Abstract
Kinetic experiments were carried out on the hydrolysis of concentrated aqueous and aqueous alkaline solutions of NaBH4 with a Co/TiO2 catalyst. The experiments in the aqueous NaBH4 solutions were performed at molal concentrations of 0.25, 1, and 4 mol/kg. In the aqueous alkaline solutions with molal NaBH4 concentrations of 0.25 and 1 mol/kg, the molal NaOH concentrations were varied in the range 0.05–8 mol/kg. The activation energies in the aqueous solution and the aqueous alkaline solutions were found to be 64.3 and 53.6 kJ/mol, respectively. Features of the kinetic curves and the possible kinetic schemes were discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Attention to the hydrolysis of alkaline solutions of sodium borohydride NaBH4 on cobalt catalysts is due to such an unexpected and so far unexplained phenomenon as an increase in the rate of hydrogen generation upon the addition of alkali to an aqueous NaBH4 solution [1, 2]. It is even more interesting that the dependence on the alkali concentration is nonmonotonic: the rate of hydrogen generation reaches a maximum at a molar NaOH concentration of 0.5–3 mol/kg, and a further increase in the concentration leads to a decrease in the rate of hydrogen generation [3–10]. At the same time, in homogeneous hydrolysis and catalytic hydrolysis on the noble metals Pt and Ru, the addition of alkali leads to a monotonic decrease in the rate of hydrogen generation [11–13].
In this work, NaBH4 solutions were studied in the molal concentration range 1–4 mol/kg because the studies were performed in the context of applied problems including the use of NaBH4 as a source of hydrogen for fuel cells. The molal NaOH concentrations were varied from 0.05 to 8 mol/kg to encompass the ranges of both an increase and a decrease in the rate of hydrogen generation. Solutions of this composition are high-concentration electrolytes, which is an additional feature in the construction of kinetic schemes of their hydrolysis. Currently, not only are there no kinetic schemes for such processes that are formulated as mathematical relations, but also there are no qualitative explanations that interpret all the observed effects from a unified position [7, 14–16]. In this work, we discussed the possible schemes of sorption and kinetic processes for describing the catalytic hydrolysis of concentrated aqueous alkaline solutions of NaBH4.
To obtain an indirect confirmation of the role of sorption processes, experiments were carried out and the activation energies were found for the aqueous solution and the aqueous alkaline solution of NaBH4. The significant decrease in the activation energy in the aqueous alkaline solution is interpreted as the effect of the sorption properties of the catalyst. The activation energy of the heterogeneous reaction proper is impossible to determine without taking into account sorption processes.
The purpose of this work was to obtain experimental data on the catalytic hydrolysis of aqueous alkaline NaBH4 solutions, analyze the specifics of the behavior of the kinetic curves, and discuss the possible mechanisms of the catalytic hydrolysis with the catalysts of the studied type.
EXPERIMENTAL
The initial substance was granulated NaBH4 powder (98%, Sigma-Aldrich).
The cobalt catalyst was synthesized by chemical reduction and impregnated onto a micron TiO2 powder [17]. The cobalt content on the TiO2 surface was 3.4 wt %.
The experiments were performed in a maximum-tightness reactor, in which all the components and parts were made of stainless steel. The reactor volume was 182.5 cm3; the reactor diameter was 5 cm. The temperature of the solution in the reactor was measured by a sensor with a Pt100 platinum resistance temperature detector (Autonics Corporation, Korea). The pressure gauges were electronic sensors (Keller, Switzerland) for operating pressures of 2.5 and 10 bar. The pressure in the reactor was varied from atmospheric pressure to the maximum pressure, which was determined by the amount of NaBH4 in the solution. Based on the maximum value, a sensor with the corresponding measurement range was selected.
The experiments were carried out in a thermostat, in which a given temperature was set. The reactor with a dry NaBH4 powder and the catalyst was placed in the thermostat. After the reactor was heated to a given temperature, a required volume of distilled water or the alkaline solution was poured into it, and the reactor was sealed. The reaction mixture was not stirred because the solution was intensively stirred by rising hydrogen bubbles. From the moment of pouring water to the end of hydrolysis, two functions were measured, one of which is the pressure in the reactor, which is proportional to the amount of the released hydrogen, and the other is the temperature of the solution. Using these functions, the experimental values of the degree of decomposition ξ and the volume of the generated hydrogen were calculated. In all the experiments, 0.05 g of the catalyst was used.
Three series of experiments were conducted to study the effect of alkali on the rate of hydrogen generation were performed at the same temperature of 30°C, which simplified the primary analysis because, in this case, the kinetic characteristics are simply constants.
