Abstract
History of the development and use of homogeneous metal complex catalysts in the chemistry of acetylene and its derivatives is considered. Achievements of the catalytic chemistry of alkynes in the creation of affective catalytic systems (based on Groups 3—11 and 13 of the Periodic Table) for alkyne dimerization, capable of producing desired dimeric isomers possessing high chemo-, stereo- and regioselectivity are analyzed. The nature of elementary stages in four mechanisms of dimerization and the relations of the nature of organometallic intermediates to the nature of catalyst (metal and ligand), substrate, and type of mechanism, and, hence, to the selectivity of dimerization process are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
CONTENTS
1. INTRODUCTION
2. BRIEF HISTORY OF DEVELOPMENT OF THE CATALYTIC CHEMISTRY OF ACETYLENE AND ITS DERIVARTIVES IN SOLUTIONS OF METAL COMPLEX CATALYSTS
3. DIMERIZATION OF ALKYNES IN SOLUTIONS OF METAL COMPLEX CATALYSTS
3.1. Complexes of Metals of Group 11 of the Periodic Table
3.2. Complexes of Ru, Os and Fe
3.3. Complexes of Rh, Ir and Co
3.4. Complexes of Ni, Pd and Pt
3.5. Complexes of Metals of Groups 4—7 of the Periodic Table
3.6. Complexes of Metals of Groups 3 and 13 of the Periodic Table.
4. CONCLUSION
1. INTRODUCTION
In 1991, monograph [1] summarized outcomes of the development of catalytic chemistry of acetylene and its derivatives with participation of homogeneous metal complex based catalysts and heterogeneous catalysts. Achievements of the acid-base and nucleophilic catalysis were generalized in monographs of Tedesсhi [2] and Trofimov [3] (see also some more recent reviews [4—6]), while some problems of “organocatalysis” were recently addressed in [7]. The kinetic aspects and mechanisms of old classical processes and new alkyne reactions discovered in the past quarter century were considered in monograph [8].
During the past 25—30 years, new catalysts, new reactions, new types of intermediates, and new mechanisms of alkyne reaction catalysis have appeared. This development of modern alkyne chemistry was described in numerous monographs [8—21] and reviews [22—45]. The most popular catalysts in modern homogeneous catalysis are based on complexes of copper, nickel, gold, ruthenium, palladium, rhodium, platinum, titanium and zirconium. In particular, the scale of research works in the field of gold complex catalysis of fine chemical synthesis of carbocycles, heterocycles, and various biologically active compounds is very impressive [9, 11, 13, 15, 18, 19, 22, 36, 44].
Increasing interest in the chemistry of alkynes, related to the appearance of more active catalysis of chemo- and stereo-selective reactions proceeding under softer compounds, is clear evidence for the revival and “golden age” of the development of acetylene chemistry. At the same time, one can hardly agree with the statement of B.M. Trost and Ch.-J. Li, editors of monograph [18], that modern chemistry of alkynes as formed in the past several decades “provides an alternative to classical stoichiometric alkyne chemistry,” Indeed, it should be recalled, first, that the classical chemistry of acetylene and its derivatives as established by works of M.G. Kucherov (1881), A.E. Favorsky, J. Nieuwland, and W. Reppe was to a considerable degree catalytic rather than purely stoichiometric and involved w the use of metal complexes in solution and on surfaces, metals, metal oxides, acid-base and nucleophilic catalysts [1–3, 9, 46–52]. Until the end of 1950s, homogeneous catalysis with metal complexes was developing mostly on the basis of acetylene chemistry. Second, note that even monographs devoted mainly to thermal stoichiometric reactions of acetylene compounds and their organometallic derivatives also considered many reactions catalyzed by Brønsted and Lewis acids and bases and metal salts [48–50, 53–55].
For the past three decades, the chemistry of acetylene transformed into the chemistry of complex acetylene compounds such as enynes, dialkynes, alkynols, aryl- and heterylacetylenes, and other derivatives, but quite impressive results were also obtained for acetylene as such—even in the form of СаС2 [45]. Considerable development took place in the chemistry of pharmaceutical chiral preparations and materials possessing special optical, electrical, thermochemical, and mechanochemical properties based on acetylene substrates. New reactions, hypotheses, and paradigms found due to progress in the chemistry of acetylenes are undoubtedly worth of consideration and generalization, together with the results of successful development and verification of “old” hypotheses concerning the mechanisms of catalytic reactions. The present review deals with issues related to the use of metal complex catalysts in the chemistry of acetylene compounds, including necessary supplementary information concerning surface-supported metal complexes and “microheterogeneous” catalytic systems.
2. BRIEF HISTORY OF DEVELOPMENT OF THE CATALYTIC CHEMISTRY OF ACETYLENE AND ITS DERIVARTIVES IN SOLUTIONS OF METAL COMPLEX CATALYSTS
It is interesting to follow the history of using various metals as metal complex catalysts in the chemistry of acetylene and its derivatives and their role in the discovery of new alkyne reactions. It is convenient to subdivide this analysis into four stages:
First stage: until beginning of 1950s.
Second stage: 1950–1970.
Third stage: 1970–1990.
Fourth stage: 1991–2018.
In 1968, monograph [52] attempted to follow the appearance of various metals in the catalytic chemistry of acetylene compounds at the first stage (20th century until 1950s) beginning with the first Kucherov’s work on the acetylene hydration (1881) and then up to about 1967. The works of M.G. Kucherov, A.E. Favorsky, J. Nieuwland, W. Reppe, W. Carothers, A.L. Klebansky, Yu.S. Zalkind and many other researchers in the first half of 20th century laid foundations of the catalytic chemistry of acetylene.
In 1925—1945, a group of chemists headed by W. Reppe in the main laboratory of IG Farben (Ludwigshafen) developed the chemistry of and technology of the four types of catalytic reactions of acetylene and its derivatives with participation of base and metal complex catalysts. These processes included ethynylation, vinylation, carbonylation, and cyclization of acetylene to benzene and cyclooctatetraene. Results of investgations in this laboratory only became widely known after the World War II due to reports of the US Technical Mission [56], Reppe’s report [47], and monograph [57] generalizing the results on acetylene carbonylation. Review [56] expressed the general opinion of participants of the 110th Meeting of ACS the outstanding results obtained by Reppe’s research group would be exceptionally important for the development of organic chemistry in the United States (see also [39]).
In a lecture made at the Moscow State Institute of Fine Chemical Technology (MIFCT) in the beginning of 1960s, academician I.L. Knunyants recalled his work with a commission for reparations in post-war Germany. Once he saw a tank marked as “Cyclooctatetraen” on railways of a chemical plant and decided that it was some hidden secret product, although he knew about this compound that was synthesized in 1911 by the Nobel Laureate R.M. Willstätter via a highly complicated 9-stage synthesis from alkaloid pseudopelletierin (isolated from pomegranate bark). What a surprise it was on finding that the tank it fact contained cyclooctatetraene produced via single-stage industrial catalytic synthesis. The history of cyclooctatetraene synthesis discovery and captive Reppe’s report [47] about his research results was also described in the lectures on organic chemistry by Yu.I. Ustynyuk [58].
In the first half of 20th century, various homogeneous conversions of acetylene were mostly catalyzed, besides acids and bases, by compounds of non-transition and post-transition metals including Hg(II), Zn(II), Cd(II), Bi(III), Sb(V), Cu(I), Ag(I), Fe(III, II), and Cr(III). Gold compounds were used to catalyze the ethynylation of carbonyl compounds (with Au2C2/SiO2 [59]) and the stoichiometric oxidation of acetylene to glyoxal (with AuCl3 [60]), while Pt(II) complexes were used only once for the catalysis of acetylene oxidation by nitric acid to oxalic acid [52].
The conversions of acetylene compounds in heterogeneous catalysis were performed using a more diverse set of catalysts including metals and alloys (Pt, Pd, Ni, Co, Fe, Cu), metal oxides (Zn, Cd, Sn, Ni, Co, Mo, Ag, V, Ti, Th) and sulfides (Zn, Cd, Mo, Th), аs well as supported salts of Co, Ni, and Ce.
Organometallic compounds of Hg(II), Cu(I), and Sb(V) formed from acetylene were the first products considered as intermediates of catalytic reactions. For example, acetylene chlorination to tetrachloroethane in SbCl5 solutions via intermediate compound SbCl4(CH=CHCl) (Nesmeyanov’s “quasi-complex“ compound) was treated as a rare case of a catalytic reaction with “clear mechanism” [61]. Complexes of Ni(0) and Ni(II) were introduced by Reppe into practical homogeneous catalysis for the synthesis of acrylic acid and its derivatives in reactions of acetylene cyclotrimerization and cyclotetramerization. In 1952, Reppe [52] reported on the synthesis of 5-ethylpyridine from acetylene and ammonia in solutions of Ni(II) and Co(II) complexes.
At the second stage (1950–1970) extensive development of the catalysis with metal complexes in general and particularly in the acetylene chemistry was accompanied by the development of coordination and organometallic chemistry. Complexes of all metals of Groups 8—10 and some metals of Groups 4—7 of the Periodic Table were introduced into practical catalysis (see Table 1) [52].
New catalysts appeared in many of the well-known reactions (hydration, hydrochlorination, isomerization, carbonylation, cyclization, etc.). First active non-mercury catalytic systems have been discovered in 1950s—1960s for the hydration of acetylene and alkynes, including CuCl–ZnCl2–H2O system (comparable with mercury catalyst in efficiency for the synthesis of acetaldehyde, but much more stable) [52, 62, 63] and RuCl3 catalyst [64]. Metal comlex catalyst compositions were modified to include new ligands such as PR3, AsR3, SbR3, \({\text{SnCl}}_{3}^{ - }\), and Py. The discovery of the oxidation of olefins in solutions of palladium complexes [65] (so-called Wacker process [66]) drawn much attention to various conversions of acetylene catalyzed by palladium complexes [52]. It was found that acetylene carbonylation in alcohol solutions of Pd(II) complexes yielded a mixture of products of the oxidative, reductive, and additive dicarbonylation (see, e.g., [67]), while the Pd(PPh3)4–CHCl3–n-BuOH system under conditions of ClPd(CHCl2)(PPh3)2 complex formation led to the selective synthesis of butyl acrylate [68—70]. Analogous processes were found to occur in solutions of Co(0) and Rh(III) complexes [52]. After the discovery of Zigler—Natta catalysts of olefin polymerization, they were successfully used not only for the polymerization of acetylene and alkynes, but also in the reactions of hydration, dimerization, and cyclization of alkynes [52]. New catalytic reactions of alkynes included the co-cyclization of acetylene with nitriles, Cadio–Chodkiewicz cross-coupling, acetylene allylation (Kurts condensation), some oxidative chlorination reactions [52] and metathesis of alkynes [71], catalytic diene synthesis of norbornadiene with participation of intermediate [Cu](C≡CH) ethynyl complex [72], and hydrosilylation of alkynes [1].
Beginning with 1950s, extensive investigations were devoted to studying the mechanisms of homogeneous catalytic conversions of alkynes, including both the well-known and new reactions (see, e.g., works by F. Bohlmann, A.N. Nesmeyanov, E.A. Shilov, R.M. Flid, R. Vestin, В.V. Sokolsky, A.A. Petrov, O.A. Chaltykian, I.L. Kotlyarevsky, S.A. Vartanyan [8, 52, 73]). Numerous studied were devoted to the organometallic and coordination chemistry of acetylene-based compounds, which provided deeper insight into the mechanisms of catalytic reactions. Important achievements at the second stage (1950–1970) included the synthesis of ethynyl (alkynide) complexes of various metals (Cu, Ag, Au, Zn, Cd, Hg, Cr, Mn, Fe, Co, Ni, Pd, Pt, and Ir), elucidation of the structures of mononuclear and cluster π-complexes of 16 metals, synthesis of linear β-substituted vinyl complexes of some metals, synthesis of μ2-derivatives М‒СН=СН–М of nickel, cobalt, and chromium, as well as metallacyclopentadienes and other σ-organometallic compounds [52]. It is also worth of mentioning numerous studies of the kinetics and mechanisms of dimerization and oxidative dimerization of alkynes and mechanisms of various cyclization and co-cyclization reactions [52].
Investigations carried out by M. Dewar in 1946—1949 made it possible to establish a relation between the hypothesis concerning participation of π-complexes of metals with acetylenes in the mechanism of their catalytic reactions and the laws of addition reaction of various molecules at the triple bond (see, e.g., works by E.A. Shilov, R.M. Flid, R. Vestin, I.I. Moiseev). At the end of the second stage (1950–1970) first attempts were made to solve the problem of acetylene activation by metal complexes (see [74]) and to develop scientific principles of catalyst selection, which led eventually (at the third stage [1]) to understanding a simple idea: a justified choice of catalysts for any reaction can only be made using a procedure of formulating hypotheses about the reaction mechanisms (and, hence, the nature of intermediates) followed by analysis of these hypotheses. A metal complex adopted to be the possible catalyst must ensure the formation and proper conversion of intermediates in the proposed mechanism. Problems related to the formulation of hypothetical reaction mechanisms and the creation of computer software for generating hypotheses have been considered in [75—78].
At the third stage (1970—1990), the class of well-known metal complex catalysts of alkyne conversion expanded to include the complexes of Sc, Y, Zr, Nb, and Ta [1]. Chloride complexes of Au(III) in solutions proved to be effective catalysts of alkyne hydration [79] and amination [80] reactions, while Au(I) and Au(III) complexes both in solutions and on carriers effectively catalyzed acetylene hydrochlorination [15, 81]. New metal complex catalysts were also found for many other well-known reactions including the hydration, hydroiodination, hydrocyanation, arylation, hydrogenation, cyclization, dimerization, polymerization, carbonylation, and hydrosilylation of alkynes [1].
At the same time, new reactions were discovered including the vynylation of olefins [82] and methane [83, 84], anti-Markovnikov (anti-M) hydration of alkynes in a polyfunctional system of three catalysts CuCl–RSH–HCl with “hard” nucleophile (Н2О) replaced by the “soft” one (RSH) [85, 86], and anti-M addition of carbamate anions to terminal alkynes in ruthenium complexes with participation of vinylidene intermediates [87]. New catalysis were also proposed for the arylation of acetylene in solutions of Co(II), Ni(II), and Pd(II) complexes with the formation of 1,2-diarylethanes [88], Sonogashira cross-coupling in solutions of palladium and Cu(I) complexes [89], and homogeneous variants of acetylene hydrodimerization to butenes and acetylene–ethylene hydro-codimerization to butenes in Pd[P(OPh)3]4 complex solutions [90]. Catalytic systems were created for the oxidative carbonylation of alkynes at С–Н bonds [91, 92] and various reactions of carbonylation [93], including the oscillatory oxidative carbonylation of acetylene [94], oxidative chlorocarbonylation of acetylene to trans-1-chloroacrylates and trans-1-chloroacrylic acid [95, 96]. It was established that the synthesis of mixed esters of saturated and unsaturated mono- and diacids could be catalyzed by Pd(I) complexes and proceeded as a result of conjugated processes [97—99]. New metal complex catalysts were found for the metathesis of alkynes under homogeneous conditions [100].
Numerous organometallic complexes were synthesized or isolated from the reaction media, including π‑complexes of 25 metals with МmAn composition (where M is a metal and A is an alkyne) and potential intermediates of catalytic reactions formed from π‑complexes (Scheme 1) and small М2–alkyne clusters (Scheme 2) [1].

Scheme 1. Organometallic compounds obtained from π-complexes of alkynes.

Scheme 2. Products of reactions of metal clusters and alkynes.
Based on the results of systematic investigations of the kinetics and mechanisms of catalytic conversions of acetylene and its derivatives in metal complex solutions, kinetic models of reactions in concentrated solutions of copper chloride complexes were established for the hydrochlorination and hydration of acetylene, methylacetylene, and vinylacetylene, anti-M-hydration of methylacetylene in a system of polyfunctional catalysts, synthesis of norbornodiene from acetylene and cyclopentadiene, hydrocyanation of acetylene, and trimerization of acetylene [1, 8]. Extensive investigations were also devoted to the mechanisms of catalytic reactions of carbonylation [101], hydrogenation, cyclization, and polymerization of alkynes, and oxidative chlorination of alkynes in aqueous systems of polyfunctional catalysts CuCl–CuCl2, CuCl2–HgCl2, and PdCl2–HgCl2 [8].
At the fourth stage (1990s to present) in development of the catalytic chemistry of acetylene compounds to which the present review is devoted, most significant progress has been achieved in conducting catalytic reactions using Ni(II), Pt(II, IV), Ru(0, II, III, IV), Pd(0, I, II), and Au(I, III) complexes, as well as clusters and nanoparticles of these metals, and numerous new ligands involving these metals have appeared. A characteristic feature of this stage is the use of extremely large variety of acetylene substrates in the synthesis of complex biologically active compounds [9, 12–19, 22, 24, 27, 34, 35, 44].
New processes were found for the dicarbomylation of acetylene and alkynes to anhydrides of saturated diacids [93, 102—104], new oscillatory reactions were discovered in the catalytic systems of oxidative carbonylation of alkynes [8, 104–106] and new reactions were found for the catalytic oxidation of acetylene by nitric acid to glyoxal in solutions of Hg, Pd, Pt, Rh, Ir, and Au in the presence of sodium nitrite catalyst [107, 108], and new variants were discovered for the reactions of cycle formation, isomerization, cycloisomerization [11–15, 17–19, 24] and, in particular, very important reaction of cycloisomerization of unconjugated enynes [11, Ch. 3]. Considerable progress was achieved in the creation of new catalysts for the reactions of alkyne addition to compounds with Е–Н, Е–Е, and E–E' bonds (Е = S, Se, Te, P, B) and methods of controlling the selectivity of these processes [23, 28, 29, 33, 109–111]. Catalytic systems based on palladium complexes were found for extremely active and regioselective process of methylacetylene methoxycarbonylation to methyl methacrylate 112, 113] and systems based on ruthenium complexes were proposed for regioselective anti-M-carboxylation of alkynes [114, 115]. Reaction of the oxidative iodination of acetylene (and terminal alkynes) with the formation of (Е,Е)-1,4-di-iodobutadienes [116] and some other catalytic reactions of alkynes were discovered in solutions of platinum(IV) complexes. Remarkable success was achieved in regioselective synthesis of the products of classical dimerization of alkynes [18]. Specific reactivity of gold complexes was established in the catalytic synthesis of very complicated molecular structures [117].
Some commercially important directions of С2Н2 application reported in 2013–2018 and the prospects of its use in synthetic organic chemistry were considered in review [45].
At the present (fourth) stage of metal complex catalysis development, extensive investigations have been devoted to studying the mechanisms of both traditional and new catalytic reactions. It was established that an important role in some processes is played by the catalytic cycles involving metal nanoclusters, “giant” clusters, and nanoparticles and the multiplicity of catalytic centers in “homogeneous” processes [8, 118—120]. It was found that the stages of formation and deactivation of active centers play an important role in the mechanisms of catalytic reactions, i.e., in the possible realization of chain mechanisms in homogeneous catalysis with metal complexes [8, 118].
The conceived series of reviews will consider catalytic reactions of alkynes in which achievements of the catalysis with metal complexes in acetylene chemistry are most clearly pronounced as manifested by the development of high-selectivity processes with a variety of catalysts and mechanisms. The first review is devoted to one of the oldest catalytic reactions—dimerization and linear oligomerization of terminal alkynes. Alkyne dimers (enynes) are important substrates in the synthetic chemistry of biologically active compounds and in the chemistry of materials. Synthetic aspects of the dimerization reaction were described in detail by S. E. Garcıa-Garrido in monograph [18, p. 301]. The kinetic and mechanistic aspects of this reaction and the influence of the nature of intermediates on the regioselectivity of reaction will be considered in Section 3 below (including recent results reported in 2014–2018). Oxidative conversions of alkynes will be considered in the second review of this series.
3. DIMERIZATION OF ALKYNES IN SOLUTIONS OF METAL COMPLEX CATALYSTS
The formation of enyne hydrocarbons via dimerization of terminal alkynes can be classified into reactions of various types, including oligomerization (first member in the series), ethynylation, and vinylation (addition of HZ molecules to triple bonds). In the reaction of catalytic homodimerization of terminal alkynes, three enynes I–III (E-, Z-, and heme-isomer, respectively) and two cumulene 1,4-substituted butatrienes IV and V can be formed (Scheme 3). In the case of cross-dimerization, the number of chemo-, regio-, and stereo-isomers increases to 18. For this reason, the creation of catalytic systems (metal, ligand, solvent) ensuring selectivity of the dimerization process is a topical task, the solution of which for some systems is among remarkable achievements of the chemistry of catalysis with metal complexes [18].

