as possible sampling points were noted. 37 points were then established for study within the drainage system. These selected study points were areas where the tributary channels flow into larger channels. Water samples (2 l) were collected at each of the established thirty seven (37) points for chemical analysis. Physico-chemical parameters (pH, temperature, total dissolved solids and electrical conductivity) of the water samples were measured in situ after using a portable HANNA Combo EC/pH meter after water samples were collected. Chemical analysis of samples collected started at the Geochemistry Laboratory of the Department of Applied Geology, Federal University of Technology, Akure as soon as the water samples arrived the laboratory. The analytical methods used in the determination of water chemistry were in accordance with American Public Health Association (APHA 1989) standard procedures. The dissolved anions were analysed by titration and turbidimetry methods using UV-spectrophotometer while atomic absorption spectrophotometer (AAS) was used to determine the cation concentrations. The cations analysed for are Na+, K+, Ca2+, Mg2+ and Fe2+ while the anions are PO42− NO3−, SO42−, HCO3−, and Cl−. Sulphate, Phosphate and Nitrate ions were analyzed for in the water samples using JENWAY 6705 Ultraviolet/Visible Spectrophotometer at wavelengths of 420 ηm and 425 ηm, respectively, while bicarbonate and chloride was analysed by titration. The atomic absorption spectrophotometer AA320N with Pelkin Elmer configuration was used for analysis of the dissolved cations (Na+, K+, Ca2+, Mg2+ and Fe2+). Ion balances were calculated (meq/l) and examined for the water samples as a quality check for the chemical analyses.
Water type classification
Piper diagram was employed for this study using GW-chart free software package developed by USGS. The Piper diagram was used to determine the predominant water facie type in the stream. The concentration of eight major ions (Na+, K+, Mg2+, Ca2+, Cl−, CO32−, HCO−3 and SO42−) are represented on the trilinear diagram by grouping the K+ with Na+ and the CO32− with HCO3−, thus reducing the number of parameters for plotting to six. On the Piper diagram, the relative concentration of the cations and anions are plotted in the lower triangles, and the resulting two points are extended into the central field to represent the total ion concentrations.
Statistical analysis
This was carried out using Microsoft Excel and IBM SPSS software to establish relationship between the chemical variables. This relationship will provide information about the evolution of the waters. Cluster analysis (CA) and correlation analysis was performed on the data matrix for advanced characterization of chemistry of the studied water samples.
Pearson correlation table was employed for the correlation analysis. A pair of chemical variables having correlation coefficient lower than 0.5 suggests weak relationship, while greater than 0.5 and depicts a strong relationship. The correlation analysis employed in the interpretation of the results presented on the correlation table was carried out after Jeyaraj et al. (2006).
Cluster analysis was done by employing hierarchical methods as hierarchical clustering does not require the number of clusters to be fixed in advance. The ward’s method was used to carry out cluster analysis, while the hierarchical agglomerate clustering with Euclidean distance was used as a dissimilarity measure where cluster are formed sequentially by starting with the most similar pair of objects and forming higher clusters step by step.
Gibbs plot
Gibbs diagram was employed to establish the relationship between water composition and lithological characteristics, identify the dominant geochemical processes and mechanisms prevailing in waters sourced. These relations, geochemical processes and mechanisms have a strong effect on the overall quality of water usage and can also guide towards its remediation if water quality is found unsuitable for many varied purposes (Gibbs 1970; Olofinlade et al. 2018). The Gibbs diagram consists of three different zones, namely evaporation, atmospheric (precipitation) and rock–water interaction dominance (Gibbs 1970). Accurate Gibbs plots for the sampled waters were obtained by the plots of TDS against ionic ratios (Na/(Na + Ca) and Cl/(Cl + HCO3).
Results and discussion
The measured physical parameters and chemical analyses results of the sampled stream waters in the study area are summarized in Table 1 as range of values and mean values.