The components of the solution were characterized by the numbers of moles Ni, provided that the initial amount of water was always 1 kg; i.e., \(N_{{{\text{H}_{2}}\text{O}}}^{0}\)≡ 1000/18. The initial ternary solution was described by the numbers \(N_{{{\text{H}_{2}}\text{O}}}^{0},\) \(N_{{{\text{NaBH}_{4}}}}^{0},\) \(N_{\text{NaOH}}^{0}\); i.e., \(N_{{{\text{NaBH}_{4}}}}^{0}\) and \(N_{\text{NaOH}}^{0}\) are the molal concentrations of NaBH4 and NaOH, respectively, in the initial solution. As the hydrolysis proceeds, not only does the amount of various boron-containing ions change, but also the amount of water molecules does; i.e., the current numbers Ni will somewhat differ from the strictly defined molal concentrations.
RESULTS OF EXPERIMENTS IN AQUEOUS SOLUTIONS
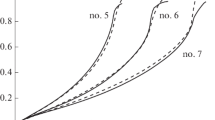
Figure 1 illustrates the dependences of the rate of hydrogen generation \({{Q}_{{{\text{H}_{2}}}}}\) in the experiments with the aqueous solutions on the degree of hydrolysis \(\xi \). Using the degree of hydrolysis instead of time makes it convenient to compare the results of different experiments in which the hydrolysis takes different times.
The range \(0 \leqslant \xi \leqslant 1\) in Fig. 1 can be divided into three subranges \(\xi < 0.2\), \(0.2 < \xi < 0.9\), and \(\xi > 0.9\). In the central subrange, most of the hydrolysis occurs, the rate of hydrogen generation is almost constant, and the observed deviations from the constant are most noticeable for the solution containing 4 moles of NaBH4, but they are also small. The drop in the rate of hydrogen generation late in the hydrolysis can be assigned to the transition of the process from kinetic to diffusion mode. Features of the kinetics of the hydrolysis of solutions of various concentrations are most pronounced early in the hydrolysis.
At the point \(\xi \)≈ 0, the measured rate of hydrogen generation \({{Q}_{{{\text{H}_{2}}}}}\) characterizes not only the kinetic features of the hydrolysis, but also the specifics of the methodology of our experiments because, at the initial moment, the NaBH4 powder dissolves and the circulation of catalyst particles in the bulk of the solution is established. Taking it into account, let us compare the measured \({{Q}_{{{\text{H}_{2}}}}}\) values (97, 350, and 1250 mL min–1 (g of catalyst)–1) with the ratios of the corresponding concentrations. The ratio of the concentrations is 4, and the ratio of the rates is ~3.6. This suggests that, in the aqueous solutions in the zeroth approximation \({{Q}_{{{\text{H}_{2}}}}}\left( 0 \right)\) ~ \(N_{{{\text{NaBH}_{4}}}}^{0}\).
The boundary of the transition from the initial subrange to the plateau \(\xi \) = 0.2–0.3 is interesting in that the three solutions at this point have different concentrations; i.e., the change in the mode of hydrogen generation is due not to the total concentration but to some finer characteristic of the solution. The \(\xi \) value close to 0.25 suggests that the transition point may be related to the almost complete hydrolysis of the \(\text{BH}_{4}^{ - }\) ion. The NaBH4 hydrolysis scheme is a sequence of four irreversible reactions [18, 19]:
The end product of the hydrolysis is a \(\text{NaB}{{\left(\text{OH} \right)}_{4}}\) solution.
It is widely hypothesized that the slowest step in the homogeneous process is the first step \({\text{BH}}_{4}^{ - }\xrightarrow{{ \uparrow {{{\text{H}}}_{{\text{2}}}}}}{\text{B}}{{{\text{H}}}_{{\text{3}}}}{\text{O}}{{{\text{H}}}^{ - }}\). It was reported [18] that the rate of the third step is approximately twice as high as the rate of the hydrolysis of the main ion, and the rates of the second and fourth steps are approximately 1000 times higher than the rate of the first step. In most of the proposed kinetic models, steps 2–4 are considered fast, i.e., instantaneous. In this approximation, the homogeneous process after the initial period of establishing a quasi-stationary distribution of components of the solution is accompanied by a self-consistent monotonic decrease in the number of four boron-containing ions:\({{N}_{{\text{BH}_{4}^{ - }}}}\), \({{N}_{{{\text{BH}_{3}}\text{OH}_{{}}^{ - }}}}\), \({{N}_{{{\text{BH}_{2}}\left(\text{OH} \right)_{2}^{ - }}}}\), and \({{N}_{{\text{BH}\left(\text{OH} \right)_{3}^{ - }}}}\).