Scheme 3. Products of alkyne homodimerization.
3.1. Complexes of Metals of Group 11 of the Periodic Table
This review of catalysts and reactions of alkyne dimerization naturally begins with metals (Cu and Au) of Group 11 of the Periodic Table, which have been historically the first catalysts of this process (for acetylene dimerization) [46, 121, 122]. The corresponding coordination and catalytic chemistry was studied in much detail, especially in the chemistry of alkynes [1, 8, 12, 14, 34, 51, 123]. In recent 20—25 years, gold complexes replenished arsenal of the catalytic chemistry of alkynes. Specific features of the coordination chemistry of Au(I) and Au(III) complexes are now quite sufficiently studied, including the nature and reactivity of gold-containing intermediates of catalytic reactions [10, 11, 13, 15, 18, 33, 124–127].
Fundamental differences of the physical and chemical properties of Cu(I) and Au(I) are related to peculiarities of the electron structures of copper and gold atoms. Filling of the 4f shell (lanthanide contraction) and the 4d and 5d shells of Au atom and, accordingly, the large numbers of electrons on atomic orbitals (AOs) at a high positive charge (79) of the nucleus lead to specific relativistic effects (REs) that determine to a considerable degree the reactivity of gold atoms in oxidation states 0, I, and III (see [124, 125] and references therein).
Briefly, the REs lead to the need for taking into account increase in the effective electron mass on s-AO due to high rotation speeds of remote electrons. This increase in the electron mass is accompanied by a decrease in the Bohr radius and, accordingly, the atomic and ionic radii. The RE refers to all s- and (to a lower degree) р-electrons. The lanthanide contraction and RE-induced contraction of AOs lead to the following consequences [124, 125]:
(i) The contraction of 6s-AO stabilizes vacant AOs and enhances the electron-acceptor properties of Au(I) and Au(III), thus increasing the Lewis acidity of Au(I) and Au(III).
(ii) The REs strongly increase the redox potentials of gold: Au+/Au0 (~1.68 V), Au3+/Au0 (1.5 V), Au3+/Au1+ (~1.4 V), Au2+/Au1+ (>1.3 V), particularly in comparison to copper ions (0.15 V for Cu2+/Cu1+). Note that analogous patterns are observed in Ni–Pt and Zn–Hg series, since the RE is also significant for Pt and Hg.
(iii) The Au+ ion is very unstable and, in the absence of ligands containing nitrogen, sulfur, and phosphorus atoms, readily exhibits disproportionation (dismutation) via reaction 3Au+ ⇄ Au3+ + \(2{\text{Au}}_{{{\text{met}}}}^{0}\) with an equilibrium constant close to to1010.
(iv) The first ionization potential of Au atom ((.22 eV) is significantly increased as compared to that of silver (7.57 eV) and copper (7.72 eV).
(v) The electronegativity of Au(I) reaches a level of 2.4 (vs. 1.8 for Cu(I) and 1.9 for Ag(I)).
(vi) The 5d-AOs of Au become more diffuse, but the repulsion of electrons and their energies decrease, which is accompanied by increasing electron—nucleus binding energies and decreasing donor properties of Au atom and ion. Therefore, the ability of forming dative bonds and participating in oxidative addition reactions significantly decreases.
(vii) Decreased repulsion of 5d-electrons on diffuse AOs in Au(I) complexes makes s-, p-, and d-AOs closer to each other, which facilitates their hybridization and increases stability of the linear geometry of LAuX and L2Au+X– complexes.
(viii) There is more clearly pronounced aurophilicity manifested by the formation of Au(I)–Au(I) bonds as compared to the case of cuprophilicity.
(ix) High electrophilicity of Au(I) increases the strength of Au(I)–L and Au(I)–X bonds, including those in numerous π-complexes of alkynes [125, 126], which makes π-complexes of alkynes capable of adding various nucleophiles (Nu) including olefins and even alkynes. For example, π-complex Au(С2Н2)+ is about 10 kcal/mol stronger than the analogous complex of Au(С2Н4)+.
(x) Dative Au+ → L contacts in π-complexes are less pronounced as compared to the case of copper ions, but are more significant in σ-complexes such as Au=СНR+ and Au≡СR+, which leads to the appearance of stable cationic carbene intermediates, e.g.,
(xi) The ability of Au(I) to form π-complexes, ethynyl compounds (alkinides), and Au(I)–Au(I) bonds (aurophilicity) allowed obtaining a large number of various clusters and supramolecular associates [11, 15, 125] and conducting unique syntheses of complex organic molecules [117]. Naturally, all the aforementioned properties of Au(I) led to its wide use in catalysis of alkyne dimerization processes.
Let us first consider the process of cuprocatalytic dimerization. The discovery of terminal alkyne dimerization reaction had a long and cautionary history that began in 1905 with the work of F. Strauss [121]. On studying the Glaser reaction [1, 8, 51, 52] of oxidative condensation of Cu(I) ethynyl compounds with various oxidants, it was established that boiling of PhC≡CCu ion acetic acid in the absence of oxygen led to the precipitation of PhC≡CCu ∙ Cu(OAc) and formation of С16Н12 hydrocarbon (1,4-diphenylbutenin) according to the following stoichiometric equation:
Here, the formation of phenylacetylene dimers results from the acidolysis of PhC≡CCu and dimerization of phenylacetylene catalyzed by Cu(I) acetate. In 1959, the Strauss’ observation was confirmed [122] and alkyne dimerization product was obtained from initial RC≡CCu in hot acetic acid solution (aerated for the oxidation of metallic copper appearing due to Cu(I) acetate dismutation) with the formation of 1% diacetylene derivative.
Upon return to the RC≡CCu–АсОН system in 1997 [128], it was established that the dissolution of Cu2О in boiling acetic acid with addition of terminal alkyl alkynes led to the formation of Z- and E-1,4-dialkylbutenins (II and I, respectively). This process terminated with the appearance of RC≡CCu and Cu(OAc)2, but alkyne did not exhibit dimerization in case of using a stronger dichloroacetic acid. By analogy with the structure of tetrameric complex [PhC≡CCuPMe3]4 [129], it was suggested that the product of phenylacetylene insertion at ≡С–Cu bond formed via dimeric complex [Cu(PhC≡CH)2]+[Cu(C≡CPh)2 ∙ AcOH]–, but the proposed mechanism was not confirmed by any evidence.
At the second and fourth stages of the development of metal complex catalysis of acetylene and its derivatives, detailed investigations were devoted to acetylene dimerization in the catalytic system discovered in 1931 by Nieuwland [46] in concentrated aqueous solutions of CuCl–NH4Cl(KCl)–HCl [1, 8, 52, 123]. For this system (Nieuwland catalyst), a correct kinetic model was proposed with a quite well substantiated mechanism of acetylene dimerization. Now let us briefly consider the main stages of studying Nieuwland catalysts.
1. Studies of the solubility of CuCl in aqueous NH4Cl and KCl solutions in a temperature interval of in the range 25–100°С showed that the [CuCl]/[NH4Cl] ratio at 80–100°С reached a maximum of 1.1–1.5 [8, 73, 123], which unambiguously pointed to the formation of polynuclear anions \({\text{C}}{{{\text{u}}}_{m}}{\text{Cl}}_{n}^{{(n - m) - }}.\) Chaltykian [73] noticed that, at CuCl concentrations below 4–5 m and NH4Cl concentrations below 6–7 m (where m is molality), the catalytic system ceases to be homogeneous and various acetylenide compounds precipitate from the solution.
2. Investigations of the equilibrium reactions of complex formation between CuCl and Cl– ion using the Hedström potentiometric method (see [130—134]) with the aid of electronic spectroscopy [134] showed that concentrated solutions contained about twenty anionic complexes \({\text{C}}{{{\text{u}}}_{m}}{\text{Cl}}_{n}^{{(n - m) - }}\) with m = 1–5 and n – m = 1–4. For example, at 25°С, [CuCl] = 4 m, [NH4Cl] = 6.5 m, and cationic background (CB) [NH4Cl + NH4NО3] = 14 m, the total molar fraction of mononuclear complexes is ~0.4 and the molar fraction of six complexes with m = 2–5 accounts for ~0.35 [132].
It is the large molar fraction of mononuclear complexes in the CuCl–KCl–Н2О system [135] that probably leads to the crystallization of KCuCl2 and KCuCl3 complexes from solutions on cooling. Note that systems used for the dimerization of acetylene to vinyl acetylene at 75°С contained 6.7 М CuCl and 6.4 М KCl or 8 М CuCl and 7.6 М KCl. In these solutions, the content of chloride ions is insufficient for the formation of even \({\text{CuCl}}_{2}^{ - }\) complexes, not speaking of \({\text{CuCl}}_{3}^{{2 - }},\)which is evidence for the existence of significant amounts of polynuclear anions in solution. The formation of binuclear complexes was established even at low CuCl concentrations (e.g., for [NaCl] \( \leqslant \) 5 M and [CuCl] \( \leqslant \) 1.6 M [136]) by the solubility method. Numerous crystalline polynuclear complexes were isolated from the Nieuwland catalytic system (see, e.g., [123, 137]), including (NH4)2Cu3Cl5 · 1/3(H2O) · 1/5(CuCl).
3. For studying the reaction kinetics and mechanisms of acetylene dimerization in multicomponent media such as Nieuwland catalysts with very complicated complexity function, a special method [8] was developed based on potentiometric measurements in situ, which allowed determining the activities of Cu+ (\({{a}_{{{\text{C}}{{{\text{u}}}^{ + }}}}}\)) and Cl– (\({{a}_{{{\text{C}}{{{\text{l}}}^{ - }}}}}\)) ions (proportional to the concentrations of these ions at CB = const) and, which is more important, maintaining their constant levels during variation of \({{P}_{{{{{\text{C}}}_{2}}{{{\text{H}}}_{2}}}}}\) and concentration of H3O+ ions [8, 138–141]. The concentration of H3O+ ions was determined by measuring (also in situ) the Hammett acidity h0 in samples of solution (taken from reactor) by the indicator method in a hermetic temperature-controlled cell [138, 140].
Investigations of the process kinetics [138, 141–145] in a gradient-free flow reactor [146] with determining \({{a}_{{{\text{C}}{{{\text{u}}}^{ + }}}}}\), \({{a}_{{{\text{C}}{{{\text{l}}}^{ - }}}}}\), h0, and \({{a}_{{{{{\text{H}}}_{2}}{\text{O}}}}}\) values [130] at constant CB gave correct dependences of the reaction rate W on \({{P}_{{{{{\text{C}}}_{2}}{{{\text{H}}}_{2}}}}}\) and h0\( \propto \) [H3O+] at \({{a}_{{{\text{C}}{{{\text{u}}}^{ + }}}}}\) = const, \({{a}_{{{\text{C}}{{{\text{l}}}^{ - }}}}}\) = сonst and led to the following formula [138, 141]:
Since the value of constant \(K_{\text{a}}^{/}\) was independent of \({{a}_{{{\text{C}}{{{\text{u}}}^{ + }}}}}\)and \({{a}_{{{\text{C}}{{{\text{l}}}^{ - }}}}}\) ([CuCl]), it was possible to analyze the behavior of kH = f(\({{a}_{{{\text{C}}{{{\text{u}}}^{ + }}}}}\), \({{a}_{{{\text{C}}{{{\text{l}}}^{ - }}}}}\)) depending on [CuCl] by approximately assuming that catalysis involves a single active complex \({\text{C}}{{{\text{u}}}_{m}}{\text{Cl}}_{n}^{{(n - m) - }}\) [142]. This analysis showed that the catalytic activity was inherent in polynuclear complexes with m = 3 and 4, and dominating contribution of \({\text{C}}{{{\text{u}}}_{4}}{\text{Cl}}_{5}^{ - }\) [142]. A more strict analysis [145] led to a conclusion that m values were within 4.3–4.7 and the active complexes included anions \({\text{C}}{{{\text{u}}}_{4}}{\text{Cl}}_{5}^{ - },\)\({\text{C}}{{{\text{u}}}_{4}}{\text{Cl}}_{6}^{{2 - }},\)\({\text{C}}{{{\text{u}}}_{5}}{\text{Cl}}_{6}^{ - }\), and \({\text{C}}{{{\text{u}}}_{5}}{\text{Cl}}_{7}^{{2 - }}.\) The mechanism of acetylene dimerization can be described by the following set of reactions (I)–(VI):
Here, the initial copper chloride complex is represented by only one molecule of water coordinated by copper atoms. Substitution of acetylene for this molecule leads to the formation of π-complex (π1) that is converted into ethynyl complex (σ1).
The ethynyl complex (σ1) is converted via π-complex (π2) into σ-organometallic compound (σ2) as a result of the π-coordinated acetylene insertion at Cu–С≡ bond (reaction (IV)). Compound σ2 is subject to fast protolysis with the formation of monovinyl acetylene (MVA).
It is also possible that the formation of σ2 via reaction (IV) involves a water molecule leading to an aqua-complex at stage (V), while the protolysis of σ2 and attachment of Cl– (reaction (VI)) proceed via two fast stages. Using the condition of quasi-stationary reactions in this mechanistic scheme with a single route, we obtain a kinetic equation for the rate of MVA synthesis. Estimation of the model parameters and their statistical analysis [142] showed that a minimum dispersion of reaction rates was achieved under assumptions that k–1\( \gg \)\({{k}_{2}}{{a}_{{{{{\text{H}}}_{2}}{\text{O}}}}}\) and \({{k}_{{ - 3}}}{{a}_{{{{{\text{H}}}_{2}}{\text{O}}}}}\)\( \gg \)k4, which yielded the following relation:
where keff = k4K1K2K3(\(K_{1}^{/}\))2Kpb; Kа = k4K3\(K_{1}^{/}\)/bk–2; \(K_{1}^{/}\) is the equilibrium constant of acetylene dissolution; Kp is the equilibrium constant of \({\text{C}}{{{\text{u}}}_{m}}{\text{Cl}}_{n}^{{(n - m) - }}\)anion formation from Cu+ and Cl–; \({{a}_{{{{{\text{H}}}_{3}}{{{\text{O}}}^{ + }}}}}~\) = bh0\({{a}_{{{{{\text{H}}}_{2}}{\text{O}}}}}\); and b is the coefficient of proportionality.
It follows from Eqs. (1) and (2) that the process of acetylene dimerization at temperatures within 50–100°С [143] proceeds without a rate-limiting stage (see discussion of the limiting stage notion in [147]). Under the reaction conditions studied, the rates of [Cu]C≡CH complex protodemetalation (W–1) and the rate of [Cu]C4H3 formation are comparable. For \({{h}_{0}}a_{{{{{\text{H}}}_{2}}{\text{O}}}}^{2}{{a}_{{{\text{C}}{{{\text{l}}}^{ - }}}}}\)\( \gg \)\({{K}_{{\text{a}}}}{{P}_{{{{{\text{C}}}_{2}}{{{\text{H}}}_{2}}}}}\) (k–2\({{a}_{{{{{\text{H}}}_{3}}{{{\text{O}}}^{ + }}}}}{{a}_{{{\text{C}}{{{\text{l}}}^{ - }}}}}\)\( \gg \)k4), the rate-limiting stage is reaction (IV), while for \({{h}_{0}}a_{{{{{\text{H}}}_{2}}{\text{O}}}}^{2}{{a}_{{{\text{C}}{{{\text{l}}}^{ - }}}}}\)\( \ll \)\({{K}_{{\text{a}}}}{{P}_{{{{{\text{C}}}_{2}}{{{\text{H}}}_{2}}}}}\), the process rate is controlled by the formation of compound σ1 via reaction (II).
The composition of ethynyl complex CumCln– 1-(С2H)(n –m)– was established with the aid of spectrophotometric and potentiometric techniques [148]. Under assumption that dominating contribution to the catalytic process is determined by a single “yellow” complex of this type, a minimum dispersion of function F related to the total optical absorption of these complexes in the wavelength interval of 350—420 nm would be observed for m = 4.3 and n = 5.0. These investigations also showed that copper acetylenide Cu2C2 is well-soluble in concentrated CuCl solutions, but only provided weak acidity (рН > 5 and h0 < 0.2). For this reason, the synthesis under adopted conditions (h0 = 0.2–0.5 in solution of CuCl (6.6 m) and NH4Cl (8.84 m) at an optimum temperature of 80°С [143]) yields MVA at a much lower concentration as compared to that of ethynyl complexes.
Valuable information on the nature of second organometallic intermediate [Cu]C4H3 (σ2) considered as a product of acetylene insertion at [Cu]–C≡ bond in the π-complex was obtained from investigations of the influence of the concentration of various metal chlorides on the process rate in the CuCl–NH4Cl–HCl–Н2О system [144]. It was found that metal chlorides can be subdivided into three groups with respect to their influence on the reaction rate:
– ZnCl2, CdCl2, and BiCl3 increase the reaction rate by increasing \({{a}_{{{\text{C}}{{{\text{u}}}^{{\text{ + }}}}}}}\) and, hence, the concentration of active chloride anions and σ1 complexes;
– BеCl2, MgCl2, CaCl2, SrCl2, BaCl2, FeCl2, CoCl2, MnCl2, and CrCl3 decrease the reaction rate by increasing \({{a}_{{{\text{C}}{{{\text{l}}}^{ - }}}}}\) and decreasing \({{a}_{{{\text{C}}{{{\text{u}}}^{{\text{ + }}}}}}}\) (similar to the effect of NH4Cl and KCl);
– HgCl2, SnCl2, and CuCl2 sharply decrease the rate of dimerization reaction. The effect of SnCl2 and HgCl2 is related to the growth in solution acidity as a result of SnCl2 hydrolysis and the formation of organomercuric compounds stable with respect to protolysis, which appear due to the transfer of organic groups via reaction
with R = C≡CH, CH=CHC≡CH, or C≡CCН=CH2.
It was established that the main factor increasing the Hammett acidity to h0 = 2 upon mercury chloride addition is the formation of ClHgCH=CHC≡CH compound on solution, which decomposes udder the action of concentrated HCl with the formation of MVA. The introduction of CuCl2 leads to conversion of the proposed intermediate compound [Cu]C4H3 (σ2) to 2-chlorovinyl acetylene (CVA) CH2=C(Cl)C≡CH. Measurements of the kinetics of competitive reactions of MVA and CVA formation [149, 150] provided information on the reaction mechanism after irreversible formation of [Cu]C4H3. In addition, it was found that, in the presence of CuCl2 under stationary conditions ([CuCl] = 12 m, [NH4Cl] = 12 m, [CuCl2] = 10–3–10–2 М, and continuous regeneration of CuCl2 via anodic oxidation of CuCl with established linear dependence of \({{a}_{{{\text{C}}{{{\text{u}}}^{{\text{ + }}}}}}}\) = const[Cu(II)]Σ), the rates of MVA and CVA formation at \({{a}_{{{\text{C}}{{{\text{u}}}^{{\text{ + }}}}}}}\) = const and \({{a}_{{{\text{C}}{{{\text{l}}}^{ - }}}}}\) = const under conditions corresponding to the mechanism of MVA synthesis according to Eqs. (I)–(VII) and their common intermediate [Cu]C4H3 (σ2) conversion according to Scheme 4 are given by the following formulas:

Scheme4. Mechanism of intermediate [Cu]C4H3 (σ2) conversion in the system with [CuCl] = 12 m, [NH4Cl] = 12 m, and [CuCl2] = 10–3–10–2 М.
Equations (3) and (4) show that the ratio of reaction rates is WCVА/WМVА = kIII[Cu((II)Σ]/h0 and their sum WCVА + WМVА equals the rate of MVA formation in the absence of CuCl2. The mechanism of [Cu]C4H3 conversion into CVA is considered in monograph [7].
4. Solid π-complexes of CuCl and Cu2SO4 with acetylene, complexes of CuCl with с MVA, as well as CuC≡CH and solid “yellow” acetylenide complexes that were described during the first two stages of acetylene chemistry development in works of M. Berthelot, R. Chavastelon, В. Manchot, J. Osterlof, O.A.Chaltykian, R. Westin, A. L. Klebanskii, I. M. Dolgopolskii, L.G. Zurich, A.A. Ginzburg, I.K. Khristich, and R. Nasta are considered in monograph [52].
It should be also noted that various important issues of organometallic and coordination chemistry related to the Nieuwland catalytic system were developed in 1990–1999 by the Lviv group of crystal chemists (M.G. Mys’kiv at al.) [137, 151—160] and by other researchers [123, 129, 161–165]. These investigations elucidated the structures of polynuclear π-complexes of acetylene, MVA, divinyl acetylene (DVA), and ethynyl complexes with CuC≡CH, CuC≡CR, and Cu2C2 fragments, which help representing the structural (crystallochemical) aspects of acetylene dimerization mechanism as considered in [8, 123].
5. Scheme 5 presents the structures of four-nuclear copper complexes as probable intermediates of the dimerization process, which were constructed based on the fragments of structures of the corresponding crystalline Cu(I) complexes (i.e., crystallochemical reconstruction of the reaction mechanism). According to the kinetics of dimerization reaction, formation of the acetylene π-complex B from A at stage (I) proceeds as a result of the chloride bridge fracture or the replacement of coordinated water molecule (cross-hatched) with transferred bridging Cl– ion (without displacement of chloride ion). The coordination note in complex B is structurally analogous to one of the possible π-complexes [123], e.g., NH4Cu3Cl4(C2H2) [152] or NH4Cu8Cl9(C2H2)4 [154]. During the formation of ethynyl intermediate C (σ1), Cl– ions can either go to solution simultaneously with Н3О+ (stage (II)) or escape at the next quasi-equilibrium stage (without changing the kinetic equation).