Physico-chemical characteristics
The pH values of the sampled waters varied from 7.16 to 7.75 with a mean value of 7.38. This shows that water in the river is slightly alkaline. Acidification of stream water can result from anthropogenic contributions of acid mine drainage (Herlihy et al. 1990) or the deposition of nitric and sulfuric acids from the atmosphere (Angelier 2003). However, it is important to note that acidification of streams can also be natural sourced in areas with considerable humic inputs (Allan 1995). But, since it has been established that the geology of an area normally determine the pH of the local waters. The alkaline nature of the water is due to the presence of carbonates in sufficient quantities (Todd 1995).
Electrical conductivity (EC) qualitatively reflects the status of inorganic pollution and is a measure of concentration of charged ions in waters and is strongly influenced by the geology in the catchment (Cunningham et al. 2010; Olson 2012). Electrical conductivity (EC) of the waters ranged from 123 to 520 µs/cm, with a mean of 259.82 µs/cm. Concentration of TDS values are also the indicator of erosion rate in the river basin. The TDS values ranged from 62 to 260 mg/l CaCO3. This shows that water in the area is generally fresh.
Anionic chemistry
HCO3− was the most dominant anion in the stream waters, while NO3− was the least. The order of major anion concentrations was HCO3− > SO42− > Cl− > NO3−. The dominance of bicarbonate confirms the continental environment for the water as established earlier by Berner and Berner (1987). Most bicarbonate ions in water are derived from the carbon dioxide in the atmosphere, and the soil as well as from solution of carbonate rocks (Hamid et al. 2020). Bouwer (1978) indicated that HCO3− is mainly produced due to the action of CO2 from the atmosphere and released from organic decomposition. Carbonic acid weathering of silicate rocks is believed to be the source of HCO3− in the river. The concentration of bicarbonate varied from 10 to 155 mg/l with the highest concentration associated with the areas underlain with granite gneiss. The concentration of sulphate varied from 1.667 to 90.833 mg/l. Natural sources of sulphate in stream-water come from the process of chemical weathering and dissolution of sulphate minerals (Alewell et al. 1999). Other primary natural sources (Alewell et al. 1999; Singleton 2000) of sulphate include atmospheric precipitation while anthropogenic sources include power plants, phosphate-based fertilizers, etc. Sulphate concentration in the stream waters are probably of anthropogenic sources since sulphate-rich minerals are not common in rocks at the study site. Chloride in stream water originates from both natural and anthropogenic sources. The natural sources of Cl− ions include sedimentary rocks and other common evaporate minerals (chloride salts) (Pradhan and Pirasteh 2011; Ramesh and Jagadeeswari 2012). The concentration of chloride varied from 3.545 to 42.540 mg/l with the highest values at the metropolitan sections of the study area. The high concentrations can be attributed to anthropogenic sources which are expectedly influenced by domestic activities along the stream course. Nitrate (NO3−) had the least concentration among the major anions tested for in all the sampled waters. The sources of nitrate and phosphate in streams are linked to agricultural activities practised near surface water bodies (Hynes 1970; Schuetz et al. 2016; Hamid et al. 2020). The dissolved ions are washed into the surface water bodies through action of precipitation with evident of irrigation return flow. The concentration of nitrate varied between 0.039 and 1.256 mg/l. The concentration of phosphate in the water samples ranged from 0.110 to 14.586 mg/l with a mean value of 2.2 mg/l. These high concentrations of PO4− can be attributed to the vegetation and agricultural practices notable at the southeastern parts of the study area toward Ehinala village where application of fertilizers on farmlands was rampant.