It can be assumed that the rate ratio that is characteristic of homogeneous hydrolysis changes in the case of heterogeneous reactions on the considered Co/TiO2 catalyst. The slowest heterogeneous step can be, e.g., the third one: \({\text{B}}{{{\text{H}}}_{{\text{2}}}}\left( {{\text{OH}}} \right)_{2}^{ - }\xrightarrow{{ \uparrow {{{\text{H}}}_{2}}}}{\text{BH}}\left( {{\text{OH}}} \right)_{3}^{ - }\). In this case, until the moment of the almost complete hydrolysis of the \(\text{BH}_{4}^{ - }\) ion, the rate of hydrogen generation decreases and then stabilizes at a value determined by the slowest step.
RESULTS OF EXPERIMENTS IN AQUEOUS ALKALINE SOLUTIONS
Figure 2 presents the dependences of the rate of hydrogen generation \({{Q}_{{{\text{H}_{2}}}}}\) in the experiments with the aqueous alkaline solutions on the degree of hydrolysis \(\xi \). The numbers at the curves are the molal concentrations \(N_{\text{NaOH}}^{0}\).
Rate of hydrogen generation at 30°C in the presence of 0.05 g Co/TiO2 in the aqueous alkaline solutions with molal NaBH4 concentrations of (a) 0.25 and (b) 1.0 mol/kg. The numbers at the curves indicate the molal NaOH concentration. The dotted lines show the curves from Fig. 1 (\({{N}_{\text{NaOH}}}\)= 0).
The rate of hydrogen generation \({{Q}_{{{\text{H}_{2}}}}}\) is seen to depend nonmonotonically on both \(N_{\text{NaOH}}^{0}\) and \(\xi \). \({{Q}_{{{\text{H}_{2}}}}}\) is maximum at \(N_{\text{NaOH}}^{0}\) ≈ 1–2 mol. The \(\xi \) range in Fig. 2 can be divided into three subranges \(\xi < 0.1\), \(0.1 < \xi < 0.8\), and \(\xi > 0.8\). The behavior of the kinetic curves in the early hydrolysis subrange in Fig. 2 is fundamentally different from the behavior of the curves in Fig. 1 (increase instead of decrease). This may be explained by features of the sorption–desorption processes in highly alkaline solutions, but a physical reason can also be assumed. Within the framework of this hypothesis, the observed increase in the rate of hydrogen generation in the subrange \(\xi < 0.1\) is more tightly related to the dissolution processes and the formation of the initial circulation in the solution. The fact is that, with an increase in the alkali concentration, the viscosity of the solution increases, as a result of which both dissolution and convection slow down.
In the subrange \(0.1 < \xi < 0.8\), there is a common trend in the behavior of the functions \({{Q}_{{{\text{H}_{2}}}}}\left( \xi \right)\) for the solutions with \(N_{\text{NaOH}}^{0}\) > 2, namely, the decrease in the hydrogen generation rate with increasing \(\xi \). Conversely, for the solutions with \(N_{\text{NaOH}}^{0}\) < 1, the functions \({{Q}_{{{\text{H}_{2}}}}}\left( \xi \right)\) either increase or are near-constant. With regard to the behavior of the functions \({{Q}_{{{\text{H}_{2}}}}}\left( \xi \right)\) in the subrange \(\xi > 0.8\), we still tend to explain it by the transition of the hydrolysis to diffusion mode.
The experimentally determined features of the hydrolysis of the aqueous and aqueous alkaline solutions of NaBH4 can be used for constructing kinetic schemes of the process on the studied catalyst.
DISCUSSION OF POSSIBLE SCHEMES OF KINETIC PROCESSES
The acceleration of catalytic hydrolysis on cobalt catalysts by the addition of alkali is an unexpected fact because homogeneous hydrolysis is always slowed down by the addition of alkali. The introduction of NaOH into an aqueous solution of NaBH4 does not change the list of solution components, but increases the number of \({\text{Na}^{ + }}\) and \({\text{OH}^{ - }}\) ions. In aqueous NaBH4 solutions, the concentration of \({\text{OH}^{ - }}\) ions is established self-consistently with the chemical processes of hydrolysis and changes with changing \(\xi \). In aqueous alkaline solutions in the considered \(N_{\text{NaOH}}^{0}\) range (0.05–8 mol), the number of \(N_{\text{NaOH}}^{0}\) ions is almost equal to the number of initial moles of NaOH, which affects the equilibrium of reversible reactions in solution.