Scheme5. Crystallochemical) aspects of acetylene dimerization mechanism [123].
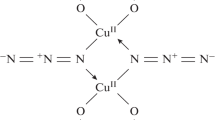
Among a huge variety of ethynyl complex structures [123], intermediate C may be compared to (PyH)4Cu9Cl12(C≡CH) presented in Fig. 1 [123, 160]. A very rich coordination chemistry has been found among ethynyl derivatives of propargyl alcohol, (NH4)2Cu4Cl5(C≡CCH2OH) ∙ H2O, which are presented in Fig. 2 [123, 156].
It should be noted that Nieuwland catalyst solutions contain pH-dependent ethynyl and symmetric “yellow” acetylenide complexes \(({\text{C}}{{{\text{u}}}_{2}}{{{\text{C}}}_{2}}){\text{C}}{{{\text{u}}}_{p}}{\text{Cl}}_{q}^{{(q-p)-}}.\) Increasing acidity (рН < 1.5) leads to the breakage of acetylenide complexes and increase (to within certain limits) in the concentration of intermediate C.
The insertion of acetylene from a π-complex via Cu–C≡ bond into intermediate D (Scheme 5) leads to the formation of fragment [Cu](CH=CHC≡CH) in Х1 (stage (IV)), probably via intermediate D*. The mechanism of this stage that is responsible for the stereochemistry of dimerization products of terminal alkynes is discussed in review [123]. The stage of protolysis of intermediate Х1 (stage (VI)) with the formation of E poses no questions and is well-known in the chemistry of σ-organometallic Cu(I) compounds [166].
Kinetic models of acetylene trimerization (synthesis of 1,3-hexadien-5-yne) and codimerization of acetylene and MVA (synthesis of 1,5-hexadien-3-yne, DVA) were also constructed using the method described above [167, 168].
6. In addition to the well-known types of coordination of alkynide ligands RC≡C– with copper atoms (VI, VII, VIII, IX), copper chloride complexes revealed another two structures (X and XI) [123]:

Rather interesting variants of coordination were also found upon the interaction of Сu2C2 with copper chloride anions [158]:

π,6-Coordination nodes in (NH4)8Cu29Cl29(C≡C)4 · 7H2O complex structure.
Quite recently, additional results important for understanding the mechanism of MVA synthesis were obtained [169, 170] by analysis of the 1Н and 13С NMR spectra of copper chloride solutions purged with acetylene. It was found that passing acetylene through a solution of 7.0 М CuCl and 6.66 М KCl at 50°С led to the appearance of signals corresponding to acetylene π-complexes, MVA, and DVA (Table 2) and the concentration of π-complexes varied with the reaction time. In addition, acetylene exhibited rapid H/D exchange in D2O solutions with the formation of HC≡CD, DC≡CD, and deuterated MVA exhibiting significant kinetic isotope effect (KIE) in which the rate of С2Н2 π-complex consumption and acetylene (in the gas phase) was ~3 times lower in the case of D2O. These observations are quite natural in the framework of the kinetically and spectrophotometrically studied mechanism of acetylene dimerization (see above) involving the kinetically reversible stage
Kinetic data [169, 170] determining the first order of reaction (VIII) with respect to the concentration of С2Н2 π-complex and acetylene concentration in the gas phase caused major doubts because they were obtained from the values of chemical shifts under nonstationary conditions and in the absence of control over the acidity of solution. In particular, reversible reaction (VIII) cannot have the first order (in (C0/C)–t) coordinates) with respect to the concentration of π‑complex even at an almost constant concentration of Н3О+, while the kinetically irreversible stage would not be accompanied by rapid H/D exchange (even if this exchange actually proceeded via the formation of σ-ethynyl Cu(C2H)complex and its protolysis). For example, data obtained previously [171] on the kinetics of isotope exchange in AcOH showed that the rate of RC≡CCu formation was ~100 times lower than the rate of H/D isotope exchange. The possible mechanisms of isotope exchange are discussed in [1]. Another questionable issue was the role of diffusion constraints in determining the reaction order with respect to gaseous acetylene.
Increase in the stability of Nieuwland catalysts in aqueous [172] and nonaqueous [173] solutions using controlled variation of their acidity (by adding HCl during the process) has been used in practice for a long time. In aqueous systems, the concentrations of “yellow” acetylenide complexes and [Cu](C2H)–, as well as their solubilities, depend on the concentration of acid (HCl) that is consumed for the side processes of hydrochlorination pf acetylene, MVA, and acetylene trimers and for the oxidative chlorination of all alkynes due to the presence of oxygen traces and, hence, CuCl2.
Now let us consider the process of aurocatalytic dimerization. Based on investigations of the activity of Au(I) complexes in reactions of alkyne dimerization under variable conditions in various solvents [174], it was established that the dimerization of n‑BuO(CH2)3C≡CH ester in boiling toluene led to the formation of isomers II and III in a (4.5–20) : 1 ratio. This ratio depends on both the nature of ligands and the nature of a base added to the catalytic system, so that the yield of a heme-isomer can reach 91–96%. The function of catalysts was performed by gold complexes LAuNTf2, where \({\text{NTf}}_{2}^{ - }\) is a non-coordinated anion and L is a carbene (NНС) or phosphine ligand:

Ligands in Au(I) complexes.
The best results were obtained using ligand XIV with additions of solid NaOAc.
Investigation of the dimerization of nine aliphatic alkynes containing various substituents in the alkyl groups led to a proposed reaction mechanism analogous to that for Cu(I) complex solutions, which is referred to below as the ethynyl mechanism. The cis-insertion of π-complex-bound alkyne at [Au]–C≡ bond (according to Markovnikov) or the interaction of [Au]–C≡CR with π-complex [Au](RC≡CH)+ (due to aurophilicity) lead to predominant formation of RC≡C–C(R)=CHAuL intermediate. Then, replacement of AuL+ with proton (probably from acetic acid) leads to isomer III. The formation of isomer II as a side product via anti-M insertion can be evidence of the possible anti-M addition of RC≡C– anion to alkyne. Taking into account the ability of Au+ to stabilize carbene and vinylidene ligands, it is also possible to suggest the participation of vinylidene ligand in a mechanism of dimerization with the formation of dimer II. The conversion of RC≡CAu(L)(=C=CHR)– can probably also lead to isomer II. However, this mechanism would require the appearance of tri-coordinated gold atom that is not characteristic of Au+.
Thus, already at the third stage of development of the modern alkyne chemistry, effective catalysts for highly selective processes of alkyne dimerization were developed based on Ni(II), Rh(I), Cr(IV), Sc(III), Y(III), and Ti(IV) complexes [1]. In 1990—2018 (fourth stage), new metal complex catalysis of alkyne dimerization were proposed and new intermediates and reaction mechanisms were found (see also review [18]).
3.2. Complexes of Ru, Os and Fe
Let us begin with the most thoroughly studied processes of alkyne dimerization catalyzed by metal complexes of Group 8 of the Periodic Table, firstly, by ruthenium (Ru) complexes.
Investigations of the selectivity of alkyne homodimerization and catalytic activity of Ru complexes with various mono- and polydentate ligands (including cyclopentadienyl and carbene complexes, see Table 3) showed that Ru(II) and Ru(0) complex solutions contained precursors of active catalysts representing selectively (≥95%) formed isomers I and II (Scheme 6) with Е/Z ratios determined by the sterical and electronic properties of ligands and alkyne substituents.

Scheme 6. Products of alkyne dimerization in catalytic systems based on ruthenium complexes.
Heme-isomer III is a product rarelyencountered in selective homodimerization, which is formed in solutions ofcomplexes 15 (Table 3, R = PhCH2) and 17 (Table 3, R = n-hexyl).Other rare isomers are cumulenes IV and V (Table 3, Scheme 6). The reactionselectivity is especially significantly influenced by geometry of thecoordination sphere of Ru atom, that is, by the degree of its screening.
The contributions of sterical effects and the nature of ligands to the regio- and stereoselectivity of dimerization were most thoroughly traced in the case of rather “closed” complex 15 (Table 3, η5-С5M5)RuLH(H2) [182, 183]. For L = PPh3 (15a) and large R (tBu and SiMe3), isomer I is formed at 95–98% selectivity. In case of L = PMe3 (15c) and the same substituents R, a mixture of isomers I–III is formed, and only the reaction with R = Ph gives isomer II at 90% yield.
Isomer V (>95%) is obtained in solutions of complex 15b for L = PCy3 and R = PhCH2. The reactions of alkyne cross-dimerization will be considered below, after analysis of the mechanisms of reactions catalyzed by ruthenium complexes.
General notions of the mechanisms of alkyne dimerization were formulated based on the results of investigations of the dimerization of acetylene and alkynes in solutions of Cu(I) complexes (Section 3.1), Ni(II) and Ni(0) complexes [1], and so-called vinylidene hypothesis proposed by Yamazaki [175] in his first work devoted to the catalysis of alkyne dimerization by Ru(II) complexes.
A. Ethynyl mechanism:
B. Vinyl mechanism:
The order of formation of intermediates can be different—whereby the ethynyl group is formed first, followed by vinyl (alkenyl) group—but the key intermediate must contain both groups.
C. Vinylidene mechanism:
Vinylidene group can form via different pathways:
(a) H[M](C≡CR) \( \to \) [M](=C=CHR).
(b) [M](η2-HC≡CR) \(\xrightarrow[{ - {\text{BH}^{ + }}}]{{{\text{BH}^{ + }}}}\) [M](=C=CHR).
(c) [M](η2-HC≡CR) \( \to \) [M](=C=CHR).
In the framework of this mechanism, different variants of decomposition of intermediate [M]-C4 can lead to regio- and stereoselective formation of isomers I, II, IV and V.
An analysis of works devoted to the synthesis of enynes in solutions of Ru(II, IV) complexes [175–191], which frequently reported the results of studying particular aspects of the dimerization mechanisms, and works devoted directly to these mechanisms in reactions catalyzed by ruthenium complexes [192–200] reveals the main problems and paradigms existing in this field of the chemistry of dimerization processes. The most profoundly substantiated and developed mechanism of catalysis with Ru complexes is the vinylidene mechanism C [176–178, 181, 192, 195, 197, 200], which are briefly illustrated below by several examples.
Dobson et al. [192] studied the reactions between HRu(O2CCF3)(CO)(PPh3)2 complex and terminal alkynes and diarylacetylenes with the formation of vinyl derivatives and, in addition, synthesized a hypothetical model intermediate for the dimerization of alkynes with 1,4-diphenylbut-1-en-3-in-2-yl ligand from HRu(O2CCF3)(CO)(PPh3)2 hydride complex and 1,4-diphenylbutadyine (i.e. [M]-C4 or σ-С4). The structure of this Ru(С4НPh2)(O2CCF3)(CO)(PPh3)2 complex contains [Ru]–C(=CHPh)–C≡CPh fragment (XII, σ-С4):

It was pointed out that this intermediate could not form via alkyne insertion at [Ru]–C≡CPh bond, that is, in the framework of mechanism A. Based on the previously (1968—1972) described vinylidene complexes of Fe and Pt (see review [200]), it was suggested that intermediate XII could appear as a result of the formation of vinylidene complex of alkyne with subsequent coupling between vinylidene group and ethynyl ligand (nucleophilic attack of RC≡C– group at α‑atom of the vinylidene group). In the case of formation of isomer III, vinylidene mechanism C can be excluded.
The complex with fragment XII was found [176, 178, 194] among the intermediates formed directly from alkyne and ruthenium complex during the dimerization of alkynes. In addition, [177, 193] complexes with [M]-C4 fragment were also synthesized, but their structure XIII was different from that of complex XII:

The transition from complex XII to XIII can be explained by taking into consideration two mesomers of asymmetric η3-allyl complex XIII:

Thus, complexes of types XII and XIII can be considered as key intermediates in the vinylidene mechanisms leading to dimers I, II, IV and V [179, 180, 190, 194, 197, 198].
Vinylidene complexes of ruthenium were probably first obtained from alkynes in 1979 by Bruce and Wallis [201] and then studied in detail (including structures) [176–178, 195] and described in reviews [115, 180, 200–203] and monograph [204]. These reviews considered the possible mechanisms of vinylidene ligand formation and, in addition, it was established that mono- and diethynyl complexes can form from alkynes and vinylidene complexes [177, 178, 181, 184] via the following reaction:
Bullock [195] studied the kinetics of π-methylacetylene complex isomerization to vinylidene complex via the following reaction:
Reaction (X) turned out to be monomolecular (first order with respect to π-complex) with ∆Н0 = 23.4 kcal/mol and \(\Delta {{S}^{ \ne }}\) = 3.9 ± 0.9 cal mol–1 K–1. At 80–110°С, the vinylidene complex converted into CpRuL2(MeCN)+\({\text{PF}}_{6}^{ - }\) complex with methylacetylene evolution. It is interesting to note that the conversion of π-complex into vinylidene complex takes place only at 60–90°С.
Wakatsuki et al. [197] performed quantum-mechanical modeling of the monomolecular transformation of acetylene π-complex with Ru(II) to vinylidene complex with the analysis of various conformations of RuCl2(PH3)2(η2-C2H2) π-complex (XIV) and RuCl2(PH3)2(=C=CH2) complex (XV). A comparison of the results of MP2/6–31G* calculations obtained for the thermal isomerization process
(∆Н0 = –48.7 kcal/mol and ∆Ea =1.07 kcal/mol) to experimental data (∆Н0 = –47.4 ± 4 kcal/mol and ∆Ea = 2 kcal/mol) showed good coincidence [197]. Calculations performed by the same method for various basis sets gave the values about ∆Н0 = – 51.7 kcal/mol and ∆Ea = 0.1 kcal/mol. For the transformation of complex XIV into XV

it was found that ∆Н0\( \cong \) 0 and the barrier of transient state TS2 of reaction XIV → XV was 34.5 kcal/mol. The transient state TS2 (a) forms via intermediate (b) as

with agostic bond

Estimates obtained for the transformation of π‑complex RuCl2(PH3)2(η2-C2H2) (XIV) into a product of oxidative addition of С2Н2 to Ru(II) with the formation of octahedral ethynyl complex Н[Ru](C≡CH) showed that the energy of this complex is much higher than the energy of transient state TS2 so that this transition in the given system is even thermodynamically hardly probable.
Now it is expedient to consider the issue of metal (ruthenium) degree of oxidation (DO) in vinylidene complexes, since carbene ligand is frequently treated as neutral ligand (singlet carbene) donating σ-electron pair to the metal atom. This approach is valid for Fischer carbene complexes. In the case of Schrock carbene complexes (М=СН2 and М=С=СНR), carbene (according to all formal rules) is a ligand with charge –2 that forms σ- and π-bonds with the metal—that is, increases the metal DO by 2 (for commonly accepted approaches to DO definition see [205]). Therefore, the ruthenium DO in a vinylidene complex becomes IV (i.e., increases by 2 as compared to that in the initial π-complex, where it was II) as in [197]. Analogous situation takes place in imides [M2+](=NR2–), nitrides [M3+](≡N3–), and phosphides [M3+](≡P3–) of transition metals. Note also that Ru=С= bonds (1.76 and 1.84 Å [202] and 1.8 Å [181]) are significantly shorter than Ru–С≡ bonds (2.08 and 2.05 Ǻ). The Ru–С bond length in [Ru]–С4 is within 2.07–2.48 Å [177, 192, 193], while the Ru=С= bond length determined via optimization of geometry [197] amounts to 1.63–1.66 Å (depending on the conformation) and the Ru–С≡ bond length in ethynyl complex is 1.95 Å.
The cumulene products of alkyne dimerization are formed as a result of the chain of transformations of intermediates XII and XIII with the shift of Ru atom from position 2 (XII) to 4 (via XIII) as shown in Scheme 7 (where A = alkyne):
Scheme7. Mechanism of synthesis of cumulene isomers IV and V.
It was established as long ago as in 1991 [179] that cumulene Z–H3SiCH=C=C=CHSiH3 has an energy higher by 17–19 kcal/mol than that of the corresponding enynes (I and II), so that the isomerization of cumulenes IV and V to isomers I and II is thermodynamically possible. This possibility was confirmed in experiments with HRuCl(PiPr3)2 complex [190]. Therefore, some stages in Scheme 7 can be kinetically reversible.
In view of the possible realization of the ethynyl mechanism of dimerization (A), it is interesting to note that the process can begin with the formation of a vinylidene complex, which subsequently converts into the ethynyl complex via reaction (IX) [181]. The corresponding mechanism of dimerization is presented in Scheme 8.
Scheme8. Dimerization mechanism А in catalysis with ruthenium complexes.
Note that the last stage–demetalation of intermediate [Ru](σ-C4) with the formation of product I in this mechanism takes place as a result of the metathesis of σ-bonds [Ru]–C and Н–С≡, but the source of H atom in some Ru-based catalytic systems still remains unclear. Bassetti et al. [187] solved the problem of demetalation in the framework of mechanism C at the stage of [Ru](σ-C4) intermediate protolysis in a system containing AcOH.
There are only few examples of the cross-dimerization of alkynes catalyzed by Ru complexes. Yi and Liu [185] used a reaction of RC≡CH addition to internal alkynes R1C≡CR catalyzed by Cp*Ru(C≡CPh)(PPh3) complex (16, Table 3) to obtain PhC≡C–C(R1)C=CHR2 product with 44–90% yield from a vinylidene precursor. A high chemoselectivity was only achieved with R1 = tBu, iBu, SiMe3 and R2 = CO2Et, COMe and amounted to 100% in reaction with tBuC≡CH and MeС≡СMe. This investigation revealed an interesting intermediate (XVI) in mechanism A with agnostic bond [Ru](Н–СН2–):