Cationic chemistry
Calcium was the most abundant cation, while potassium had the least concentrations in all sampled waters. The order of major cation concentrations was Ca2+ > Mg2+ > Na+ > K+. Calcium concentrations in the sampled water varied between 12.28 and 58.66 mg/l. Calcium ions dissolve readily from silicate and carbonate rich rocks, leached ions and other sources. Mallick (2017) reported that Ca concentration in natural freshwater falls below 10 mg/l, although water in the proximity of carbonate rocks and limestone may contain calcium ranging 30–100 mg/l. The possible source of calcium is Ca-rich plagioclase feldspar which is a common mineral in the granite and gneiss that occur in the study area. Other source of calcium in the stream waters may be the fertilizers used in the area for agricultural purposes. The concentration of magnesium varied from 9.385 to 35.29 mg/l. Presence of magnesium in the stream water is due to weathering of pyroxenes and amphiboles in charnockitic rocks (Hamid et al. 2020). Erosion of rocks and minerals is the most common source of Ca and Mg. Leaching of soil by erosion, pollution from sewage and industrial waste can also contribute to Ca and Mg (Nikanorov and Brazhnikova 2012). Concentration of sodium in the samples varied from 24.37 to 53.705 mg/l with the highest values found in areas underlain by granite gneisses. Most salts of Na are not active in chemical reactions even if they are readily soluble in water (Pradhan and Pirasteh 2011). Weathering of Na–K-bearing minerals/rocks, ion (cation) exchange process, pollution from industrial effluent and domestic sewage, and/or agricultural activities are responsible for the dominance of Na–K in water bodies (Hamid et al. 2020). Sodium in the stream-water could have been sourced from weathering of Na-rich plagioclase feldspars as well as leachate from decaying municipal wastes, some industrial brines and sewage in the area of study. Potassium is the least abundant cation in the water samples with an average concentration of 8.872 mg/l. Potassium minerals are resistant to weathering (Pradhan and Pirasteh 2011) have a weak migration potential (Nikanorov and Brazhnikova 2012). The major source of potassium in Ala River is probably the dissolution and hydrolysis of orthoclase feldspar and biotite. Iron (Fe) in the stream water occurred as a trace element, with its concentration ranging from 0 to 1.516 mg/l. The probable sources of Fe in the stream water may be the weathering of pyroxenes, amphiboles or biotite as well as decaying organic wastes and plant debris.
Water types
To obtain the principal water types present, all the water samples analyzed were classified with Piper diagram using the Ca2+, Mg2+, Na+, K+, HCO3−, Cl−, and SO42− concentrations (Piper 1944). Two facies groups of water emerged from the classification according to the position they plotted on the diamond field of the diagram (Figs. 3 and 4).
About 57% of the samples plotted in the alkaline earth metal facies (Ca–Mg) range as well as in the bicarbonate facies range (Fig. 2). The dominant water type for the water in the study area was the Calcium-bicarbonate type CaHCO3 which is typical of continental environment. The alkaline earth metals (Ca + Mg) exceeded the alkalis (Na + K). The presence of high calcium, high sulphate, low chloride and high bicarbonate belong to the CaHCO3 facies with appreciable continental influences. This hydrochemical facies type suggests that the effects of chemical processes in the lithologic environment were the prevailing factors that determined chemistry of the water. About 43% of the water samples plotted within mixed calcium–magnesium–chloride water type facies range (Fig. 3). Water of this chemistry is associated with domestically-sourced (anthropogenic) contamination activities. From these results, it is evident Ca and Mg are the dominant cations, while HCO3 and SO4 are the dominant anions. This suggests that dissolution of silicate minerals are prominent in Akure hydrological environment. The activities such as oxidation of pyrite and dissolution of halites from saline and alkaline soils that usually influenced sulphate concentration (Subramanian et al. 1987) are not evident in the study area. Therefore, the mixed calcium–magnesium–chloride water type does not indicate any geogenic processes. This water type is an indication to the human activities contributing to the chemistry of the water.
Sources of major ions
In semi-arid regions, precipitation, evaporation and rock–water interaction are the common hydrogeochemical processes and the water chemistry of these areas is generally dependent on these processes (Kumar and James 2016). Gibbs (1970) plot was employed to illustrate the dominant geochemical processes affecting chemistry of the stream waters (Fig. 5). All the sampled waters plotted in the zone of rock-water interaction. The water–rock interaction dominance indicates that there is a strong interaction between the chemistry of the underlying rock lithologies and the chemistry of the water, thus revealing the processes of weathering and dissolution of silicate minerals as the dominant factors controlling the stream-water chemistry.