The main homogeneous reversible reaction in NaBH4 solutions is the formation and decomposition of metastable neutral complex \(\left[ {\text{BH}_{4}^{ - } \cdot {\text{H}^{ + }}} \right]\). The direct reaction can be written in two ways:
Similarly, reverse processes can occur via two routes:
Further, the notation \(\left[ \text{Comp} \right] \equiv \left[ {\text{BH}_{4}^{ - } \cdot {\text{H}^{ + }}} \right]\) is used. When reactions (II)–(V) are in equilibrium, the number of molecules of the complex \({{N}_{{\left[ \text{Comp} \right]}}}\) is proportional to the number of ions \({{N}_{{\text{BH}_{4}^{ - }}}}\), and the functional dependence on the number of ions \({{N}_{{{\text{OH}^{ - }}}}}\) is determined by the relation between the rates of reactions (II)–(V). Three cases can be distinguished:
– if reactions (III) and (IV) dominate, \({{N}_{{\left[ \text{Comp} \right]}}}\) is independent of \({{N}_{{{\text{OH}^{ - }}}}}\);
– if reactions (II), (IV), or (III), (V) dominate, \({{N}_{{\left[ \text{Comp} \right]}}}\) ~ \({{N}_{{\text{H}_{2}^{{}}\text{O}}}}{\text{/}}{{N}_{{{\text{OH}^{ - }}}}}\);
– if reactions (II) and (V) dominate, \({{N}_{{\left[ \text{Comp} \right]}}}\) ~ \({{\left( {{{N}_{{\text{H}_{2}^{{}}\text{O}}}}{\text{/}}{{N}_{{{\text{OH}^{ - }}}}}} \right)}^{2}}\).
During the hydrolysis of the aqueous solutions of NaBH4, the number of ions \({{N}_{{{\text{OH}^{ - }}}}}\) is established self-consistently with the number of complex \(\left[ \text{Comp} \right]\) molecules and molecules of boric acid, which is formed by the dissociation reaction:
At the beginning of the hydrolysis at \(\xi \) ≈ 0, there is no boric acid, and the number of ions \({{N}_{{{\text{OH}^{ - }}}}}\) is approximately equal to the number of complex molecules \({{N}_{{\left[ \text{Comp} \right]}}}\). Correspondingly, there are three asymptotics: \({{N}_{{{\text{OH}^{ - }}}}}\) ≈ \({{N}_{{\left[ \text{Comp} \right]}}}\) ~ \(N_{{{\text{NaBH}_{4}}}}^{0}\), \({{N}_{{{\text{OH}^{ - }}}}}\) ≈ \({{N}_{{\left[ \text{Comp} \right]}}}\) ~ \(\sqrt {N_{{{\text{NaBH}_{4}}}}^{0}} \), and \({{N}_{{{\text{OH}^{ - }}}}}\) ≈ \({{N}_{{\left[ \text{Comp} \right]}}}\) ~ \(\sqrt[3]{{N_{{{\text{NaBH}_{4}}}}^{0}}}\). In the experiments in Fig. 1, the rate of hydrogen generation at \(\xi \) ≈ 0 increased by a factor of 3.6 with increasing \(N_{{{\text{NaBH}_{4}}}}^{0}\) by a factor of 4. This fact gives rise to the hypothesis that \({{N}_{{\left[ \text{Comp} \right]}}}\) should be calculated from Eqs. (III)–(V), with process (IV) being more intense than process (V). On the other hand, the above-discussed specifics of the experimental procedure used can strongly affect the \({{Q}_{{{\text{H}_{2}}}}}\left( 0 \right)\) values, i.e., “mask” the actual asymptotics. Additional information on the behavior of \({{N}_{{\left[ \text{Comp} \right]}}}\) during the hydrolysis of the aqueous solutions can be tried to be obtained by developing a procedure for measuring the pH of these solutions during hydrolysis.
In the aqueous alkaline solutions, \({{N}_{{{\text{OH}^{ - }}}}} \approx N_{\text{NaOH}}^{0},\) and this means a decrease in \({{N}_{{\left[ \text{Comp} \right]}}}\) with increasing \(N_{\text{NaOH}}^{0}\), except in the case of the domination of reactions (III) and (IV), where \({{N}_{{\left[ \text{Comp} \right]}}}\) is independent of \(N_{\text{NaOH}}^{0}\). If the number of complex molecules in the solution decreases, then their role in the sorption–desorption processes decreases.