Katayama et al. [188] described the cross-dimerization of alkynes ArC≡CH with Me3SiC≡CH catalyzed by vinylidene complex RuCl2L2(=C=CHPh). In the case of p-TolC≡CH and L = PiPr3, this reaction in the presence of amines gave predominantly Me3SiC≡CCH=CH(p-Tol) (isomer II) with a yield of 82–93% (depending in the nature of amine) while the content of heme-isomer III varied within 7–14%. The formation of isomer II was considered as a result of the operation of mechanism C, while isomers I and III were formed by mechanism A.
Investigation of alkyne reactions with osmium(II) complexes by Dobson et al. [192] showed that Os(O2CCF3)2(CO)L2, OsH(O2CCF3)(CO)L2 and Os{C(C≡CPh)=CHPh)}(O2CCF3)(CO)L2 complexes catalyzed phenylacetylene oligomerization with the formation of С64Н48 and С90Н80 oligomers and osmium hydride complex catalyzed the formation of isomeric vinyl products of phenylacetylene addition to tolane and methylphenylacetylene.
Various osmium hydride complexes (H[Os], H2[Os], H3[Os] and H4[Os]) react with alkynes to form diverse organometallic compounds (see [206] and references therein) containing alkenyl, alkylcarbyne (alkylidyne), vinylidene, and alkynide ligands, respectively. Complex HOs(=C=CHPh)(η2-O2CCH3)L2 catalyzes PhC2H dimerization to a mixture of isomers I and II. Barbaro et al. [207] thoroughly studied the mechanism of dimerization with the participation of HOs(PP3)(N2)BPh4 complex (PP3 = P(CH2CH2PPh3)3, XVII). It was established that, similar to ruthenium complexes (see above), osmium complex XVII in reaction with Me3SiC≡CH forms (via vinylidene complex H[Os](=C=CHSiMe3)) an intermediate of type ХIII (Os[η3–Me3SiC3=CHSiMe3][BPh4], XVIII) and 1 eq. styrene. In excess of PhC2H, the catalytic reaction proceeds with the formation of isomer II at ~90% yield. Measurements of 1H and 31P (1H) NMR spectra in situ and modeling intermediates and their reactions established the following sequence of phenylacetylene conversions in the presence osmium hydride complexes:
It was found that the catalytic cycle begins and ends with the formation of [Os](C≡CR)+ and involves the formation of active centers
and the catalytic cycle proper
It is interesting to note that, as long ago as in 1985, Gotzig et al. [208] established that the oxidation of complex Os(C≡CPh)L2 (L = PMe3) by silver ion (AgPF6) also yields Os(σ-C4)L4PF6 product as a result of the oxidative coupling of ethynyl groups with detachment of H from solvent and reduction of Ag+ to Ag0.
Reactions of simple hydride complexes HOsCl(CO)(PiPr3)2 with alkynes in the presence of Et2NH led to the formation of butatrienes IV and V, which were stable in the case of R = tBu and Cy [209]. In the case of R = Ph, the obtained butatrienes exhibited polymerization with the formation of oligomers [–CH(Ph)C≡CC(H)Ph)–]n. In the case of R = Me3Si, the butatriene exhibited isomerization to enyne II. It was shown that the role of amine consists in the formation of active intermediates:
Investigations of various osmium pincer complexes showed (see [210] and references therein) that Os complexes were a promising alternative to other metal complex catalysts. For example, a pincer complex with POP ligand, H4Os[POP] (XIX), where РОР = [Xant(PiPr3)2] (XX)

is an active catalyst of alkyne dimerization to isomers II with a yield of 94% (R = Ph, TOF = 100 h–1) and 90% (R = tBu, TOF = 30 h–1). The formation of active catalyst starts from complex XIX and proceeds via the formation of two olefin molecules and liberation of Н2 molecule:
The catalytic cycle is presented in Scheme 9 [210]:

Scheme9. Catalytic cycle with H4Os[POP] (XIX) precursor (reproduced with permission from Inorg. Chem. 52, 6199 (2013) [210]. Copyright (2013) Am. Chem. Soc.).
Catalysts based on iron complexes were rarely used in investigations of alkyne dimerization, but yet gave some interesting results [211–216]. Complexes FeCl2L and HFeClL, HFe(H2)L+ with L = PP3 and [P(CH2CH2PMe2)3] showed generally low activity in alkyne dimerization [211], where Z-isomer (II) was formed from PhC2H selectively but slowly, at a catalyst TOF about 1 turn per day.
However, there were found and studied four isomers of intermediate complexes (PP3)Fe(η3-PhC3C=CHPh)+\({\text{BPh}}_{4}^{ - }\) leading to predominant formation of isomer XXI:

Midya et al. [212, 213] studied simple systems based on FeCl3L complexes (L = dimethylethylenediamide, DMEDA) in the presence of KOtBu and other bases. Reactions with 17 arylacetylenes revealed the formation of a mixture of isomers I and II, with predominant synthesis of isomer I (78–89%). Investigation of the influence of various amines as ligands and radical inhibitors (ТЕМРО) [213] led to the creation of an original mechanism of dimerization with the participation of arylvinyl radicals and a change in the degree of iron atom oxidation (Fe(II) → Fe(III) → Fe(IV) → Fe(II)). The role of KOtBu reduces to the formation of radical anions from ArC2H and subsequent formation of arylvinyl radicals from these anions:
The obtained intermediates interact, e.g., with Fe(III) to form [Fe(IV)](C≡CAr)(CН=CHAr) complex decaying into Fe(II) and isomer I.
Investigation of the reactions of iron pincer complex HFe(BH4)(iPr-PNP) showed selective formation of isomer II (94%) in homodimerization of phenylacetylene and 13 other arylalkynes [214]. In addition, highly selective cross-coupling between ArC2H and Me3SiC2H (Z–isomer II) at 79–99% yield) without any additions. The active catalyst [Fe](BH4)(C≡CAr) formed from the initial complex with Н2 evolution and BH4 ligand retention in the coordination sphere of Fe atom during the catalytic process.
An interesting aminocarbene ligand (XXII) was used by Bhunia et al. [215] in the presence of KOtBu:

Complex Fe(CО)4L with this ligand catalyzed phenylacetylene dimerization at high selectivity with respect to isomer I (82–84%) and large turnover number (6500). The work included discussion of the mechanism of II → I isomerization via metal carbene intermediate and quantum-chemical analysis of the mechanism. However, for unclear reasons, the consideration referred to trans-addition of the ethynyl fragment to alkyne π-complex.
Recently, Liang et al. [216] studied the catalysis of alkyne dimerization by pentamethyl cyclopentadienyl complexes Fe–Ср*Fe(HL)Cl and Ср*Fe(L), where HL = XXIII,

and established extremely high selectivity of reaction with respect to heme-isomer III (>99%) in the case of Ср*Fe(L) complex, which was also highly selective in the cross-dimerization of propargyl alcohol with arylalkynes in which isomer III (ArC≡CC(CH2OH)=CH2) was formed at 90–94% yield. Similar to the case of other metals, the nature of ligands was a significant factor determining selectivity of the process.
In concluding this section, it should be noted that the interest in catalysis with iron complexes in various reactions of organic synthesis has incredibly increased: recent (2015) updated review [217] contains 1651 references.
3.3. Complexes of Rh, Ir, and Co
Catalysts based on Rh(I) complexes have been used in alkyne dimerization (and oligomerization) reactions already at the second and third stages of development of the chemistry of acetylene and its derivatives. Probably, Singer and Wilkinson [218] carried out the first reaction of alkyne dimerization with Rh(I) complex (Wilkinson’s complex RhCl(PPh3)3). It turned out that phenylacetylene was rapidly polymerized and substituted α-hydroxypropargyl amines rapidly dimerized with the formation of isomer I (73%) and π‑complex with RhCl(PPh3)2. Based on these results, the so-called ethynyl dimerization mechanism A was proposed with the formation of an intermediate product of oxidative addition of RC≡CH to Rh(I).
Albano and Aresta [219] studied activity of the cationic complex of cis-Rh(diphos)(CO)2BPh4 in the nonselective oligomerization of МеС2Н into СН2Cl2 and obtained 50% dimers (I/III = 3), 8% linear trimers, 42% isomeric trimethylbenzenes at 60оС. Analogous dimers are formed from α- and β-hydroxy substituted terminal alkynes. At 80°С, the reaction with β-hydroxy derivatives yielded same isomers in III/I ratio from 55/45 to 75/25 [220]. Kinetic measurements showed second order with respect to the alkyne concentration for these substrates. It was also found that additions of AsPh3 and PPh3 ligands to Rh(C8H14)2Cl complex influence the ratio of trimers and dimers: for [L]/[Rh] = 6 and variable [AsPh3]/[PPh3], the ratio of trimers and dimers changes from 1/19 до 6.7/1 [220].
In 1990, it was established [221] that reaction with rhodium complex RhCl(PМе3)3 in acetone yielded a sum of isomers I and III (95–98%) but (in contrast to alkyl alkynes) phenylacetylene did not exhibit dimerization. The analysis of 1Н and 31Р (1Н) NMR spectra measured during 1-pentyne reaction (–65°С) showed evidence of the formation of a π-complex. At –10оС, this complex converted into π-complex isomers Rh(PМе3)3(RC≡CH\()_{2}^{ + }\)Cl– and a mixture of ethynyl complexes НRh(PМе3)3(C≡CR)(RC≡CH)+Cl– (а, b) with the isomer ratio (а)/(b) ≈ 1.5, which is close to the ratio of concentrations of isomeric dimers I/III. An increase in the temperature to 25°С leads to the formation of ethinylvinyl intermediates (c, d),

the first of which, complex (с), leads to product III, while complex (d) leads to product I. The obtained results confirmed realization of the vinyl dimerization mechanism B. Boese and Goldman [222] also confirmed cis-addition of alkyne at Rh–Н bond (in both Markovnikov and anti-Markovnikov mode) and showed that reaction with dimeric complex (RhClL2)2 at 25–50°С yields isomers I and III. The complex with R = Ph yields 100% isomer III, complex with R = tBu yields 100% isomer I, and R = n–Pr leads to 37% isomer I and 63% isomer III.
Investigation of the reaction with π-allyl rhodium complex Rh(η3-C3H5)(PiPr3)2 [223] showed evidence of the operation of dimerization mechanism C via the formation of ethinylvinylidene intermediates. In 2005, same researchers [224] continued studying the intermediates and laws of alkyne dimerization and showed that the ethinylvinylidene intermediate converts via reaction with CO into a type XII complex (σ-C4):
The obtained E-isomer under the action of acids forms isomeric dimers (R = Ph):
Isomerization of the E-isomer of type XII under the action of a strong acid leads to a cumulene product [Rh](RC=C=C=CHR) subsequently converting into dimer IV. Same work thoroughly studied conversions of the ethinylvinylidene intermediate into product XII (E, Z) and then into dimers I, II and butatrienes during thermal and photochemical processes. Interesting conversions were also observes in the case of unsubstituted acetylene:
Here, the oxidative addition of MeI to complex RhCl(CO)L2 stimulates the reaction of dimerization with active complexes of Rh(III) [225]. Besides a small amount of alkyne RC≡CMe (acetone, tetrahydrofuran, СН2Cl2) formed in quantitative amounts in excess MeI, the process of dimerization by mechanism A in the presence of sodium methylate leads to products I and III (80/20, %). The nature of products and selectivity of dimerization significantly depend on the nature of solvent. In acetonitrile, phenylacetylene and methyl iodide yield only methylphenylacetylene, while the reaction of alkynes in methanol with К2СО3 addition in the presence of methyl iodide yields product II (80–99%). Formation of this product was considered as evidence of the operation of mechanism C.
Investigation of the reaction of alkyne addition to diesters of acetylenedicarboxylic acid [226] in solutions of pincer complexes Rh(III)(NCN)(OAc)2(H2O), where NCN is bis-(oxasolinyl)phenyl, allowed Z-isomer of the dimerization product to be obtained with 98–100% yield. There are two pathways of ethynyl intermediate formation in the framework of mechanism A:
In the presence of H2, the ethynyl ligand is formed as a result of the metathesis of σ-bonds H–[Rh] and Н–С≡ according to equation
The rather seldom dimerization product III is formed selectively (90%) in the case of N-substituted propargylamines RNHCH2C≡CH in solutions of RhClL3 and [RhCl(COD)]2 complexes in the presence of 1,1-bis-(diphenylphosphino)ferrocene (dppf) [227]. Isomers III and I in a percentage ratio of 56/8 form from arylalkynes in the case of 8-oxyquinolinate complexes of Rh(I) with COD and in the presence of PPh3 [228]. Catalysis of alkyne dimerization was also observed in solutions of Rh(I) carbene complexes [Rh(NHC)(Cl)(COE)]2 and Rh(NHC)(Cl)(COE)(Py) (XXIV), where COE is cyclooctene and NHC is 1,3-bis-(2,6-diisopropylphenyl)-2-imidasole [229]. For example, complex XXIV catalyzes the formation of 82% III, 4% trimers, and 10% 1,2,4-Ph3С6Н3 from phenylacetylene in С6D6 at 40°С. In excess Py, the same complex leads to 92% III and 6% trimers. In the case of R = tBu, Me3Si, the reaction at alkyne conversion 90–100% leads to product III at 84–98% yield. Intermediates of this process were synthesized and the NMR spectra of π-complexes with PhCH2C≡CH and product I (tBu) and of ethynyl complex Rh[NHC](Cl)(H)(C≡CSiMe3)Py2 were obtained. It has been concluded that complex XXIV is an active universal catalyst operating in the framework of mechanism A for 3—6 h at 100% conversion with 92–95% selectivity.
Catalysts based on Ir and Co complexes were only used in several investigations of the catalysis of dimerization reactions. For example, complex Ir(η2-SNC5H4)(PPh3)2 [230] catalyzed alkyne dimerization by mechanism B with the formation of isomer I (94–100% for R = Ph, tBu, and n-Bu) via intermediate НIr(η2-SNC5H4)(С≡СR)L2.
Detailed experimental and quantum-chemical investigation [231] of the activity of pincer iridium hydride complexes Ir(РСР)(Н)2 (РСР = (2,6-СН2РtBu2)2С6Н3) gave interesting results. Reaction of this metal hydride complex with norbornene (NBE) yields norbornane and π-complex Ir(РСР)(NВЕ), which acts as the active catalyst of PhC2H dimerization with the formation of product I in the framework of mechanism B:
This investigation led to some important observations and conclusions:
(i) The ethynyl hydride complex was more stable (by 29 kcal/mol) than its phenyl analog H[Ir]Ph.
(ii) The reaction of alkyne addition at H–[Ir] bond is fast and yields 1,2-addition product [Ir]–С(Ph)=CH2, but this process is reversible.
(iii) As a result of β-elimination H–[Ir], the 1,2-addition product converts into a π-complex and then slower transforms into the 2,1-addition product [Ir](C≡CPh)(CH=CHPh), which exhibits fast reductive elimination to yield product I.
(iv) Constraints in the formation of С–С bonds is a general feature of the intermediates of type [Ir](C≡CR)(С(R)=CH2) which are related to the screening of Ir atom with substituents of PCP ligands.
(v) Computer simulations showed that the vinyl group in transient state of stage (3) must rotate by 90о for attaining the necessary orientation. This rotation is hindered by significant screening with substituents in the case of α-substituent in the vinyl group. Although the reductive elimination with the formation of С–С bonds reduces stress in the coordination sphere of the metal, the sterical hindrances inhibit the process of dimer elimination.
It should be noted that the observed effects do not require reversibility of the stage of alkyne addition at the М–Н bond. The products of 2,1- and 1,2-addition can also be formed in parallel. In this case, the product of 2,1-addition forms slowly but rapidly converts into a dimer, while the product of 1,2-addition appears rapidly but then slowly transforms into a dimerization product. Naturally, reversibility of the stage of addition would be useful, since it hinders the accumulation of waste intermediates (see kinetics of the asymmetric hydrogenation of olefins [8]).
Investigation of the hydrosilylation of alkynes in solutions of Co(I) complexes [232] established that СоСl(РМе3)3 complex catalyzed phenylacetylene dimerization with the formation of isomer I (78%) and alkyne cyclomerization to 1,3,5-Ph3С6Н3 (19%), while СоСl(РМе3)2(СО)2 complex predominantly catalyzed the formation of isomers I ((76%) and II (13%). The reactions of alkyne dimerization and cycle formation were also catalyzed by CoBr2(dppe) complexes in the presence of Zn0 and Mg0 reducers [233]. The yield of dimer I from phenylacetylene amounted to 47% and the yield of cyclic trimer was 41%.
In concluding this section, it should be noted that the tryad of metals under consideration participates in the catalysis of alkyne dimerization with predominant formation of isomers I, III, IV and linear trimers in complexes with various ligand environments and metals in oxidation states I and III.
3.4. Complexes of Ni, Pd, and Pt
Catalysts based on Ni(0, II) complexes were already studied in reactions of alkyne dimerization and oligomerization [1, 52] at the second and third stages of development of the chemistry of acetylene compounds [234—237] beginning with W. Reppe’s works on carbonylation and cyclization of acetylene and alkynes (see [1, 47, 56]).
A comparison of the reactivity of metals in Group 10 of the Periodic Table (see review [43]) shows that the activation energy of the stage of reductive elimination in reaction
increases in the following order (kcal/mol): Ni (16.5) < Pd (24.9) < Pt (45.8). The same sequence describes growth in the activation energy of the reverse reaction of oxidative addition (kcal/mol): Ni (20.9) < Pd (43.9) < Pt (49.3). The strongest π-complexes with alkynes (and alkenes) are also formed by Ni(0) [43]. The most frequently encountered nickel complexes can be ordered as Ni(II) > Ni(I) > Ni(III) [43]. As for palladium complexes with various metal oxidation states, their accessibility and occurrence can be presently ordered (by data of the author of this review) as Pd(II) > Pd(0) > Pd(I) > Pd(IV) > Pd(III).
The aforementioned properties of nickel complexes also account for the high activity of Ni(CO)2(PPh3)2 complexes in the reactions of linear and cyclic oligomerization of terminal alkynes [234, 235] and polymerization of unconjugated terminal alkynes [236]. In the case of alkyl substituents С2–С5, dimeric products were found to contain isomers I and III [235], while linear trimers contained three branched isomers with structures determined using spectroscopic and chemical methods.
Results of a series of investigations devoted to alkyne reactions in the Zigler catalyst system NiX2–AliBu3 were summarized in [237], including interesting data on the mechanism of formation of Е-2,4-dialkyl-1,3-butadiene from alkynes simultaneously with trialkylbenzenes (after the hydrolysis of organoaluminum intermediates). It was shown how alkylhydride complexes HNi(iBu)Ln and \({\text{NiL}}_{n}^{0}\) are formed from Ni(II) complexes with acetylacetonate and salicylaldiminate ligands. Based in the results of isotope experiments with RC≡CD and H2O/D2O, it was concluded that, in addition to the vinyl mechanism of С–С bond formation in the diene obtained upon the hydrolysis of intermediates iBu2AlC(R)=CH–C(R)=CH2 and RCH=CH–C(R)=CHAlIBu2, a part of the diene was formed upon the hydrolysis of cyclic products (XXV) obtained by oxidative addition of alkynes to a complex of \({\text{NiL}}_{n}^{0}\) and the corresponding cyclic organoaluminum compounds