Correlation matrix
The compositional relationships among the dissolved ions were used to assess the origin of solutes and the processes that resulted in the composition of the water (Table 2). The Na/Cl relationship gave a good correlation (r = 0.509). Generally, Na–Cl relationships are used to ascertain origin of salinity in water (Sami 1992; Carol et al. 2009). Salinity may also originate from the dissolution of chloride-rich minerals. If there exists a positive correlation between sodium and chloride, then their origin is likely to be from a common source (Kumar and James 2016). Figure 6 describes the relationship between Na and Cl, all the samples except two deviate from the 1:1 equiline towards the Na-field. This obviously indicates silicate weathering as a source of high sodium ratio in water. Potassium and chloride also gave good relationship.
In the study area, the sources of salinity may be from anthropogenic activities along the river course. This salinity may indicate pollution within the water body, as large contents of chloride in freshwater is an indicator of pollution (Venkatasubramani and Meenambal 2007). The relationship between Ca and HCO3 was plotted to study the dominant sources for the ions. HCO3 showed a poor correlation (0.39) with Ca (Table 2), which indicates that both ions are from different sources. Figure 7 indicates carbonic acid weathering of silicate minerals led to the presence of HCO3, while Ca is sourced from carbonate weathering of the granites and gneisses in the study area. This shows that magnesium dominates over calcium in many locations. In Fig. 8, the points fall evenly across all fields. This further indicates that silicate weathering and carbonate mineral weathering are the prominent geochemical processes acting in the surface. The relationships between cations showed strong correlations between 0.533 and 0.776. This suggests that the cations in the stream water share a common dominant source. Magnesium is often associated with calcium in all natural waters. Magnesium poses no major concern with regards to public health, and limits of concentration set for the water are based mainly on palatability, corrosion and incrustation criteria (Mallick 2017). Sodium (Na+) and potassium (K+) showed correlation of 0.72; also indicating a similar source. Na in stream water is sourced from plagioclase feldspars in granite rocks and K from orthoclase feldspar and muscovite minerals present in granites. An important source of Na and K in surface water is weathering of silicate minerals (Kumar and James 2016). Common igneous rock minerals such as pyroxenes, amphiboles, Ca and K feldspars, are prone to weathering. This forms a rich source of sodium and potassium (Jacks 1973; Bartarya 1993).
Cluster analysis
For hierarchical analysis, ward linkage method revealed the results of parameters and are presented in two groups in Fig. 9. Group one accounted for about 65% of the total water samples, while Group two accounted for about 35% of the waters. The grouping was observed to have been based on samples with similar parameters. As observed in the piper plot, it was discovered in the cluster analysis that Group two constituted samples with high possibility for contamination while the group one samples are of geogenically sourced. The sulphate concentration for this group two ranged between 66.167 and 90.833 mg/l, while Group one has range of values between 1.667 and 10.750 mg/l. The samples that constitute group two are found in the river sections in the municipal part of the study area indicating the influence of domestic activities on the river chemistry.
Conclusion
Geochemical studies was carried out towards the identification of different geochemical processes influencing the chemistry of a major river that drained Akure Area. The results of the chemical analysis of the water samples showed that the chemical composition of the stream waters can be linked to the geology of the area. The abundance of bicarbonates and the dominance of CaHCO3 facie type in the study area confirms the continental environment of the water. Detailed analysis based on statistical analysis and Gibbs plot revealed that carbonic acid weathering of silicate rocks is the dominant geochemical processes influencing the chemistry of the water. The cluster analysis revealed the alteration in the chemistry of the surface water body through contamination from anthropogenic sources such as effluents from municipal wastes, agrochemical run-off from farm land, atmospheric precipitation among others. The study also accentuates the importance of statistical analysis in geochemical studies; as regression analysis on the cations showed that the stream waters are related to a similar source. The results based on chemical analysis and different statistical analyses employed indicates that the chemistry of the stream water is largely controlled by geochemical mechanisms. The information obtained from this study can, therefore, be used as reference for assessing the possible environmental impacts of future developments around the river and designing a remedial activity for pollution in the river.