The heterogeneous reactions occur on neutral particles of the solution that are adsorbed on the catalyst. Particles of different sorts can compete with each other for adsorption sites. An increase in the initial concentration \(N_{{{\text{NaBH}_{4}}}}^{0}\) and/or \(N_{\text{NaOH}}^{0}\) leads to a change in the concentrations of components of the solution and, correspondingly, in the fractions of adsorbed particles of different sorts. In the aqueous alkaline solutions of NaBH4, neutral particles are water and boric acid molecules, as well as complex \(\left[ \text{Comp} \right]\) molecules. In the course of the hydrolysis, with increasing \(\xi \), the number of complex molecules \({{N}_{{\left[ \text{Comp} \right]}}}\) decreases, and the number of boric acid molecules \({{N}_{{\text{B}{{{\left( \text{OH} \right)}}_{3}}}}}\) increases. The number of water molecules also slightly decreases. Although, in the solution, we have \({{N}_{{\left[ \text{Comp} \right]}}}\) \( \ll \) \({{N}_{{\text{BH}_{4}^{ - }}}}\) \( \ll \) \(N_{{{\text{H}_{2}}\text{O}}}^{0},\) the molecules of the complex in the adsorbed state, \({{\left[ \text{Comp} \right]}^{\text{S}}}\), can be sufficiently stable and active to affect the hydrolysis.
For the irreversible heterogeneous reactions, several variants of reactions on adsorbed water \({\text{H}_{2}}{\text{O}^{\text{S}}}\) molecules and adsorbed complex \({{\left[ \text{Comp} \right]}^{\text{S}}}\) molecules can be proposed, but there are no variants of reactions involving \(\text{B}\left(\text{OH} \right)_{3}^{\text{S}}\) molecules, which can affect the hydrolysis in a passive way, namely, by occupying adsorption sites of the catalyst. To describe the sorption–desorption processes, let us introduce the following notation: \({{\varepsilon }_{\text{empt}}}\) is the fraction of the free surface, \({{\varepsilon }_{{{\text{H}_{2}}\text{O}}}}\) is the fraction of the surface that is occupied by \({\text{H}_{2}}{\text{O}^{\text{S}}}\) molecules, \({{\varepsilon }_{{\text{B}{{{\left( \text{OH} \right)}}_{3}}}}}\) is the fraction of the surface that is occupied by \(\text{B}\left(\text{OH} \right)_{3}^{\text{S}}\) molecules, and \({{\varepsilon }_{{\left[ \text{Comp} \right]}}}\) is the fraction of the surface that is occupied by complex \({{\left[ \text{Comp} \right]}^{\text{S}}}\) molecules.
If the slowest reaction in chain (I) is the first reaction, it is sufficient to consider only heterogeneous processes involving the \({\text{BH}}_{4}^{ - }\) ion (the other three reactions are fast). Irreversible reactions on \({\text{H}_{2}}{\text{O}^{\text{S}}}\) and \({{\left[ \text{Comp} \right]}^{\text{S}}}\) can be written as
In such a model, both \({\text{BH}}_{4}^{ - }\) ions and complex \(\left[ \text{Comp} \right]\) molecules exist throughout the hydrolysis, and their concentrations asymptotically tend to zero by its end. The rate of reactions (VII) is proportional to the amount \({{\varepsilon }_{{{\text{H}_{2}}\text{O}}}}\) and the flux of \({\text{BH}}_{4}^{ - }\) ions or complex \(\left[ \text{Comp} \right]\) molecules from the solution to the catalyst. As long as the rate flux \({{R}_{{{\text{H}_{2}}\text{O}}}}\) of the heterogeneous reaction is lower than the diffusion flux of particles initiating the reaction (\({\text{BH}}_{4}^{ - }\) or \(\left[ \text{Comp} \right]\)), the reaction is under kinetic mode: \({{Q}_{{{\text{H}_{2}}}}}\) ~ \({{\varepsilon }_{{{\text{H}_{2}}\text{O}}}}{{R}_{{{\text{H}_{2}}\text{O}}}}\). If the ratio of the rates is inverse, then the process is under diffusion mode from the very beginning: \({{Q}_{{{\text{H}_{2}}}}}\) ~ \({{\varepsilon }_{{{\text{H}_{2}}\text{O}}}}{{R}_{{{\text{H}_{2}}\text{O}}}}{{N}_{{{\text{BH}}_{4}^{ - }}}}\). In any case, the hydrolysis by reaction (VII) is completed under diffusion mode. The rate \({{R}_{{{\text{H}_{2}}\text{O}}}}\) of the heterogeneous reaction is a function of temperature only, and in the isothermal process it is a constant. The sorption characteristic \({{\varepsilon }_{{{\text{H}_{2}}\text{O}}}}\) depends not only on temperature, but also on the ratio of the sorbed components of the solution, i.e., on the initial composition of the solution and on the degree of hydrolysis. Note also that the temperature dependences of \({{R}_{{{\text{H}_{2}}\text{O}}}}\) and \({{\varepsilon }_{{{\text{H}_{2}}\text{O}}}}\) are opposite: with an increase in temperature, the rate increases, whereas the adsorption decreases, i.e., the effect of the characteristic \({{\varepsilon }_{{{\text{H}_{2}}\text{O}}}}\) manifests itself in a decrease in the activation energy determined from the rate of hydrogen generation.