In the case of Ni(CO)2(PPh3)2 complex with a large ratio of AliBu3/Ni = 60, the process of 1-hexin dimerization yields, in addition to dienes, isomer III at 17% yield with a mixture of isomeric tributylbenzenes.
Ogoshi et al. [238] carried out a selective room-temperature synthesis of dimer I in a highly active catalytic system Ni(COD)–PtBu3–C6D6 in which the product yield for 5 min reached 88—98% (depending on the nature of alkyne substituent). Some alkynes exhibited side conversion into trimers and oligomers. This catalyst gave the first example of Bu3Sn(C≡CH) dimerization with 88% yield of isomer I. The proposed dimerization mechanism B consisted in the cis-addition of alkyne at H–Ni(C≡CR) bond with the formation of (RCH=CH)Ni(PtBu3)(C≡CR) intermediate complex. In the absence of solvent, the obtained dimer can react with arylbromide (in the presence of Pd(0) complexes) in dimethylformamide, leading to products of the Migita–Stille reaction (cross-coupling with participation of SnR4).
Palladium complexes as catalysts of alkyne dimerization were mostly studied (unlike nickel complex catalysts) at the fourth stage of development of the chemistry of acetylene compounds, but the variety of metal complex types, alkynes, and the possibility of controlling both regio- and stereoselectivity of reactions attracted much research attention to palladium catalysts. Note that catalysis with palladium complexes in the chemistry of alkynes is generally well developed: recent (2014) updated review [41] on this subject contains more than 600 references.
As long ago as in 1984, Sabourin [239] showed that palladium acetate in solvents such as CH3CN, C6H6, and Et3N in the presence of phosphonyl ligands (e.g., iPr2(Ph)P=O) catalyzes the formation of dimers III and trimers of propargyl alcohols; the ratio of dimers to trimers significantly grows on adding Cu(I) and with increasing number of substituents at γ-carbon atom bound to ОН group. Under optimum conditions, this ratio can reach 99/1.
The nonselective cross-dimerization also proceeds with the formation of four possible isomers of geminal dimers. It was suggested that the process could involve an intermediate RC≡CPdH complex, but no any grounds were provided. It is interesting to note that acetylene reaction in this system yields only monovinylacetylene.
Trost et al. [240] carried out a systematic investigation of the catalytic properties of Pd(OAc)2 complexes with phosphine ligands in reactions of homodimerization and cross-coupling (addition of terminal alkynes to disubstituted acetylenes), where phosphine was represented by sterically loaded tris-(2,6-dimethoxyphenyl)phosphine (TDMPP).
The homodimerization of various alkynes with alkyl, aryl, and alkylhydroxyl substituents and unconjugated olefin groups led to geminal isomers III with up to 90% yields. The cross-coupling of donor alkynes with those containing electron-acceptor substituents (electron-withdrawal groups, EWGs)

led to enynes with 70–95% yields, which was evidence of the important role of EWG substituent (СО2СН3, SO2Ph) in stabilization of a carbanion bound to с Pd(II). This stabilization probably also takes place in the case of TMSC≡CH homodimerization with the formation of isomer I:

Analysis of various hypotheses led to selecting the ethynyl mechanism A (Scheme 10, A = alkyne). It should be noted that all variants involved the nucleophilic addition of RC≡C– according to the Markovnikov rule (except for TMSC≡CH) with the anticipated formation of Pd(IV) (see review [241]).

Scheme10. Homodimerization according to mechanism А in solutions of palladium complexes (A = alkyne).
The depalladation of enyne intermediate [Pd](σ-C4) can also be produced by AcOH evolved at the first stage or directly by alkyne—as synchronous reaction of nucleophile-assisted electrophilic substitution (Seia)—so-called metathesis of σ-bonds. The possibility of alkyne activation by Pd(0) complex of Pd2(dba)3 ∙ CHCl3 with TDMPP (1 : 1) was also checked, and it was found that the coupling reaction proceeds slowly and not completely. The oxidation of Pd(0) with allyl acetate in this system leads to acceleration of the process and provides 94% alkyne conversion.
However, Herrmann et al. [242] succeeded to find conditions for the selective dimerization of arylalkynes with the formation of isomer I and showed that PdL4 and Pd2(dba)3 ∙ CHCl3 complexes with P(o-Tol)3 ligands also form isomer III, while the same complex Pd2(dba)3 with TDMPP ligand leads (along with III) to product I at 70% yield (for PhC2H), and 91% yield (for 4-NCC6H4C2H). The best yield of linear E-isomer I was obtained for Pd/L = 1/5. In contrast to the aforementioned work [240], where allyl acetate was used for the oxidation of Pd(0) to Pd(II), Herrmann et al. [242] used π-allyl Pd(II) complex for its reduction with diethylamine to Pd(0) and allylamine in the presence of 5-fold excess of TDMPP with the formation of bidentate complex

and obtained isomer I at 93% yield. It should be noted that the introduction of ortho-substituents into ArC≡CH phenyl ring leads irrespective of their nature (Ме, МеО, F) to complete arrest of the dimerization process. It was suggested [242] that the resioselective addition of ArC≡CH via ArC≡C–Pd bond is favored by agostic interaction of the ortho-Н-aryl group with Pd in the intermediate ArC≡CPd(L)H(η2-ArC≡CH). In addition, it was shown that Pd(II) did not exhibit reversible addition via о-С–Н(D) bond with the formation of Pd(IV) and it was believed that the agostic interaction rationally explained excellent regioselectivity observed in the system.
Rubina and Gevorgyan [243] used carbene complexes PdI2(NHCR2) with R = Me, tBu, and 2-ethylphenyl in Et3N solution in the presence of PPh3 for studying the dimerization of phenylacetylene. Complex PdI2(NHCR2)(PPh3) with R = 2-ethylphenyl catalyzed the formation of dimer I at 95% yield per sum of dimers. Additions of CuI led to the appearance of 1,4-diphenyldiyne formed quantitatively in the presence of 5 mol % CuI. Another method for in situ synthesis of carbene ligands was proposed [244] based on the reaction of Pd(OAc)2 with imidazolium chloride (R = Cy, Tol, Mes, iPr)

In dimethylacetamide at 80°С in the presence of Cs2CO3, hept-1-yne yielded 90% of isomer I and small amounts of isomers II and III. Close results were also obtained in dimethylformamide, tetrafuran, and dioxane. Note that К2CO3 changed the regioselectivity of dimerization that led to the formation of dimer III at 89% yield. The catalytic system was active with respect to various alkynes. The influence of the nature of cation in carbonates on the regioselectivity was not explained. In reactions of ArC≡CH in the presence of К2CO3, the percentage ratio of isomers I/III varied from 70/26 to 60/34.
A rather universal catalyst for the synthesis of isomers I from a very wide circle of alkynes (22 compounds) was proposed [245] based on bis-carbene Pd(IPr)2 complex (Pd(0) with IPr ligands):

This complex in toluene at 60°С in the presence of TDMPP leads to the formation of isomer I at 51–94% yield. The absence of ortho-H in aryls does not prevent catalytic reaction (in contrast to that with a catalytic system described in [242]). The proposed vinyl mechanism B includes the hydropalladation of alkyne according to Markovnikov with allowance for the hydride character of H atom in Pd–Н bonds. This mechanism was confirmed by calculations in the framework of density functional theory (DFT). Note that the predominant hydropalladation of alkynes in ethynyl hydride complexes of Pd(II) and Pt(II) was demonstrated in the reactions of these complexes with RCO2C≡CCO2R [246, 247]. Even the reaction in dioxane at 100°С did not reveal carbometalation products [246]. In continuation of the initial work [245], the investigation of catalytic properties of the proposed system was expanded [248] in view of observations [244] of a strong influence of additions and the nature of bases on the regioselectivity of dimerization. It was established that, in the systems studied in toluene with variable coordination sphere of Pd(0) complexes, the additions of cesium and potassium carbonates did not affect regioselectivity of the process (isomer I), while the addition of carboxylate anions sharply changed the product nature to isomer III (at 100% yield for cesium pivalate). This result was rationally explained by the influence of bases on the regioselectivity of dimerization. A base that splits a proton from intermediate RC≡C[Pd]H changes the process from hydropalladation of alkyne (Markovnikov reaction) with the formation of product I (mechanism B) to carbometalation of alkyne in anionic intermediate (anti-M reaction) with the formation of isomer III (mechanism A). This hypothesis was confirmed by DFT calculations.
Alternative regioselectivity of hydropalladation with a vinyl mechanism of dimerization was observed during the synthesis of vinyl ethynyl intermediate PhC≡CPd(L)2–[C(Ph)=CH2] via reaction of Pd(PEt3)4 with PhC≡CH in the presence of acid Ph2P(O)OH in С6D6 at 25°С [249], there the intermediate product structure was determined by X-ray diffraction (XRD). Palladium complex Pd2(dba)3 in the same system in the presence of dppe ligands also selectively catalyzed the formation of isomer III at 40–93% yield depending on the substituents in alkyne. Note that, in the case of cross-coupling of PhC≡CH with PhC≡CR in the presence of Ph2P(O)OH, the palladium atom also binds to a carbon atom possessing the maximum effective negative charge with the formation of intermediates (for R = P(O)(OEt)2 and COCH3)

There are the following reasons for the influence of a Brønsted acid on the regioselectivity of alkenyl group formation: (a) less pronounced hydride character of H-atom in НPdLn+ complex formed as a result of Pd(0) complex protonation and (b) outer-sphere acid (HA) protonation of π-complex (Scheme 11).

Scheme11. Dimerization mechanism B with outer-sphere acid (HA) protonation of π-complex.
Chen et al. [250] convincingly demonstrated that the reactions of Ph2C2 with RCO2H and M(РEt3)4 (M = Ni, Pd, Pt) in C6D6 at 25°С yield entirely cis-alkenyl products. In the case of palladium complex, reaction with PhC≡CH yields only the anti-M type product:

Investigations of Pt(0) complexes showed that hydrometalation proceeds via the reaction of π-complex with RCO2H acid, i.e., according to variant (b). In particular, Pt(II) hydride complex formed in the reaction of PtL4 with AcOHdoes not interact with Ph2C2 with the formation of alkynyl complex for 20 h at 25°С and for additional 10 h at 60–100°С, while the π-complex of Pt(0) reacts with AcOH at 25°С. Probably, Pd(0) complex obeys an analogous mechanism for the formation of alkenyl intermediates. The synthesis of cis-alkenyl complex in the case of Ph2C2 is most probably determined by the electrophilic proton substitution for metal in the π-complex with a metalacyclopropene structure [1].
3.5. Complexes of Metals of Groups 4—7 of the Periodic Table
There were only separate cases of testing metals from Groups 4—7 as catalysts of alkyne dimerization reactions [251–255]. Kawata et al. [251] reported that Re2(CO)10 complex in the presence of Bu4NF in toluene at 80°С catalyzed the formation of isomer I at 50% yield, while ReBr(CO)3(THF)2 complex led to selective synthesis of product I with 98% yield. Various arylalkynes with both electron-donor and electron-acceptor substituents were also dimerized at high yields. Catalysis of ethylpropionate reaction under identical conditions led to 1,3,5-isomeric cyclotrimer at 60% yield.
Already at the second stage of development of the chemistry of acetylene compounds, it was shown [252] that alkylacetylenes exhibited dimerization in the Cr(tBu)4–ZnEt2 system at 30°С with the formation of isomer III at almost quantitative yield for a ratio of ZnEt2/Cr(tBu)4 = 3. In the case of using AlEt3 instead of ZnEt2, only trialkylbenzenes were formed.
Zigler catalyst systems based on Ti(IV) cyclomethylcyclopentadienyl complexes also proved to be active in the homo- and cross-dimerization of alkynes [253]. In particular, various alkynes exhibited dimerization with the formation of isomer III at regioselectivity >99% and yields within 92–99% in the \({\text{Cp}}_{2}^{*}{\text{TiC}}{{{\text{l}}}_{2}}\)–iPrMgBr system with Et2О at 30оС. It is interesting to note that the use of unsubstituted η5-С5Н5 ligands led to the oligomerization of alkynes. Various combinations of RC≡CH and R/C≡CH were studied in the reaction of cross-dimerization and it was found that R/C≡CС(R)=СН2 dimer was formed at maximum regioselectivity (92%) for R/ = Me3Si and R = n-Bu. Akita et al. [253] used isopropylmagnesium bromide-reduced \({\text{Cp}}_{2}^{*}{\text{TiC}}{{{\text{l}}}_{2}}\) to obtain \({\text{Cp}}_{2}^{*}{\text{TiH}}\) complex that was subsequently converted via reaction with RC≡CH (by various mechanisms) into \({\text{Cp}}_{2}^{*}{\text{TiC}} {\equiv} {\text{C}}{{{\text{R}}}^{/}}\) complex. The latter complex was suggested to be an active catalyst of alkyne dimerization by ethynyl mechanism with Markovnikov variant of R/C≡C– nucleophile addition to alkyne in Ti(III) π-complex. The synthesized ethynyl complex (via LiC≡CR/) successfully catalyzed the dimerization process. It was shown that the addition of RC≡CH at Ti–C≡CR bond proceeds via cis-addition.
Horton [254] showed that isomer III could also be obtained in solutions of cationic complex Zr(IV), \({\text{Cp}}_{2}^{*}\)ZrMe+A– (A = B[4-C6H4F] (XXVI)). The reaction of complex XXVI with 5- to 10–fold excess of RC≡CH (R = tBu, Pr) in C6D5Br at –30оС led to the formation of organometallic compound XXVII

together with alkyne oligomers. In the case of R = Me3Si, compound XXVII reacts with excess alkyne at 25оС to form new product XXVIII at 50% yield by adding third ≡CH molecule at Zr–СН= bond. It was established that complex XXVII, as well as XXVI, catalyzes the dimerization and trimerization of alkynes for 5 min at 25оС. The catalyst turnover number for tBuС≡СН was 1300 at a selectivity of isomer III formation above 99.5% (TOF = 15600 h–1). In the case of R = Ме, the reaction yielded 72.5% trimers. It was suggested that the formation of dimer III from intermediate XXVII (or trimer from XXVIII) proceeded via metathesis of σ-bonds in the reaction with alkyne with the formation of active catalyst [Zr](C≡CR)+. Insertion of the third alkyne molecule at Zr–СН= bond in compound XXVI proceeds as anti-M addition:

Similar to other catalytic systems, the ligand environment of zirconium atom significantly influences regioselectivity of the dimerization process. For example, Z-isomer II was obtained at 92% yield from solutions of complex XXIX

with bis-ureate ligand, which converted in the presence of aniline in toluene into a binuclear bridged amide—imide dimeric complex capable of catalyzing alkyne dimerization [255]. Based on the results of determination of the structure of this complex (Z-isomer II) by XRD and 1Н NMR spectroscopy, the regioselectivity of a reaction involving two Zr atoms in the intermediate complex and trans-addition of nucleophilic RC≡C– to π-complex fragment [Zr](НC≡CR) in anti-Markovnikov reaction direction (Scheme 12).

Scheme12. Dimerization mechanism A in reaction with initial complex XXVIII [255].
3.6. Complexes of Metals of Groups 3 and 13 of the Periodic Table
The classical work [256] devoted to studying the metathesis of σ-bonds in reactions of \({\text{Cp}}_{2}^{*}\)ScR (R = H, alkyl, aryl, vinyl, alkenyl) with H2, C6H6, RCH=CH2, and RC≡CH showed that \({\text{Cp}}_{2}^{*}\)ScCH3 reacted with propyne within several minutes at T < 0°С with the formation of \({\text{Cp}}_{2}^{*}\)ScC≡CCH3 complex capable of catalyzing the selective dimerization of propyne at 25°С with the formation of isomer III. The proposed dimerization mechanism includes the insertion of propyne at Sc–С bond (Markovnikov type reaction) with the formation of \({\text{Cp}}_{2}^{*}\)Sc[CH=C(CH3)C≡CCH3] complex and protolysis of this alkenyl intermediate by propyne via mechanism of s-bond metathesis—i.e., without preliminary alkyne coordination by scandium in the state of oxidation degree III. In contrast to Zr and Hf complexes capable of readily inserting propyne at the М–Н bond, \({\text{Cp}}_{2}^{*}\)ScH rapidly reacts with propyne to form a propinyl complex and Н2. Reactions of these Sc complexes with acetylene [257] at –78°С revealed the formation of acetylenide compounds \({\text{Cp}}_{2}^{*}\)ScC≡CH and \({\text{Cp}}_{2}^{*}\)ScCºCSc\({\text{Cp}}_{2}^{*}\). The symmetric product is synthesized at increased concentration of \({\text{Cp}}_{2}^{*}\)ScC≡CH via reaction
also as a result of the metathesis of Sc–C and ≡C–H bonds. In the presence of excess acetylene, the oligomerization and polymerization processes proceed via cis-addition of acetylene at Sc–C bonds. Note that the metathesis of σ-bonds was repeatedly assumed in interpretation of the mechanisms of alkyne dimerization in various catalytic systems.
The catalysis of alkyne dimerization by analogous yttrium (Y) complexes in nonpolar solvents also proceeded regioselectively with the formation of isomer III product [258]. The reaction of \({\text{Cp}}_{2}^{*}\)YCH(SiMe2)2 with propyne in benzene at 25°С yielded \({\text{Cp}}_{2}^{*}\)YC≡ССН3 and 2-methyl-1-penten-3-yne (isomer III), while the reaction with phenylacetylene gave an analogous dimer at 89% yield. The TOF for propyne dimerization was 5400 h–1.
The catalytic properties of yttrium are frequently considered in comparison to those of other rare-earth metals such as lanthanides and actinides [259–263]. For example, phenylacetylene reactions with \({\text{Cp}}_{2}^{*}\)МCH(SiMe2)2 (М = Y, Ln, Ce) complexes [259] were accompanied by the formation of alkyne oligomers and \({\text{Cp}}_{2}^{*}\)МC≡СPh]n complexes, but the regio- and chemoselectivity depended on the nature of alkyne substituent R and the nature of metal. In the case of Y complexes and RC≡CH, the reactions only afforded dimer III (R = tBu, Pr) or a mixture of dimers III and I (R = SiMe3, Ph), while Ln and Ce complexes for R = SiMe3 and Ph also yielded products III and I, but in combination with trimers.
Analysis of the IR and 1Н NMR spectra of oligomeric ethynyl complexes of Ce [259] led to a conclusions about their dimeric nature with ethynyl bridging ligands, significant contribution of the π-system of ethynyl ligand to the coordination of metal, and close rates of tBuC≡CH dimerization in solutions of the initial \({\text{Cp}}_{2}^{*}\)СеCH(SiMe2)2 complex and ethynyl complex.
Another original mechanism leading to the formation of 1,4-enynes (mechanism D, not encountered previously) was discovered in investigations of the properties of ethynyl complexes of Sm [260, 261], Ce, and Nd [261]. XRD data showed that, in contrast to the aforementioned dimeric structure with ethynyl bridging ligands [\({\text{Cp}}_{2}^{*}\)Ln(μ-C≡CR)]2 [259], any processes leading 10 \({\text{Cp}}_{2}^{*}\)Sm(C≡CR)(THF) complex resulted eventually in the formation of [\({\text{Cp}}_{2}^{*}\)Sm]2(μ-η2,η2-RC=C=C=CR) complex (XXX). The action of two phenylacetylene molecules on complex XXX leads to PhCH=CHC≡CPh. Probably, the metathesis of Sm–C and Н–С≡ bonds in complex XXX leads to the formation of \({\text{Cp}}_{2}^{*}\)SmC≡CPh and complex XXXI, after which 1,3-shift [Sm] within π-allyl complex XXXI results in the formation of complex XXXII

and the protolysis of complex XXXII by the second alkyne leads to a product of reaction according to equation (XIV):
The synthesis of intermediate complex XXX resembles the Glaser—Zalkind reaction of oxidative dimerization of alkynes (see [51]) where one possible mechanism involves the dimerization of ethynyl complexes RC≡CMXn via bicyclic σ-organometallic compound XXXIII [1]:

In the case of Co(II) oxidant and catalysis with Cu(I) complexes, the mechanism of oxidative dimerization of alkynes can be represented by Scheme 13 [1].