References
Alewell C, Mitchell MJ, Likens GE, Krouse HR (1999) Sources of stream sulfate at the Hubbard Brook Experimental Forest: long-term analyses using stable isotopes. Biogeochemistry 44:281–299
Allan JD (1995) Stream ecology: structure and function of running waters. Chapman and Hall, London
Angelier E (2003) Ecology of streams and rivers. Science Publishers Inc, Enfield
APHA (1989) Standard methods for the examination of water and wastewater, 17th edn. American Public Health Association, Washignton, p 268
Bartarya SK (1993) Hydrochemistry and rock weathering ina sub-tropical lesser himalayan river basin in Kumaun, India. J Hydrol 146:149–174. https://doi.org/10.1016/0022-1694(93)90274-D
Berner KB, Berner RA (1987) The global water cycle: geochemistry and environment. Prentice-Hall, New Jersey, p 397
Bouwer H (1978) Groundwater hydrology. McGraw-Hill, New York
Boyd CE (2000) Water quality: an introduction. Springer, Berlin
Carol E, Kruse E, Mas-Pla J (2009) Hydrochemical and isotopic evidence of groundwater salinization processes on the coastal plain of Samborombon Bay, Argentina. J Hydrol 365:335–345
Charlton R (2008) Fundamentals of fluvial geomorphology. Routledge, New York
Chorover J, Derry LA, McDowell WH (2017) Concentration-discharge relations in the critical zone: implications for resolving critical zone structure, function, and evolution. Water Resour Res 53:8654–8659. https://doi.org/10.1002/2017WR021111
Cunningham MA, Menking KA, Gillikin DP, Batur P (2010) Influence of open space on water quality in an urban stream. PhysGeogr 31(4):336–356. https://doi.org/10.2747/0272-3646.31.4.336
Federal Office of Statistics (1992) Statistical news, June 1992
Feth JH, Roberson CE, Polzer WL (1964) Sources of mineral constituents in water from granitic rocks, Sierra Nevada, California and Nevada: water supply paper 1535–1. USGS, Reston, p 70
Garrels RM, Mackenzie FT (1967) Origin of the compositions of some springs and lakes. In: Stumm W (ed) Equilibrium concepts in natural water systems, vol 67. American Chemical Society, Washington, pp 222–242
Gibbs RJ (1970) Mechanism controlling world water chemistry. Sciences 170:795–840
Gupta S, Banerjee US (2012) Geochemistry of the River Damodar—the influence of geology and weathering environment on the dissolved load. Int J GeomatGeosci 2(3):853–867
Hamid A, Bhat SU, Jehangir A (2020) Local determinants influencing stream water quality. Appl Water Sci 10:24
Han G, Liu C (2004) Water geochemistry controlled by carbonate dissolution: a study of the river waters draining karst-dominated terrain, Guizhou Province, China. Chem Geol 204:1–21
Herlihy AT, Kaufmann PR, Mitch ME (1990) Regional estimates of acid mine drainage impact on streams in the Mid-Atlantic and southeastern United States. Water Air Soil Pollut 50:91–107
Huang F, Wang X, Lou L, Zhou Z, Wu J (2010) Spatial variation and source apportionment of water pollution in Qiantang River (China) using statistical techniques. Water Res 44:1562–1572
Hynes HBN (1970) The ecology of running waters. Liverpool University Press, Liverpool
Ibama B, Wocha C, Dike EC (2015) Development of waterways for effective transportation system and recreation enhancement in selected navigable creeks and waterways in Rivers State. Int J Sci Eng Res 6(9):1016–1026
Jacks G (1973) Chemistry of groundwater in a district in southern India. J Hydrol 18:185–200