In the case of reactions (VIII), the numbers of \({\text{OH}^{ - }}\) ions and \({\text{H}_{2}}\text{O}\) molecules change slightly during the hydrolysis, and their concentrations do not tend to zero, whereas \({{\varepsilon }_{{\left[ \text{Comp} \right]}}}\) decreases, tending to zero as the degree of hydrolysis increases. This means that the hydrogen generation via this route is most likely to occur under kinetic mode (\({{Q}_{{{\text{H}_{2}}}}}\) ~ \({{\varepsilon }_{{\left[ \text{Comp} \right]}}}{{R}_{{\left[ \text{Comp} \right]}}}\)), but at a rate that continuously decreases in proportion to the decrease in \({{\varepsilon }_{{\left[ \text{Comp} \right]}}}\), which looks like a first-order reaction.
If one assumes that, in a certain range of degrees of hydrolysis, the rate of the third reaction in chain (I) is lower than that of the first one, then the kinetic scheme becomes more complicated, and it is necessary to model the evolution of both the \({\text{BH}}_{4}^{ - }\) ion and the \({\text{B}}{{{\text{H}}}_{2}}\left( {{\text{OH}}} \right)_{2}^{ - }\) ion. The \({\text{B}}{{{\text{H}}}_{3}}{\text{O}}{{{\text{H}}}^{ - }}\) and \({\text{BH}}\left( {{\text{OH}}} \right)_{3}^{ - }\) ions can still be considered short-lived. The most probable route of hydrolysis for the \({\text{B}}{{{\text{H}}}_{2}}\left( {{\text{OH}}} \right)_{2}^{ - }\) ion is reaction (VII); i.e., the process on adsorbed water molecules. The number of unknown kinetic constants increases, and accordingly so does the difficulty of tuning the model of this sort. In any case, to model the catalytic hydrolysis, it is necessary to describe the sorption–desorption processes; they can be written as
where \({\text{M}_{{{\text{H}_{2}}\text{O}}}}\), \({\text{M}_{{\left[ \text{Comp} \right]}}}\), and \({\text{M}_{{\text{B}{{{\left( \text{OH} \right)}}_{3}}}}}\) are particles that initiate the desorption of adsorbed particles of \({\text{H}_{2}}{\text{O}^{\text{S}}}\), \({{\left[ \text{Comp} \right]}^{\text{S}}}\), and \({\text{B}}\left( {{\text{OH}}} \right)_{{\text{3}}}^{{\text{S}}}\), respectively.
The desorption occurs by collisions of adsorbed particles with particles of the solution, and the probability of desorption depends on the type of particles. The main particles are water molecules. The role of boron-containing ions as initiators of desorption is assumed to be insignificant. In electrolytes, according to their role in hydration of ions, water molecules are classified into “free” ions (\({\text{H}_{2}}{\text{O}^{\text{F}}}\)) and ions associated with \({\text{Na}^{ + }}\) and \({\text{OH}^{ - }}\) (let them be denoted as \({\text{H}_{2}}{\text{O}^{\text{I}}}\)). This classification of water molecules is a way of taking into account the specifics of high-concentration electrolytes without the help of a model of “activities.” We used a similar approach previously [20] to calculate the pH of sodium metaborate solutions. The number of \({\text{H}_{2}}{\text{O}^{\text{I}}}\) molecules depends on the number of \({\text{Na}^{ + }}\) and \({\text{OH}^{ - }}\)ions. To simplify the model, the same hydration number \(k\) can be used:
where Min is the function that selects the minimum value of the two values given in square brackets.