Scheme13. Probable mechanism of the oxidative dimerization of alkynes.
The decay of an analogous bicyclic complex in reactions of complexes \({\text{Cp}}_{2}^{*}\)LnC≡CPh (Ln = Sm, Ce, Nd) can also lead to 1,4-enyne by Scheme 14.

Scheme14. Possible mechanism D of alkyne dimerization with participation of two ethynyl intermediates.
Investigation of the catalytic properties of monocyclopentadienyl complexes of Y, Yb, and Lu in the reactions of alkyne dimerization [262] showed that these metal complexes provided very high selectivity in the formation of isomers II from alkynes with aryl and alkyl substituents. Especially high activity was observed for Lu complexes XXXIV

(where TНF is tetrahydrofuran), which ensured 100% selectivity with respect to isomer II at 99% conversion (80°С, C6D6). There was convincing evidence that a real catalyst in this case represented dimeric ethynyl bridging complex XXXV (studied by XRD).

It was suggested that π-bound alkyne inserted at Lu–С≡ bond with subsequent metathesis of σ-bonds. Review of catalysis with complexes of Sc, Y, La, Ce, Yb, Lu, and Pr [263] showed that, depending on the ligand environment of metal atom in oxidation state III and the nature of substituents in alkyne, the formation of both isomers I and II with 100% regioselectivity and a small amount of oligomers is possible. Dimeric ethynyl complexes of Lu precipitated on cooling solutions to room temperature and could be readily separated from reaction products [263]. Same researchers also demonstrated the possibility of dimerization of 1,2-diethinyl aromatic compounds in solutions of mononuclear Lu and Pr complexes with the formation of polymers with molecular masses Мn = 5300–16 000 D at 95–99% yield and 95–100% selectivity.
Ge at al. [264] showed the role of sterically constrained ligands in the control of stereo- and regioselectivity in the case f catalysis with yttrium complexes. While the reaction of \({\text{Cp}}_{2}^{*}\)YCH(SiMe2)2 complex with heteroaromatic alkynes led to predominant formation of isomers I and III [259], the use of sophisticated macrocyclic imide ligand L with four nitrogen atoms in Y(L)CH(SiMe2)2 complex afforded only isomer II with 100% selectivity.
At the end of this section, let us consider two elements of Group 3 belonging to 6d-metals of actinide (An) subgroup, thorium (Th) and uranium (U) [265–268], and aluminum (Al)–nontransition metal of Group 13 [269].
Haskel et al. [265] synthesized and studied Th and U complexes \({\text{Cp}}_{2}^{*}\)Аn(μ-C≡CR)2 possessing approximately equal catalytic activity in reactions of alkyne oligomerization: tBuC≡CH at 80оС formed isomer III at 98–100% yield, while Me3SiC≡CH regio- and stereoselectively converted into Е,Е-1,4,6-tris-(trimethylsilyl-1,3-hexadien-5-yne) at 90–95% yield. The first stage in the mechanism of Me3SiC≡CH trimerization was suggested to be alkyne insertion at [Аn]–C≡ bond according to first-order equation with resoect to both alkyne and [Аn]. The slow stage represented metathesis of σ-bonds with products formed from alkenyl-ethynyl intermediates. Analysis of the 1Н NMR spectra and stages of process termination in the presence of Н2О and D2O [265] suggested the presence of intermediates leading to enyne III and trimer \({\text{Cp}}_{2}^{*}\)Th(C(R)=CHCR=CRC≡CR)2 (compound XXXVI).

Same research group established [266] that the selectivity of formation of dimer III or trimer could be readily controlled by additions of amines RNH2 (R = Me, Et, 2-6-dimethylbenzene, tBu) and Me2NH not involved in the stoichiometry of dimerization reaction. According to [266], the role of amines consists in the formation of active amide complexes \({\text{Cp}}_{2}^{*}\)Th(NHR)2 and in the participation in protolysis of М–СН= bond in the intermediate complex (as confirmed by the results of isotope investigations with RNH2/RND2 pairs) leading to dimer or trimer, with the regeneration of amide ligand in the active complex. In the case of thorium catalyst, the kinetic equation has the following form:
which is explained by the formation of a non-active diamide–amine complex \({\text{Cp}}_{2}^{*}\)Th(NHR)2(RNH2) where the size and inductive properties of substituent R and the nature of substituent R/ in R/С≡СН significantly influence the chemoselectivity of the process. However, the problem of separately assessing the role of sterical effects of substituent R in amine and the influence of inductive effects on the reactivity of N–H bond in amine is yet unsolved.
Detailed investigation [267] of the catalytic properties of uranium complex [(Et2N)3U]+\({\text{BPh}}_{4}^{ - }\) in alkyne dimerization established predominant formation of isomer III, although the reaction with alkyne containing bulky substituents proceeded with the formation of isomer II. Analysis of the reaction mechanism showed that the first stage involving the formation of (Et2N)2UС≡СR+(А1) and Et2NН is weakly reversible, with the subsequent formation of uranium π-complex (В1) (Et2N)2U(С≡СR)(η2-RС≡СН)+\({\text{BPh}}_{4}^{ - }\) (R = tBu). Study of the kinetics of tBuС≡СН dimerization to isomer III established the first order with respect to [U]Σ and variable order (from 1 to 0) with respect to alkyne. Assuming that the formation of intermediate (А1) from [(Et2N)3U]+BPh4 and alkyne was shifted rightward, the reaction mechanism was proposed that included a two-stage cycle with intermediates А1 and (Et2N)2U–С(Н)=С(R)С≡СR+ (С1) and the aforementioned complex В1 not participating in this cycle (see [267] for notations).
Unfortunately, the kinetic equation (А11) obtained in [267] did not correspond to adopted assumptions. Under quasi-stationary conditions, stage (1) of the formation of π-complex В1 from А1 is always equilibrium (d[B1]/dt ≈ 0), while the cycle of stages (2) and (3) leads to the following equation for reaction rate W:
where [S] is the concentration of substrate (alkyne). Assuming that k3[S] ⪢ k–2, we obtain W = k2[S][A1] and [U]Σ = [A1] + [B1] = [A1](1 + K1[S]), while the experimental observations gave
Under the assumption of [U]Σ ≈ [B1] used in [267], the reaction order with resoect to substrate will be zero rather than variable. Equation (7) indicates that the rate-controlling stage in the system under consideration is reaction (2) (see [147] for the critical analysis of the notions of “rate-controlling,” “rate-determining,” and “limiting” reaction stage).
Dash et al. [268] studied the alkyne dimerization catalysis by dimeric bridged cyclopentadienyl complexes of thorium with a more open coordination sphere of the metal atom and snowed that the formation of isomer III with this catalyst was significantly faster and more selective as compared to the reaction catalyzed by \({\text{Cp}}_{2}^{*}\)ThМе2 complex. Indeed, Me2Si\({\text{Cp}}_{2}^{{//}}\)ThBu2 complex (Cp// = C5Me4) proved to be ~200 times more active for R = Bu and ~105 more active for R = iPr in comparison to the nonbridged complex. It was suggested that the observed reaction acceleration was related to the facilitated stage of С1 type intermediate (see above) demetalation by alkyne. In the case of bridged Cp-complexes, the catalyst represents a Me2Si\({\text{Cp}}_{2}^{{//}}\)Th(С≡СR)2 complex, which is quantitatively formed from a precursor at the irreversible stage.
Same research group also showed [269] that compounds of aluminum (nontransition metal of Group 13) in the form of methyl alumoxane (MAO) selectively catalyzed the dimerization of various alkynes (except Me3SiС≡СН) with the formation of isomer III (93–99%) in boiling benzene (С6H12), tetrahydrofuran (Et2O), and without solvent. It was suggested that alkyne regioselectively inserted at [Al]–C≡ bond of ethynyl compound [Al](C≡CR) (A), formed as a result of the metathesis of σ–bonds via reaction
The stage of demetalation of intermediate B with the formation of product III also resulted from the metathesis of σ-bonds with participation of alkyne according to Scheme 15. The catalyst TOF in this process is not large (not exceeding 5 h–1).

Scheme15. Mechanism of alkyne dimerization in AMO solutions (reproduced with permission from Org. Lett. 2 (6), 737 (2000) [269]. Copyright (2000) American Chemical Society).
4. CONCLUSION
The review of catalysts, mechanisms, and intermediates of alkyne dimerization reactions provides a basis for several generalizations. Considering present achievements in the field of alkyne homodimerization, it can be pointed out that a proper М–L system can be selected for any terminal alkyne, which will ensure chemo-, regio-, and stereoselectivity of the synthesis of one or several possible isomers (Scheme 4): I (E-), II (Z-), III (heme-isomer), IV and V (1,4-substituted butatrienes).
The selectivity of dimerization is controlled by three factors: (i) the nature of metal and the electron and sterical properties of ligands (catalyst); (ii) the nature of alkyne substituents (substrate); and (iii) reaction mechanism realized in the given reaction medium (mechanism). Even the same initial metal complex can feature various reaction mechanisms (see, e.g. catalysis with ruthenium complexes [188]). Solutions of various complexes with additions of acids or bases maintain dimerization via mechanisms A, B, C (Section 3.2) and D (Section 3.6). Some metals behave as catalysts featuring different mechanisms depending on the degree of oxidation and the set of ligands, e.g., Rh (A, B, C), Ru (C and A), Pd (A and B), Ir (B and C).
Examples of catalysis with Ru complexes (Table 3) show how difficult is to separate and explain factors determining selectivity of the dimerization process. For example, the same complex of Ru(II) provides the ratio of isomers I/II = 95/5 for alkyne with R = Ph and 5/95 for alkyne with R = n-Bu. For R = tBu, the catalysis with Ru(II) and Ru(0) complexes leads to isomers IV and V, respectively. An obvious fact is that isomer III cannot be formed via mechanism C. A simple explanation of stereoselectivity in the formation of products I and II obtained via mechanism C using Ru(II) vinylidene complex consists in variable geometry of this complex and its dependence on the sterical characteristics of ligands and alkyne substituents [18, 182, 183]:

At present, intermediates in various mechanisms have been studied in much detail: in situ, in compounds isolated from reaction medium (XRD), and on model compounds. Table 4 presents intermediates encountered in dimerization reactions. Summarizing data from Table 4, we can make the following conclusions:
1. Ethynyl (alkynide) complexes [M](C≡CR) (1) represent intermediates formed at various stages of the process in all the four dimerization mechanisms (А, В, С, and D) for metals of Groups 3–11 and 13 of the Periodic Table. Both mono- and bis-ethynyl complexes [M](C≡CR)2 are known [210].
Ethynyl ligands can form from alkynes by the following mechanisms:
– Substitution of metal ion for hydrogen ion
–Oxidative addition of alkyne to [M] with increase in the metal DO by two units
In the presence of ligands such as H or R in the initial complex, ethynyl groups can also form as a result of the metathesis of σ-bonds with evolution of Н2 or RH (see, e.g., [269]).
2. Compounds 2 and 3 are typical intermediates of ethynyl mechanism A, the formation of which has been repeatedly confirmed (Table 4). Complex 3 leads to dimers I and III (alkyne сis-insertion at metal—ethynyl bond) and dimer II (trans-addition of ethynyl group to π-complex). Complex 4 converts into dimer III. Regioselectivity of the insertion reaction (M or anti-M) depends on the distribution of electron density in [M]–C≡ fragment and π-complex 2 and, to a greater extent, on sterical factors.
The formation of isomers I (Е) and II (Z) during dimerization catalyzed by Cu(I) acetate in АсОН [128] in the framework of ethynyl mechanism A сan be understood provided that the reaction involves polynuclear Cu(I) complexes (by analogy with Scheme 5). The trans-addition of ethynyl group to π-complex is also probable in the case of binuclear complexes of Zr (Scheme 12 [255]) and Lu (complex XXXIV [262]). The formation of intermediate 3 in the case of Cu(I) (anti-M) can be explained by sterical constraints for alkylacetylene and by possible changes in the distribution of electron density due to a contribution from the mesomer with vinylidene carbanion, which modifies nucleophilic properties of the RC≡C– group so that it becomes a “softer” nucleophile:
Electron density distribution in the initial alkyne, except for that in the presence of electron-acceptor substituents (EWGs), is not a factor determining the regioselectivity of nucleophile addition to alkyne in the π-complex where this distribution can be significantly changed. The nucleophilic attack as such modifies the distribution of effective charges on carbon atoms in the π-complex, the more stronger, the “softer” the nucleophile is. For a very simple model reaction of soft nucleophile Н– addition to propyne, ab initio calculations of the potential energy surface showed [271, 272] that the difference of activation energies of СН3СН=СН– addition according to Markovnikov and in the opposite mode (M versus anti-M) was rather small and amounted to 1.7 kcal/mol [271] or 1.2 kcal/mol [272], respectively, the anti-M reaction being faster. Note that the terms of reaction “in the Markovnikov” (M) and “anti-Markovnikov” (anti-M) direction are used in the classical sense, so that the electropositive end E of ENu molecule in the non-activated terminal alkyne adds to the most hydrogenized carbon atom (1, M) and the nucleophilic group Nu adds to the least hydrogenized atom (2, anti-M). For detailed consideration of the problems related to application of the Markovnikov rule, see review [273].
3. Complexes 5 and isomeric compounds 6 represent intermediates of the vinyl mechanism B. The order of vinyl fragment appearance in intermediate 6 can vary in accordance with the mechanism of vinyl ligand formation:
– cis-insertion of alkyne at [M]–Н bond with variable regioselectivity;
– outer-sphere protonation of the π-complex, with the metal DO increase by two units [1, 249, 250];
– electrophilic substitution of metal in π-complexes with characteristics approaching those of the metalacyclopropene structure [1]

In order to confirm the mechanism of Н+(D+) trans-addition to alkyne in the π-complex, it is necessary to check that (i) the π-complex MLn(RC≡CH) has no free coordination vacancies for the formation of [M]–Н(D) bond and (ii) the cis-alkenyl ligand does not exhibit isomerization to trans-isomer [1].

The formation of enynes as a result of alkyne insertion at the [M]–alkenyl ligand bond was not established, probably because this is a rarely encountered case of [M]–Н bond β-elimination from this σ-butadiene intermediate, e.g., for acetylene reaction
At the same time, it was shown [231] that the reaction of PhC2H with iridium hydride complex involves rapid formation of the anti-M product of 1,2-cis-insertion of [Ir][C(Ph)=CH2], but the process is reversible (!) and the subsequent β-elimination of [Ir]–Н bond leads to slow conversion of the π-complex of iridium into 2,1-cis-insertion product [Ir](CН=CHPh) with the formation of isomeric complex 6 (Table 4) and final dimer I. The stage of β-Н elimination with the formation of alkyne π-complexes was also confirmed by isotope methods for alkenyl complexes of Zr(IV) [274]. In particular, \({\text{Cp}}_{2}^{*}\)Zr(CH=CHCH3)2 complex converted at room temperature into zirconacyclopentene which exhibited isomerization on heating via the reaction

The mechanism of β-Н elimination from alkenyl complexes can probably be represented by stages of the formation and conversion of η2-vinyl (alkenyl) complexes [275]:

The influence of bases on the transition from vinyl mechanism of alkyne dimerization to the ethynyl mechanism was considered in [248].
4. Vinylidene complexes 7 of metals have been studied in much detail [115, 200–202, 204, 276–278] and the operation of vinylidene mechanism C in alkyne dimerization reaction was repeatedly confirmed (Table 4). The nature of products of the interaction of vinylidene ligand and ethynyl group with the formation of various intermediate structures 8 and 9—11 containing [M](σ-С4) fragments, leading eventually to enynes I and II and cumulenes IV and V. There are three main mechanisms of vinylidene ligand formation from alkynes and π-complexes:
– 1,3-shift of hydride
– 1,2-shift of hydrogen atom in alkyne
– outer-sphere protonation of ethynyl ligand
The mechanism of π-complex isomerization to vinylidene was considered in [197[ and in subsequent quantum-chemical and experimental investigations of various metal complexes including Mn(I) [279], Ru(II) [280], and Rh(I) [281–284] (cited here without detailed analysis). An interesting experimental observation with theoretical consideration was recently reported by Tenorio et al. [284], where it was shown that Cl– ion or methanol played a positive role in the transfer of H atom ([M]–Н) to ethynyl ligand (1,3-shift of H atom) with the formation of vinylidene complex of ruthenium. A very interesting mechanism of vinylidene ligand formation was discovered in [285], where the reaction of RuX(H)(H2)L2 complex and PhC≡CD led to the formation of RuX(D)(=C=CHPh) and this isotope exchange was explained by mechanism of η1-vinyl intermediate formation according to Markovnikov with subsequent α-elimination of [M]–D and the formation of vinylidene ligand as
Results of аb initio (B3LYP) calculations showed exothermicity of all stages in this process. Osmium hydride complex OsH3ClL2 similarly reacted with alkyne, but via intermediate
η2-vinyl complex which exhibited subsequent α-elimination of [Os]–Н with the formation of vinylidene ligand. The formation of η2-vinyl complexes of alkynes is especially typical of cationic osmium complexes [286].
The conversion of vinylidene group into ethynyl ligand under the action of bases on vinylidene complexes was reported in [177, 181]. Therefore, a vinylidene complex can be a precursor in the ethynyl mechanism A of alkyne dimerization [181, 207]. On the basis of data on the kinetics of vinylidene → alkyne transformations studied in solutions of ruthenium complexes, some other variants of vinylidene dimerization were considered in [287] with allowance for the contribution of a mesomeric structure with α-carbocation center facilitating the 2,1-Н-shift:
It should be also noted that the vinylidene → alkyne conversion was also observed for disubstituted vinylidene ligands Ph(R)C=C= with the formation of PhC≡CR in cationic complexes of Ru and Fe, where this conversion was reversible (toluene + PR3 or CН3CN) [288].
5. The mechanisms of demetalation of [M](σ-С4) intermediates 3, 4, and 9–11 according to the results presented in this review can be quite diverse:
– Н[M]С4 → НС4 + [M],
– [M]С4 + RC≡CH → M](C≡CR) + НС4,
– [M]С4 + НХ → [M]Х + НС4.
The mechanism involving alkynes (metathesis of σ-bonds) is frequently encountered in catalysis with complexes of various metals (Ru, Rh, Sc, Sm, Th, U, Al). Investigation of the kinetics and mechanism of alkyne dimerization catalyzed by [RuCl(μ-Cl)(η6-п-cumol)]2 complex in acetic acid confirmed the role of АсОН at the demetalation stage [289].
6. Alkyne dimerization according to mechanism D, in which fragment С4(μ2-η2, η2-С4) in intermediate complex 12 is formed from two ethynyl complexes [M](C≡CR), have veer only observed in catalysis with lanthanide complexes, although the aforementioned bicyclic intermediate found in amine solutions of Cu(ОАс)2 is also capable leading via intermediate complex 12 to cumulenes and isomers I and II