Jeyaraj T, Padmavathy S, Jebakumari S (2006) Correlation between water quality parameters for groundwater samples of Bharathi Nagar, Tiruchirappalli City. Indian J Environ Prot 22(7):755–769
Kayastha SP (2015) Geochemical parameters of water quality of Karra River, Hetauda Industrial are, Central Nepal. J Inst Sci Technol 20(2):31–36
Kematou TE, Tsinkou F, Tchouankoue JP, Ndam N, Ngounou N, Hinman NW (2013) Influences of lithology on water quality: a study of the Ngam and Mou watersheds in West Cameroon (Central Africa). Procedia Earth Planet Sci 7(2013):405–408
Koger JM, Newman BD, Goering TJ (2018) Chemostatic behaviour of major ions and contaminants in a semiarid spring and stream system near Los Alamos, NM, USA. Hydrol Process 32:1709–1716. https://doi.org/10.1002/hyp.11624
Kumar SPJ, James EJ (2016) Identification of hydrogeochemical processes in the Coimbatore district Tamil Nadu, India. Hydrol Sci J 61(4):719–731. https://doi.org/10.1080/02626667.2015.1022551
Larson AG (1983) Influence of Geology on the chemical composition of two Washington rivers. Northwest Sci 57(4):256–266
Louvat P, Allègre CJ (1997) Present denudation rates on the island of Reunion determined by river geochemistry: basalt weathering and mass budget between chemical and mechanical erosions. GeochimCosmochim Acta 61:3645–3669. https://doi.org/10.1016/S00167037(97)00180-4
Mallick J (2017) Hydrogeochemical characteristics and assessment of water quality in the Al-Saad Lake, Abha Saudi Arabia. Appl Water Sci 7:2869–2882. https://doi.org/10.1007/s13201-017-0553-1
Meybeck M (2003) Global occurrence of major elements in rivers. Treatise Geochem 5:207–223. https://doi.org/10.1016/B0-08-043751-6/05164-1
Nikanorov AM, Brazhnikova LV (2012) Water chemical composition of rivers, lakes and wetlands. Types Prop Water 2:42–80
Olabode OF, Oluwaniyi OE, Adebayo QA et al (2020) Morpho-lithostructural analysis of Ala River basin for flood risk assessment: geospatial techniques intervention. Earth Sci Inform. https://doi.org/10.1007/s12145-020-00457-6
Olarewaju VO (1988) REE in Charnockitic and Associated Granitic rocks of Ado-EkitiAkure, Southwest Nigeria. Precambrian geology of Nigeria. Nigerian Geological Survey, Abuja, pp 231–239
Ollivier P, Hamelin B, Radakovitch O (2010) Seasonal variations of physical and chemical erosion: a three-year survey of the Rhone River (France). GeochimCosmochim Acta 74:907–927. https://doi.org/10.1016/j.gca.2009.10.037
Olofinlade WS, Daramola SO, Olabode OF (2018) Hydrochemical and statistical modeling of groundwater quality in two contrasting geological terrains of southwestern Nigeria. Model Earth Syst Environ 4(4):1405–1421
Olson JR (2012) The influence of geology and other environmental factors on stream water chemistry and benthic invertebrate assemblages. All Graduate Theses and Dissertations
Oluwaniyi OE (2018) Influence of lithology on structural configuration and water chemistry of Ala drainage system, Akure Southwestern Nigeria. M.Tech thesis, Dept. App. Geology Fed. Uni. Of Tech. Akure, Nigeria, vol 116, pp 9–11
Ondo State Ministry of Economic Planning and Budget (2010) The publication of facts and figures of Ondo State of Nigeria. Research and Statistics Department, Abuja, pp 7–9
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Trans Am Geophys Union 25:914–928. https://doi.org/10.1029/TR025i006p00914
City Population (2016) Ondo State population. Population statistics, charts, map and location. https://www.citypopulation.de/php/nigeria. Accessed 10th May 2020