In expressions (IX), particles of initiators of desorption are denoted as \({\text{M}_{{{\text{H}_{2}}\text{O}}}}\), \({\text{M}_{{\left[ \text{Comp} \right]}}}\), and \({\text{M}_{{\text{B}{{{\left( \text{OH} \right)}}_{3}}}}}\), which can be individual combinations of \({\text{H}_{2}}{\text{O}^{\text{F}}}\) and \({\text{H}_{2}}{\text{O}^{\text{I}}}\). The simplest variant is to take the same expression for all \({\text{M}_{i}}\):
where the coefficient \(\beta \) is the ratio of the rates of desorption of \({\text{H}_{2}}{\text{O}^{\text{I}}}\) and \({\text{H}_{2}}{\text{O}^{\text{F}}}\) molecules. At \(\beta > 1\), the efficiency of the initiation of desorption for \({\text{H}_{2}}{\text{O}^{\text{I}}}\) molecules is higher than that for \({\text{H}_{2}}{\text{O}^{\text{F}}}\), and in this case, \({{N}_{\text{M}}}_{{_{i}}} > 0\) for any variants of aqueous alkaline solutions and hydration numbers \(k\). At \(\beta < 1\), depending on the values of the parameters \(k\) and \(\beta \), there may be a situation where \({{N}_{{{\text{M}_{i}}}}}\) = 0. For situations with such values of the parameters \(k\) and \(\beta \), the desorption model requires refinement. In the considered case of the aqueous alkaline solutions, the values \(\beta > 1\) are expected.
Equations (1) and (IX) of sorption processes represent a general case that describes all possible combinations of the intensities of these processes. At the same time, there are two limiting variants, in which the number of unknown constants is smaller. The simplest variant is the situation of a relatively low occupation of the catalyst surface with active particles:
In this case, the particles do not compete with each other. The opposite case is maximum competition, where there are almost no free sites:
The above principles for constructing a catalytic hydrolysis model reflect our approach to this problem.
Heterogeneous reactions occur on the surface of a catalyst, or rather, on particles of a certain type that are adsorbed from the solution. On active sites of the catalyst, various particles can be adsorbed; i.e., there is a competition between particles initiating reactions and “inert” particles. A generally accepted practice of generalizing the data of a series of isothermal experiments is to determine the activation energy Ea for the Arrhenius model of the rate of a chemical reaction: \(R = A\exp \left( { - {{E}_{\text{a}}}{\text{/}}{{R}_{\text{g}}}{\text{/}}T} \right)\), where Rg is the universal gas constant. In homogeneous processes, the parameter Ea characterizes the energy barrier to the reaction. In catalytic heterogeneous reactions, the formal determination of the activation energy from the product yield does not take into account the fact that the product yield rate \(R\) is the product of the area of the surface occupied by “active” particles \(({{\varepsilon }_{{\text{a}}}})\) by the probability of the chemical reaction proper: \(R = \varepsilon \exp \left( { - {{E}_{\text{a}}}{\text{/}}{{R}_{\text{g}}}{\text{/}}T} \right)\). Whereas the rate of a heterogeneous reaction depends only on temperature, \(\varepsilon \) is a function not only of temperature, but also of the composition of the solution (concentrations of components).
Conventional processing of kinetic data to determine the activation energy assumes that R is a function of temperature only. It is clear that such an activation energy implicitly depends on the sorption properties of the surface and the concentration of the solution.
This means that, using a gross approach, i.e., without separating the sorption and reaction characteristics, the activation energy is different for different concentrations \(N_{{{\text{NaBH}_{4}}}}^{0}\) and \(N_{\text{NaOH}}^{0}\).
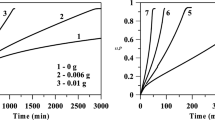
To demonstrate this fact, experiments were made in the temperature range 293–333 K in aqueous and aqueous alkaline solutions of NaBH4 with a molar concentration of 1.0 mol/kg. The molal alkali concentration is 1.0 mol/kg. The activation energy was determined using linear approximations of the kinetic curves in the range \(\xi \) = 0.1–0.9 (Fig. 3). The calculated rates of hydrogen generation were used to obtain approximated Arrhenius coefficients (Fig. 4). The activation energies for the aqueous and aqueous alkaline solutions were 64.3 and 53.6 kJ/mol, respectively. As expected, there is a dependence of the activation energy on the composition of the solution.
CONCLUSIONS
The studied rate of hydrogen generation depends nonmonotonically on both the alkali concentration and the degree of decomposition of NaBH4. The rate of hydrogen generation is maximum at \(N_{\text{NaOH}}^{0}\) ≈ 1–2 mol, which is consistent with the existing estimates of this value for a number of cobalt-based catalysts [10, 21].
A scheme of sorption and kinetic processes was presented for describing the catalytic hydrolysis of concentrated aqueous alkaline solutions of NaBH4.