as a result of the metathesis of σ-bonds with alkynes (or acidolysis). Investigation of the dimerization process catalyzed by Fe(III) complexes provided information on the possible realization of a radical mechanism of enyne synthesis [213].
Diversity of the mechanisms of one and the same reaction with variable metal catalyst and coordination sphere of the catalytic complex is a characteristic feature of the modern catalysis with metal complexes.
ABBREVIATIONS AND NOTATION
AO, atomic orbital,
Anti-М, Anti-Markovnikov direction of reaction,
CB, cationic background,
COE, cyclooctene,
CVA, 2-chlorovinyl acetylene,
DFT, density functional theory,
DMEDA, dimethylethylene diamine,
DO, degree of oxidation,
dppf, 1,1'-bis-(diphenylphosphino)ferrocene,
DVA, divinyl acetylene (1,5-hexadien-3-yne),
EWG, electron-withdrawal group,
KIE, kinetic isotope effect,
M, Markovnikov direction of reaction,
MAO, methylaluminoxane,
MVA, monovinyl acetylene,
NВЕ, norbornene,
РСР, (2,6-СН2РtBu2)2С6Н3,
RE, relativistic effect,
TDMPP, tris-(2,6-dimethoxyphenyl)phosphine,
TOF, (catalyst) turnover frequency,
XRD, X-ray diffraction
REFERENCES
Temkin, O.N., Shestakov, G.K., and Treger, Yu.A., Atsetilen. Khimiya. Mekhanizmy reaktsii. Tekhnologiya (Acetylene. Chemistry. Reaction Mechanisms. Technology), Moscow: Khimiya, 1991.
Tedeschi, R.J, Acetylene-Based Chemicals from Coal and Other Natural Resources, New York: Dekker, 1982.
Trofimov, B.A., Geteroatomnye proizvodnye atsetilena (Heteroatomic Derivatives of Acetylene), Moscow: Nauka, 1981.
Trofimov, B.A., Acetylene and its derivatives in reactions with nucleophiles: recent advances and current trends, Curr. Org. Chem., 2002, vol. 6, no. 13, p. 1121.
Trofimov, B.A. and Gusarova, N.K., Usp. Khim., 2007, vol. 76, no. 6, p. 550.
Trofimov, B.A. and Shmidt, E.Yu., Russ. Chem. Rev., 2014, vol. 83, no. 7, p. 600.
Salvio, R., Moliterno, M., and Bella, M., Alkynes in organocatalysis, Asian J. Org. Chem., 2014, vol. 3, p. 340.
Temkin, O.N., Homogeneous Catalysis with Metal Complexes: Kinetic Aspects and Mechanisms, Chichester: Wiley, 2012.
Acetylene Chemistry. Chemistry, Biology and Material Science, Diederich, F., Tykwinski, R.R., and Stang, R.J., Eds., Weinheim: Wiley, 2005.
Catalysis by Gold. Catal. Sci. Ser., Bond, G.C., Louis, C., and Thompson, D.T., Eds., London: Imperial College, 2006, vol. 6.
Modern Gold Supramolecular Chemistry. Gold-Metal Interactions and Applications, Laguna, A., Ed., Weinheim: Wiley, 2009.
Click Chemistry for Biotechnology and Materials Science, Lahann, J., Ed., Hoboken: Wiley, 2009, p. 432.
Gold Chemistry: Applications and Future Directions in the Life Sciences, Mohr, F., Ed., Weinheim: Wiley, 2009, p. 408.
Click Tiazoles. Topics in Heterocyclic Chemistry, Kosmrlj, J., Ed., Berlin: Springer–Verlag, 2012, Vol. 28.
Modern Gold Catalyzed Synthesis, Hashmi, A.S.K. and Toste, F.D., Eds., Weinheim: Wiley, 2012, p. 402.
Transition Metals Catalyzed Carbonylation Reactions, Beller, M. and Wu, X.-F., Eds., Berlin: Springer, 2013, p. 228.
Maretina, I.A. and Ionin, B.I., Alkynes in Cycloadditions, Tebby, J.C., Ed., Chichester: Wiley, 2014, p. 312.
Modern Alkyne Chemistry. Catalytic and Atom-Economic Transformations, Trost, B.M. and Li, Ch.-J., Eds., Weinheim: Wiley, 2015, p. 424.
Au-Catalyzed Synthesis and Functionalization of Heterocycles. Topics in Heterocyclic Chemistry, Bandini, M., Ed., Berlin: Springer, 2016, p. 289.
Lei, A., Shi, W., Liu, C., Liu, W., Zhang, H., and He, C., Oxidative Cross-Coupling Reactions, Weinheim: Wiley, 2017, p. 229.
Lu, W. and Zhou, L., Oxidation of C–H Bonds, Hoboken: Wiley, 2017, p. 508.
Schore, N.E., Transition metal-mediated cycloaddition reactiones of alkynes in organic synthesis, Chem. Rev., 1988, vol. 88, p. 1081.
Beletskaya, I. and Moberg, C., Element-element addition to alkynes catalyzed by the group 10 metals, Chem. Rev., 1999, vol. 99, p. 3435.
Chung, Y.K., Transition metal alkyne complexes: the Pauson–Khand reaction, Coord. Chem. Rev., 1999, vol. 188, p. 297.
Kiss, G., Palladium-catalyzed Reppe carbonylation, Chem. Rev., 2001, vol. 101, no. 11, p. 3435.
Temkin, O. and Bruk, L., Oxidative Carbonylation-Homogeneous. Encyclopedia of Catalysis, Horvath, I., Ed., New York: Wiley, 2003, vol. 5, p. 394.
Negishi, E.-I. and Anastasia, L., Palladium-catalyzed alkynylation, Chem. Rev. 2003, vol. 103, p. 1979.
Beller, M., Seayad, J., Tillack, A., and Jiao, H., Catalytic Markovnikov and anti-Markovnikov functionalization of alkenes and alkynes: recent developments and trends, Angew. Chem., 2004, vol. 43, p. 3368.
Alonso, F., Beletskaya, I.P., and Yus, M. Transition-metal-catalyzed addition of heteroatom-hydrogen bond to alkynes, Chem. Rev., 2004, vol. 104, p. 3079.
Fűrstner, A. and Davies, P.W., Alkyne metathesis, Chem. Commun., 2005, p. 2307.
Gabriele, B., Salerno, G., and Costa, M., Oxidative carbonylation, Top. Organomet. Chem., 2006, vol. 18, p. 232.
Hintermann, L. and Labonne, A., Catalytic hydration of alkynes and its application in synthesis, Synthesis, 2007, no. 8, p. 1121.
Beletskaya, I.P. and Ananikov, V.P., Addition reactions of E–E and E–H bonds to triple bond of alkyne catalyzed by Pd, Pt and Ni complexes (E = S, Se), Pure Appl. Chem., 2007, vol. 79, p. 1041.
Meldal, M. and Tornøe, C.W., Cu-catalyzed azide–alkyne cycloaddition, Chem. Rev., 2008, vol. 108, p. 2952.
Hein, C.D., Liu, X.-M., and Wang, D., Click chemistry a powerful tool for pharmaceutical sciences, Pharm. Res., 2008, vol. 25, no. 10, p. 2216.
Hashmi, A.S.K., Homogeneous gold catalysis beyond assumptions and proposals – characterized intermediates, Angew. Chem., 2010, vol. 49, p. 5232.
Hintermann, L., Recent developments in metal-catalyzed additions of oxygen nucleophiles to alkenes and alkynes, Top. Organomet. Chem., 2010, vol. 31, p. 123.
Abbiati, G., Beccalli, E.M., and Rossi, E.,Groups 9 and 10 metals-catalyzed O–H bond addition to unsaturated molecules, Top. Organomet. Chem., 2013, vol. 43, p. 231.
Schobert, H., Production of acetylene and acetylene-based chemicals from coal, Chem. Rev. 2014, vol. 114, no. 3, p. 1743.
Trotus, I.-T., Zimmermann, T., and Schüth, F., Catalytic reactions of acetylene: a feedstock for the chemical industry revisited, Chem. Rev., 2014, vol. 114, no. 3, p. 1761.
Chinchilla, R. and Nájera, C., Chemicals from alkynes with palladium catalysts, Chem. Rev. 2014, vol. 114, no. 3, p. 1783.
Quintero-Duque, S., Dyballa, K.M., and Fleischer, I., Metal-catalyzed carbonylation of alkynes: key aspects and recent development, Tetrahedron Lett., 2015, vol. 56, no. 21, p. 2634.
Ananikov, V.P., Nickel: the “spirited horse” of transition metal catalysis, ACS Catal., 2015, no. 5, p.1964.
Huple, D.B., Ghorpade, S., and Liu, R.-S., Recent advances in gold-catalyzed N- and O- functionalizations of alkynes with nitrones, nitroso, nitro and nitroxy species, Adv. Synth. Catal., 2016, vol. 358, p. 1348.
Voronin, V.V., Ledovskaya, V.S., Bogachenkov, A.S., Rodygin, K.S., and Ananikov, V.P., Molecules, 2018, vol. 23, p. 2442.
Nieuwland, J.A. and Vogt, R.R., The Chemistry of Acetylene, New York: Reinhold, 1945.
Reppe, W., Neue Entwiclungen auf dem Gebiete der Chemie des Acetylene und Kohlen Oxydes, Berlin: Springer, 1949.
Johnson, A.W., The Chemistry of Acetylenic Compounds, London: Edward Arnold and Co., 1946, Vol. 1; 1950, Vol. 2.
Raphael, R.A., Acetylenic Compounds in Organic Synthesis, New York: Academic Press, 1955, p. 219.
Ziegenbein, W., Einführungen der Ăthinyl- und Alkynyl-Gruppe in Organische Verbindungen, Berlin: Verlag Chemie, 1963.
Kotlyarevskii, I.L., Shvartsberg, M.S., and Fisher, L.B., Reaktsii atsetilenovykh soedinenii (Reaction of Acetylene Compounds), Novosibirsk: Nauka SB AS USSR, 1967, p. 354.
Temkin, O.N. and Flid, R.M., Kataliticheskie prevrashcheniya atsetilenovykh soedinenii v rastvorakh kompleksov metallov (Catalytic Transformations of Acetylene Compounds in the Solutions of Metal Complexes), Moscow: Nauka, 1968, p. 212.
Rudledge, T.F., Acetylenic Compounds: Preparation and Substitution Reaction, New York: Reinhold, 1968.
Khimiya atsetilenovykh soedinenii (Chemistry of Acetylene Compounds), Viie, G.G., Ed., Moscow: Khimiya, 1973.
Shostakovskii, M.F. and Bogdanova, A.V., The Chemistry of Diacetylenes, New York: Wiley, 1974.
Hanford, W.E. and Fuller, D.L., Acetylene chemistry, Ind. Eng. Chem., 1948, vol. 40, p. 1171.
Copenhaver, J. and Bigelow, M., Acetylene and Carbon Monoxide Chemistry, New York: Reinhold, 1949.
Ustynyuk, Yu.A., Lektsii po organicheskoi khimii. Ch. 2. Khimiya uglevodorodov. Ser. Mir khimii (Lection on Organic Chemistry. Part 2. Chemistry of Hydrocarbons), Moscow: Tekhnosfera, 2016.
Walker, L.F. and Landergan, T.E., US Patent 2487006, 1949; C.A., 1950, vol. 44, p. 5380.
Kindler, K., Chem. Ber., 1921, vol. 54, p. 647.
Fogel', I.G., Atsetilen, ego svoistva, izgotovlenie i primenenie (Acetylene, its Properties, Production and Application), Zal’kind, Yu., Ed., Leningrad: ONTI Goskhimtekhizdat, 1934.
Flid, R.M. and Temkin, O.N., USSR Copyright Certificate 117494, 1958.
Flid, R.M. and Temkin, O.N., Zh. Fiz. Khim., 1961, vol. 35, p. 452.
Halpern, I., James, B. R., and Kemp, A.L.W., J. Amer. Chem. Soc., 1961, vol. 83, p. 4097.
Moiseev, I.I., π-Kompleksy olefinov v zhidkofaznom okislenii (π-Complexes of Olephynes in Liquid-Phase Oxidation), Moscow: Nauka, 1970, p. 270.
Jira, R., Acetaldehyde from ethylene – a retrospective on the discovery of the Wacker process, Angew. Chem., 2009, vol. 48, p. 9034.
Lines, C.B. and Long, R., Preprints Division of Petrol. Chem. Inc. Am. Chem. Soc., 1969, vol. 14, no. 2, p. 159.
Kaliya, O.L., Kirchenkova, G.S., Temkin, O.N., and Flid, R.M., Kinet. Katal., 1969, vol. 10, no. 5, p. 1186.
Kaliya, O.L., Temkin, O.N., Flid, R.M., Kimel’fel’d, Ya.M., Kirchenkova, G.S., and Smirnova, E.M., Izv. Akad. Nauk SSSR, Ser. Khim., 1969, p. 2854.
Kimel’fel’d, Ya.M., Smirnova, E.M., Pershikova, N.I., Kaliya, O.L., and Temkin, O.N., Zh. Strukt. Khim., 1971, vol. 12, p. 1097.
Penella, F., Banks, R.L., and Bailey, G.C., J. Chem. Soc. Chem. Commun., 1968, p. 1548.
Mogilyanskii, A.I., Kogan, L.M., Bondarenko, I.E., Kuleshova, L.S., and Temkin, O.N., Uchenye zapiski MITKhT im M.V.Lomonosova, 1970, vol. 1, no. 2, p. 10.
Chaltykian, O.A., Copper-Catalyzed Reactions, New York: Consultants Bureau, 1966.
Shmulevich, L.A., Razvitie khimii atsetilena, Extended Abstract of Cand. Sci. (Chem) Dissertation, Moscow: Institute of the History of Science and Technology AS USSR, 1969.
Temkin, O.N., Bruk, L.G., and Zeigarnik, A.V., Kinet. Katal., 1993, vol. 34, no. 3, p. 445.
Zeigarnik, A.V., Bruk, L.G., Temkin, O.N., Likholobov, V.F., and Maier, L.I., Russ. Chem. Rev., 1996, vol. 65, p. 117.
Temkin, O.N., Zeigarnik, A.V., and Bonchev, D., Chemical Reaction Networks. A Graph-Theoretical Approach, Boca Raton: CRC, 1996, p. 286.
Zeigarnik, A.V., Valdes-Perez, R.E., Temkin, O.N., Bruk, L.G., and Shalgunov, S.I., Organometallics, 1997, vol. 16, p. 3114.
Norman, R.O.C., Parr, W.J.E., and Thomas, C.B., J. Chem. Soc. Perkin Trans., 1976, p. 1983.
Fukuda, Y., Utimoto, K., and Nozaki, H., Heterocycles, 1987, vol. 25, p. 297.
Hutchings, G.J., J. Catal., 1985, vol. 96, p. 292.
Chiusoli, G.P., J. Organomet. Chem., 1986, vol. 300, p. 57.
Grigoryan, E.A., Gyulumyan, Kh.R., Gurtovaya, E.I., Enikolopyan, N.S., and Ter-Kazaryan, M.A., Dokl. Akad. Nauk SSSR, 1981, vol. 257, no. 2, p. 364.
Noskova, N.F., Sokol’skii, D.V., Izteleulova, M.B., and Gafarova, N.A., Dokl. Akad. Nauk SSSR, 1982, vol. 262, no. 1, p. 113.
Shestakov, G.K., Temkin, O.N., Vsesvyatskaya, N.Yu., and Stepanov, A.M., Zh. Org. Khim., 1979, vol. 15, no. 2, p. 245.
Vsesvyatskaya, N.Yu., Shestakov, G.K., and Temkin, O.N., Kinet. Katal., 1986, vol. 27, p. 1330.
Sasaki, Y. and Dixneuf, P.H., J. Chem. Soc. Chem. Commun., 1986, p. 790.
Chukhadzhyan, G.A., Abramyan, Zh.I., Kukolev, V.P., Gevorgyan, G.A., Tonyan, G.M., and Melkonyan, L.N., Armyan.Khim. Zh., 1978, vol. 31, no. 8, p. 607.
Sonogashira, K., Tohda, Y., and Hagihara, N., Tetrahedron Lett., 1975, p. 4467.
Shestakov, G.K., Vasil’ev, A.M., Tishchenko, L.M., Temkin, O.N., and Flid, R.M., Kinet. Katal., 1974, vol. 15, no. 4, p. 1070.
Heck, R.F., J. Amer. Chem. Soc., 1972, vol. 94, p. 2712.
Tsuji, J., Takahashi, M., and Takahashi, T., Tetrahedron Lett., 1980, vol. 21, p. 849.
Bruk, L.G. and Temkin, O.N., Khim.Prom-sti, 1993, p. 57.
Shulyakovskii, G.M., Temkin, O.N., Bykanova, N.V., and Nyrkova, A.N., Khimicheskaya kinetika v katalize. Kineticheskie modeli zhidkofaznykh reaktsii (Chemical Kinetics in Catalysis. Kinetic Models of Liquid-Phase Reactions) Kiperman, S.L., Ed., Chernogolovka: IOC AS USSR, ICP AS USSR, 1985.
Brailovskii, S.M., Kabalina, G.A., Shestakova, V.S., and Temkin, O.N., Zh. Org. Khim., 1977, vol. 13, p. 1158.
Shestakova, V.S., Brailovskii, S.M., and Temkin, O.N., Kinet. Katal., 1978, vol. 19, p. 1585.
Bruk, L.G., Vsesvyatskaya, N.Yu., Stromnova, T.A., Alekseeva, N.F., and Temkin, O.N., Zh. Org. Khim., 1978, vol. 14, p. 473.
Temkin, O.N., Mekhryakova, N.G., Bruk, L.G., Kaliya, O.L., and Prudnikov, A.Yu., Kinet. Katal., 1979, vol. 20, p. 629.
Temkin, O.N. and Bruk, L.G., Usp. Khim., 1983, vol. 52, p. 206.
Mortreux, A. and Blanchard, M., Chem. Commun., 1974, p. 786.
Kaliya, O.L., Temkin, O.N., Mekhryakova, N.G., and Flid, R.M., Dokl. Akad. Nauk SSSR, 1971, vol. 199, no. 6, p. 1321.
Bruk, L.G. and Temkin, O.N., Proc. 9th Soviet-Japan Seminar on Catalysis, Novosibirsk: Boreskov Institute of Catalysis SB RAS, 1990, p. 83.
Bruk, L.G. Oshanina, I.V., Kozlova, A.P., Vorontsov, E.V., and Temkin, O.N., J. Mol. Catal., 1995, vol. 104, p. 9.
Temkin, O.N. and Bruk, L.G., Kinet. Catal., 2003, vol. 44, no. 5, p. 601.
Malashkevich, A.V., Bruk, L.G., and Temkin, O.N., J. Phys. Chem. A, 1997, vol. 101, no. 52, p. 9825.