Pradhan B, Pirasteh S (2011) Hydro-chemical analysis of the ground water of the basaltic catchments: upper Bhatsai region, Maharastra. Open Hydrol J 5:51–57
Probst J-L, NKounkou RR, Krempp G et al (1992) Dissolved major elements exported by the Congo and the Ubangi rivers during the period 1987–1989. J Hydrol 135:237–257. https://doi.org/10.1016/0022-1694(92)90090-I
Raburu PO, Okeyo-Owuor JB (2006) Impact of agro-industrial activities on the water quality of River Nyando, Lake Victoria Basin, Kenya. In: Proceedings of the 11th World Lakes Conference, Nairobi, pp 307–314
Rahaman MA (1988) Recent advances in the study of the basement complex of Nigeria. Precambrian geology of Nigeria. Geological Survey of Nigeria, Abuja, pp 11–43
Ramesh K, Jagadeeswari BP (2012) Hydrochemical characteristics of groundwater for domestic and irrigation purposes in Periyakulam taluk of Theni district, Tamil Nadu. Int Res J Environ Sci 1(1):19–27
Rasmussen TC (2011) Surface and subsurface waters. In: Glinski J, Horabik J, Lipiec J (eds) Encyclopedia of agrophysics. Encyclopedia of earth sciences series. Springer, Dordrecht
Ritter ME (2006) The physical environment: an introduction to physical geography. https://www.earthonlinemedia.com/ebooks/tpe_3e/title_page.html
Sami K (1992) Recharge mechanisms and geochemical processes in a semi-arid sedimentary basin, eastern cape. South Africa. J Hydrol 139(1–4):27–48
Schuetz T, Gascuel-Odoux C, Durand P, Weiler M (2016) Nitrate sinks and sources as controls of spatio-temporal water quality dynamics in an agricultural headwater catchment. Hydrol Earth Syst Sci 20:843–857
Singleton H (2000) Ambient water quality guidelines for sulphate. Ministry of Environment, Lands and Parks, Victoria
Subramanian V, Biksham G, Ramesh R (1987) Environmental geology of peninsular river basins of India. J Geol Surv India 30:393–401
Todd LP (1995) Changes in biological characteristics of Daphnia magna from chronic action of copper and nickel at low concentrations. Hydrobiol J 5:59–62
Venkatasubramani R, Meenambal T (2007) Study of the sub surface water quality in Mettupalayam taluk of Coimbatore district, Tamil Nadu. Nat Environ PollutTechnol 6:307–310
Venugopal T, Giridharan L, Jayaprakash M, Velmurugan PM (2009) A comprehensive geochemical evaluation of the water quality of River Adyar, India. Bull Environ ContamToxicol 82(2):211–217
Viers J, Dupré B, Braun J-J et al (2000) Major and trace element abundances, and strontium isotopes in the Nyong basin rivers (Cameroon): constraints on chemical weathering processes and elements transport mechanisms in humid tropical environments. Chem Geol 169:211–241. https://doi.org/10.1016/S0009-2541(00)00298-9
World Health Organization (2011) Guidelines for drinking water quality, 4th edn. WHO, Switzerland, Geneva
Xian G, Crane M, Junshan S (2007) An analysis of urban development and its environmental impact on the Tampa Bay watershed. J Environ Manag 85(4):956–976
Zhang Y, Guo F, Meng W, Wang XQ (2009) Water quality assessment and source identification of Daliao River basin using multivariate statistical methods. Environ Monit Assess 152:105–121
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oluwaniyi, O.E., Asiwaju-Bello, Y.A. Geochemical processes influencing stream water chemistry: a case study of Ala River, Akure, Southwestern Nigeria. Sustain. Water Resour. Manag. 6, 108 (2020). https://doi.org/10.1007/s40899-020-00460-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-020-00460-1