The determined activation energy values, which were found from the rate of hydrogen generation, are gross characteristics, since they do not distinguish between the rate of the heterogeneous reaction and the sorption properties of the catalyst surface. The degree of occupation of the catalyst surface with active particles depends not only on temperature, but also on the composition of the solution. As a result, in the gross Arrhenius approximation, both coefficients turn out to be functions of the solution concentration. The activation energy of the heterogeneous reaction proper is impossible to determine without taking into account sorption processes.
REFERENCES
Li, Q. and Hern Kim, H., Fuel Process. Technol., 2012, vol. 100, p. 43.
Chou, C.C., Hsieh, C.H., and Chen, B.H., Energy, 2015, vol. 90, no. 2, p. 1973.
Metin, O. and Ozkar, S., Energy Fuels, 2009, vol. 23, p. 3517.
Ingersoll, J.C., Mani, N., Thenmozhiyal, J.C., and Muthaiah, A., J. Power Sources, 2007, vol. 173, no. 1, p. 450.
Li, Q. and Kim, H., Fuel Process. Technol., 2012, vol. 100, p. 43.
Hansu, T.A., Caglar, A., Sahin, O., and Kivrak, H., Mater. Chem. Phys., 2020, vol. 239, p. 122031.
Ekinci, A., Horoz, S., Baytar, O., and Şahin, Ö., J. Optoelectron. Biomed. Mater., 2020, vol. 12, no. 2, p.25.
Didehban, A., Zabihi, M., and Shahrouzi, J.R., Int. J. Hydrogen Energy, 2018, vol. 43, no. 45, p. 20645.
Xu, J., Du, X., Wei, Q., and Huang, Y., ChemistrySelect, 2020, vol. 5, p. 6683.
Wang, L., Li, Z., Zhang, Y., Zhang, T., and Xie, G., J. Alloys Compd., 2017, vol. 702, p. 649.
Shang, Y., Chen, R., and Jiang, G., Int. J. Hydrogen Energy, 2008, vol. 33, no. 22, p. 6719.
Huang, Y.-H., Su, C.-C., Wang, S.-C., and Lu, M.-C., Energy, 2012, vol. 46, p. 242.
Demirci, U.B. and Garin, F., J. Alloys Compd., 2008, vol. 463, p. 107.
Zhang, Q., Wu, Y., Sun, X., and Ortega, J., Ind. Eng. Chem. Res., 2007, vol. 46, p. 1120.
Shen, X., Wang, Q., Wu, Q., Guo, S., Zhang, Z., Sun, Z., Liu, B., Wang, Z., Zhao, B., and Ding, W., Energy, 2015, vol. 90, no. 1, p. 464.
Xie, L., Wang, K., Du, G., Asiri, A.M., and Sun, X., Int. J. Hydrogen Energy, 2017, vol. 42, no. 2, p. 30639.
Shabunya, S.I., Minkina, V.G., Kalinin, V.I., Sankir, N.D., and Altaf, S.T., Kinet. Catal., 2021, vol. 62, no. 3, p. 350.
Mochalov, K.N. and Khain, V.S., Kinet. Katal., 1965, vol. 6, no. 4, p. 541.
Kreevoy, M.M. and Hutchins, J.E.C., J. Am. Chem. Soc., 1972, vol. 94, p. 6371.
Shabunya, S.I., Minkina, V.G., Martynenko, V.V., and Kalinin, V.I., Russ. Chem. Bull., 2019, no. 6, p. 1183.
Wei Y., Wang R., Meng L., Wang Y., Li G., Xin S., Zhao X., Zhang K., Int. J. Hydrogen Energy, 2017, vol. 42, no. 15, p. 9945.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Glyanchenko
Abbreviations and notation: ξ, degree of decomposition of NaBH4; R, rate constant, mol/s/m2; Ni, number of moles of the ith component; \({{Q}_{{{\text{H}_{2}}}}}\), rate of hydrogen generation, mL/min/g catalyst; \({{\varepsilon }_{\text{empt}}}\), fraction of the free surface; \({{\varepsilon }_{i}}\), fraction of the surface that is occupied by particles of type i; \({\text{M}_{i}}\), particles of type i, initiating the desorption of adsorbed particles; Ea, activation energy, J/mol; Rg, universal gas constant, J/mol/K; and \(k\), hydration number.
Rights and permissions
About this article
Cite this article
Shabunya, S.I., Minkina, V.G. & Kalinin, V.I. Features of Hydrolysis of Concentrated Aqueous Alkaline Solutions of NaBH4 on Co/TiO2 Catalyst. Kinet Catal 63, 585–592 (2022). https://doi.org/10.1134/S002315842205010X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002315842205010X