Gorodsky, S.N., Bruk, L.G., Istomina, A.E., Kurdiukov, A.V., and Temkin, O.N., Top. Catal., 2009, vol. 52, p. 557.
Kozlov, A.I., Brailovskii, S.M., and Temkin, O.N., Kinet. Katal., 1994, vol. 35, no. 4, p. 551.
Kozlov, A.I., Brailovskii, S.M., and Temkin, O.N., Kinet. Katal., 1995, vol. 36, no. 2, p. 225.
Ananikov, V.P., Gayduk, K.A., Orlov, N.V., Beletskaya, I.P., Khrustalev, V.N., and Antipin, M.Yu., Chem. Eur. J., 2010, vol. 16, p. 2063.
Khemchyan, L.L., Ivanova, J.V., Zalesskiy, S.S., Ananikov, V.P., and Beletskaya, I.P., Adv. Synth. Catal., 2014, vol. 356, p. 771.
Zalesskiy, S.S., Khrustalev, V.N., Kostukovich, A.Yu., and Ananikov, V.P., Organometallics, 2015, vol. 34, p. 5214.
Drent, E., Arnoldy, D., and Budzelar, P.H.M., J. Organomet. Chem., 1993, vol. 455, p. 247.
Drent, E., Arnoldy, D., and Budzelar, P.H.M., J. Organomet. Chem., 1994, vol. 475, p. 57.
Doucet, H., Hofer, J., Bruneau, C., and Dixneuf, P.H., J. Chem. Soc. Chem. Commun., 1993, p. 850.
Bruneau, C. and Dixneuf, P.H., Angew. Chem., 2006, vol. 45, p. 2176.
Mitchenko, S.A. and Shubin, A.A., Metalloorganicheskie proizvodnye atsidokompleksov platiny: sintez, reaktsionnaya sposobnost' i dizain kataliticheskikh reaktsii (Organometallic Derivatives of Platinum Acid Complexes: Synthesis, Reactivity and Design of Catalytic Reactions), Donetsk: DonGUET, 2004.
Dorel, R. and Echavarren, A., Chem. Rev., 2015, vol. 115, p. 9028.
Schmidt, A., Halaiqa, A., and Smirnov, V.V., Synlett., 2006, no. 18, p. 2861.
Eremin, D.B. and Ananikov, V.P., Understanding active species in catalytic transformations: from molecular catalysis to nanoparticles, leaching, “cocktails” of catalysts and dynamic systems, Coord. Chem. Rev., 2017, vol. 346, p. 2.
Schmidt, A.F., Kurokhtina, A.A., and Larina, E.V., Mendeleev Commun., 2017, vol. 27, p. 213.
Strauss, F., J. Liebig. Annalen Chem., 1905, vol. 342, no. 5, p. 190.
Akhtar, M., Richards, T.A., and Weedon, B.C.L., J. Chem. Soc., 1959, p. 933.
Mykhalichko, B.M., Temkin, O.N., and Mys’kiv, M.G., Russ. Chem. Rev., 2000, vol. 69, no. 11, p. 957.
Gorin, D.J. and Toste, F.D., Nature, 2007, vol. 446, p. 395.
Soriano, E. and Marco-Contelles, J., Top. Curr. Chem., 2011, vol. 32, p. 1.
Zuccaccia, D., Belpassi, L., Rocchigiani, L., Tarantelli, F., and Macchioni, A., Inorg. Chem., 2010, vol. 49, p. 3080.
Das, A., Dash, C., Celik, M.A., Yousufuddin, M., Frenking, G., and Dias, R., Organometallics, 2013, vol. 32, p. 3135.
Balcioglu, N., Uraz, I., Bozkurt, C., and Sevin, F., Polyhedron, 1997, vol. 16, no. 2, p. 327.
Cornfield, P.W.R. and Shearer, H.M.M., Acta Crystallogr. Chem., 1966, vol. 21, p. 957.
Temkin, O.N., Flid, R.M., Sukhova, T.G., Chepaikin, E.G., and Tikhonov, G.F., Zh. Prikl. Khim., 1968, vol. 41, p. 633.
Sukhova, T.G., Temkin, O.N., Flid, R.M., and Kaliya, T.K., Zh. Neorg. Khim., 1968, vol. 13, no. 8, p. 2073.
Sukhova, T.G., Borshch, N.Ya., Temkin, O.N., and Flid, R.M., Zh. Neorg. Khim., 1969, vol. 14, no. 3, p. 694.
Sukhova, T.G., Temkin, O.N., and Flid, R.M., Zh. Neorg. Khim., 1969, vol. 14, no. 4, p. 928.
Sukhova, T.G., Temkin, O.N., and Flid, R.M., Zh. Neorg. Khim., 1970, vol. 15, no. 7, p. 1849.
Nishiwaki, K.-ichiro, Kobayashi, M., Takeuchi, T., Matuoto, K., and Osakada, K., J. Mol. Catal. A: Chem., 2001, vol. 175, p. 73.
Ahrland, S. and Rawstorne, J., Acta Chem. Scand., 1970, vol. 24, p. 157.
Mykhalichko, B.M., Aksel’rud, L.G., and Davydov, V.N., Koord. Khim., 1997, vol. 42, no. 3, p. 410.
Tikhonov, G.F., Temkin, O.N., and Flid, R.M., Kinet. Katal., 1967, vol. 8, no. 3, p. 520.
Tikhonov, G.F., Temkin, O.N., and Flid, R.M., Zh. Fiz. Khim., 1966, vol. 11, no. 12, p. 3075.
Tikhonov, G.F., Shestakov, G.K., Temkin, O.N., and Flid, R.M., Zavod. Lab., 1967, vol. 33, no. 2, p. 150.
Temkin, O.N., Tikhonov, G.F., Flid, R.M., and Galeev, Yu.R., Kinet. Katal., 1967, vol. 8, no. 6, p. 1236.
Temkin, O.N., Flid, R.M., Shestakov, G.K., Ermakova, A., Tikhonov, G.F., Yarovaya, L.N., and Mikhal’chenko, V.G., Kinet. Katal., 1969, vol. 10, no. 6, p. 1231.
Shestakov, G.K., Tikhonov, G.F., Temkin, O.N., and Flid, R.M., Kinet. Katal., 1970, vol. 11, no. 3, p. 575.
Shestakov, G.K., Tikhonov, G.F., Temkin, O.N., Flid, R.M., Gershenzon, I.Sh., Brailovskii, S.M., and Dolzhnikova, S.I., Kinet. Katal., 1970, vol. 11, no. 4, p. 875.
Temkin, O.N., Shestakov, G.K., and Kozlova, N.Yu., Kinet. Katal., 1990, vol. 31, no. 4, p. 850.
Tikhonov, G.F., Shestakov, G.K., Temkin, O.N., and Flid, R.M., Kinet. Katal., 1966, vol. 7, no. 5, p. 614.
Temkin, O.N., O razlichnykh vzaimosvyazyakh kinetiki i termodinamiki (On the Various Relationships of Kinetics and Thermodynamics), Saarbrücken: Lambert Acad. Publ., 2016, p. 119.
Temkin, O.N., Sukhova, T.G., Shestakov, G.K., Tikhonov, G.F., Flid, R.M., Chepaikin, E.G., Novikova, G.M., and Borshch, N.Ya., Kinet. Katal., 1969, vol. 10, no. 5, p. 1004.
Brailovskii, S.M., Temkin, O.N., Flid, R.M., and Belova, N.G., Zh. Fiz. Khim., 1970, vol. 44, no. 4, p. 1112.
Brailovskii, S.M., Temkin, O.N., and Flid, R.M., Kinet. Katal., 1971, vol. 12, no. 5, p. 1152.
Osechkin, S.I., Mys’kiv, M.G., Zavalii, P.Yu., and Sobolev, A.N., Metalloorg. Khim., 1991, vol. 4, no. 5, p. 997.
Mykhalichko, B.M., Mys’kiv, M.G., and Aksel’rud, L.G., Koord. Khim., 1993, vol. 19, no. 9, p. 722.
Mys’kiv, M.G. and Mykhalichko, B.M., Zh. Srukt. Khim., 1994, vol. 35, no. 5, p. 120.
Mykhalichko, B.M., Mys’kiv, M.G., and Davydov, V.N., Zh. Neorg. Khim., 1999, vol. 44, no. 3, p. 411.
Mykhalichko, B.M. and Mys’kiv, M.G., Russ. J. Coord. Chem., 1998, vol. 24, no. 12, p. 880.
Mykhalichko, B.M., Russ. J. Inorg. Chem., 1998, vol. 43, no. 10, p. 1520.
Mykhalichko, B.M., Russ. J. Coord. Chem., 1999, vol. 25, no. 5, p. 336.
Mykhalichko, B.M., Mys’kiv, M.G., and Davydov, V.N., Russ. J. Inorg. Chem., 1999, vol. 44, no. 1, p. 40.
Mys'kiv, M.G., Zavalii, P.Yu., Mykhalichko, B.M., and Fundamenskii, V.S., Koord. Khim., 1988, vol. 14, no. 12, p. 1619.
Mykhalichko, B.M. and Mys’kiv, M.G., Koord. Khim., 1999, vol. 25, no. 5, p. 336.
Gamasa, M.P., Gimtno, J., and Lastra, E., J. Organomet. Chem., 1988, vol. 346, p. 277.
Reger, D.L. and Huff, M.F., Organometallics, 1990, vol. 9, p. 2807.
Osakoda, K., Takizawa, T., and Yamamoto, T., Organometallics, 1995, vol. 14, p. 3531.
Reger, D.L., Collins, J.E., and Huff, M.F., Organometallics, 1995, vol. 14, p. 5475.
Lang, H. and Weinmann, M., Synlett., 1995, p. 1.
Normant, J.E. and Alexakis, A., Synthesis, 1981, p. 841.
Vartanyan, V.S., Shestakov, G.K., and Temkin, O.N., Armyan.Khim. Zh., 1979, vol. 32, no. 4, p. 259.
Vartanyan, V.S., Shestakov, G.K., and Temkin, O.N, Armyan.Khim. Zh., 1979, vol. 32, no. 4, p. 264.
Tachiyama, T., Yoshida, M., Aoyagi, T., and Fukuzumi, S., Appl. Organomet. Chem., 2008, vol. 22, p. 205.
Tachiyama, T., Yoshida, M., Aoyagi, T., and Fukuzumi, S., J. Phys. Org. Chem., 2008, vol. 21, p. 510.
Hefner, J.G., Zizelman, P.M., Durfee, L.D., and Lewandos, G.S., J. Organomet. Chem., 1984, vol. 260, p. 369.
Lieu, J., Zuo, Y., Han, M., Wang, Z., and Wang, D., J. Nat. Gas Chem., 2012, vol. 21, p. 495.
Liu, J., Zuo, Y., Han, M., and Wang, Z., J. Chem. Technol. Biotechnol, 2013, vol. 88, p. 408. https://doi.org/10.1002/jctb.3860
Sun, S., Kroll, J., Luo, Y., and Zhang, L., Synlett., 2012, vol. 23, p. 54.
Yamazaki, H.J., J. Chem. Soc. Chem. Commun., 1976, vol. 21, p. 841.
Dahlberg, L., Frosin, K.-M., Kerstan, S., and Werner, D., J. Organomet. Chem., 1991, vol. 407, p. 115.
Bianchini, C., Perruzzini, M., Zanobini, F., Frediani, P., and Albinati, A., J. Am. Chem. Soc., 1991, vol. 113, p. 5453.
Bianchini, C., Frediani, P., Masi, D., Perruzzini, M., and Zanobini, F., Organometallics, 1994, vol. 13, p. 4616.
Wakatsuki, Y. and Yamazaki, H., J. Am. Chem. Soc., 1991, vol. 113, p. 9604.
Wakatsuki, Y. and Yamazaki, H., J. Organomet. Chem. 1995, vol. 500, p. 349.
Slugovc, C., Mereiter, K., Zobetz, E., Schmid, R., and Kirchner, K., Organometallics, 1996, vol. 15, p. 5275.
Yi, C.S. and Liu, N., Organometallics, 1996, vol. 15, p. 3968.
Yi, C.S. and Liu, N., Synlett., 1999, no. 3, p. 281.
Yi, C.S. and Liu, N., Organometallics, 1997, vol. 16, p. 3910.
Yi, C.S. and Liu, N., Organometallics, 1998, vol. 17, p. 3158.
Melis, K., Samulkiewicz, P., Rynkowski, J., and Verpoort, F., Tetrahedron Lett., 2002, vol. 43, p. 2713.
Bassetti, M., Pasquini, C., Raneri, A., and Rosato, D., J. Org. Chem., 2007, vol. 72, p. 4558.
Katayama, H., Yari, H., Tanaka, M., and Ozawa, F., Chem. Commun., 2005, p. 4336.
Chen, X., Xue, P., Sung, H.H.Y., Wiliams, I.D., Peruzzini, M., Bianchini, C., and Yia, G., Organometallics, 2005, vol. 24, p. 4330.
Lee, J.-H. and Caulton, K.G., J. Organomet. Chem., 2008, vol. 693, p. 1664.
Hijazi, A., Parkhomenko, K., Djukic, J.-P., and Chemmri, A., Adv. Synth. Catal., 2008, vol. 350, p. 1493.
Dobson, A., Moore, D.S., Robinson, S.D., Hursthouse, M.B., and New, L., Polyhedron, 1985, vol. 4, p. 1119.
Jia, G., Rheingold, A.L., and Meek, D.W., Organometallics, 1989, vol. 8, p. 1378.
Wakatsuki, Y., Satoh, M., and Yamazaki, H., Chem. Lett., 1989, p. 1585.
Bullock, R.M., J. Chem. Soc. Chem. Commun., 1989, p. 165.
Tenorio, M.J., Puerta, M.C., and Valegra, P., J. Chem. Soc. Chem. Commun., 1991, p. 1750.
Wakatsuki, Y., Koga, N., Yamazaki, H., and Morokama, K., J. Am. Chem. Soc., 1994, vol. 116, p. 8105.
Yi, C.S. and Liu, N., Organometallics, 1997, vol. 16, p. 3729.
Lee, H.M., Yao, J., and Jia, G., Organometallics, 1997, vol. 16, p. 3927.
Bruneau, C. and Dixneuf, P.H., Acc. Chem. Res., 1999, vol. 32, p. 311.
Bruce, M. and Wallis, R.C., Austr. J. Chem., 1979, vol. 32, p. 1471.
Bruce, M., Chem. Rev., 1991, vol. 91, p. 197.
Naota, T., Takaya, H., and Murahashi, S.-I., Chem. Rev. 1998, vol. 98, p. 2599.
Metal Vinylidens and Allenylidens in Catalysis: From Reactivity to Applications, Bruneau, Ch. and Dixneuf, P.H., Eds., Weinheim: Wiley, 2008.
Temkin, O.N. and Bruk, L.G., Ross. Khim. Zh., 2014, vol. 58, nos. 5–6, p. 90.
Esteruelas, M.A., Oro, L.A., and Ruiz, N., Organometallics. 1994, vol. 13, p. 1507.
Barbaro, P., Bianchini, C., Peruzzini, M., and Polo, A., Inorg. Chem. Acta., 1994, vol. 220, p. 5.
Gotzig, J., Otto, H., and Werner, H., J. Organomet. Chem., 1985, vol. 257, p. 247.
Esteruelas, M.A., Herrero, J., Lopez, A.M., and Olivan, M., Organometallics, 2001, vol. 20, p. 3202.
Alos, J., Bolaño, T., Esteruelas, M.A., Olivan, M., Oñate, E., and Valencia, M., Inorg. Chem., 2013, vol. 52, p. 6199.
Field, L.D., Messerle, B.A., Smernik, R.J., Hambley, T.W., and Turner, P., J. Chem. Soc. Dalton Trans., 1999, p. 2557.
Midya, G.C., Paladhi, S., Dhara, K., and Dash, J., Chem. Commun., 2011, vol. 47, p. 6698.
Midya, G.C., Paladhi, S., Dhara, K., and Dash, J., Org. Biomol. Chem., 2014, vol. 12, p. 1812.
Rivada-Wheelahan, O., Chakraborty, S., Shimon, L.J.W., Ben-David, Y., and Milstein, D., Angew. Chem., 2016, vol. 55, p. 6942.
Bhunia, M., Sahoo, S., Vijakumar, G., Adhikari, D., and Mandal, S., Organometallics. 2016, vol. 35, p. 3775.
Liang, Q., Osten, K.M., and Song, D., Angew. Chem., 2017, vol. 56, p. 1.
Bauer, I. and Knölker, H.-J., Chem. Rev., 2015, vol. 115, p. 3170.
Singer, H. and Wilkinson, G., J. Chem. Soc. A, 1968, p. 849.
Albano, P. and Aresta, M., J. Organomet. Chem., 1980, vol. 190, p. 243.
Schāfer, H.-A., Marcy, R., Rüping, T., and Singer, H., J. Organomet. Chem., 1982, vol. 240, p. 17.
Kovaler, I.P., Yerdakov, K.V., Strelenko, Yu.A., Vinogradov, M.G., and Nikishin, G.I., J. Organomet. Chem., 1990, vol. 386, p. 139.
Boese, W.T. and Goldman, A.S., Organometallics, 1991, vol. 10, p. 782.
Schāfer, M., Wolf, J., and Werner, H., J. Chem. Soc. Chem. Commun., 1991, p. 1341.
Schāfer, M., Wolf, J., and Werner, H., J. Chem. Soc. Dalton Trans., 2005, p. 1468.
Lee, C.-C., Lin, Y.-C., Liu, Y.-H., and Wang, Y., Organometallics, 2005, vol. 24, p. 136.
Ito, J.-ichi, Kitase, M., and Nishiyama, H., Organometallics, 2007, vol. 26, p. 6412.
Peng, H.M., Zhao, J., and Li, X., Adv. Synth. Catal., 2009, vol. 351, p. 1371.
Mochizuki, K., Sakai, K., Kochi, T., and Kakiuchi, F., Synthesis, 2013, vol. 45, p. 2088.
Rubio-Perez, L., Azpiroz, R., Di Giuseppe, A., Polo, V., Castarlenas, R., Perez-Torrente, J.J., and Oro, L.A., Chem. Eur. J., 2013, vol. 19, p. 15304.
Ogata, K. and Toyota, A., J. Organomet. Chem., 2007, vol. 692, p. 4139.
Ghosh, R., Zhang, X., Achord, P., Emye, T.J., Krogh-Jespersen, K., and Goldman, A.S., J. Am. Chem. Soc., 2007, vol. 129, p. 853.
Field, L.D. and Ward, A.J., J. Organomet. Chem., 2003, vol. 681, p. 91.
Hilt, G., Hess, W., Vogler, T., and Hengst, C., J. Organomet. Chem., 2005, vol. 690, p. 5170.
Meriwether, L.S., Colthup, E.C., Kennerly, G.W., and Reusch, R.N., J. Org. Chem., 1961, vol. 26, p. 5155.
Meriwether, L.S., Colthup, E.C., Kennerly, G.W., and Reusch, R.N., J. Org. Chem., 1961, vol. 26, p. 5163.
Colthup, E.C. and Meriwether, L.S., J. Org. Chem., 1961, vol. 26, p. 5.
Giacomelli, G., Caporusso, A.M., and Lardici, L., J. Org. Chem., 1979, vol. 44, p. 231.
Ogoshi, S., Ueta, M., Oka, M., and Kurosawa, H., Chem. Commun., 2004, no. 23, p. 2732.
Sabourin, E.T., J. Mol. Catal., 1984, vol. 26, p. 363.
Trost, B.M., Sorum, M.T., Chan, C., Harms, A.E., and Rühter, G., J. Am. Chem. Soc., 1997, vol. 119, p. 698.
Canty, A.J., Acc. Chem. Res., 1992, vol. 25, p. 83.
Herrmann, W.A., Böhm, V.P.W., Gstöttmayr, G.V.K., Grosche, M., Reisinger, C.-C., and Weskamp, T., J. Organomet. Chem., 2001, vols. 617–628, p. 616.
Rubina, M. and Gevorgyan, V., J. Organomet. Chem., 2001, vol. 123, p. 11107.
Yang, C. and Nolan, S.P., J. Org. Chem., 2002, vol. 67, p. 591.
Jahier, C., Zatolochnaya, O.V., Zvyagintsev, N.V., Ananikov, V.P., and Gevorgyan, V., Org. Lett., 2012, vol. 14, p. 2846.
Tohda, Y., Sonogashira, K., and Hagihara, N., J. Organomet. Chem., 1976, vol. 110, p. 53.
Yasuda, T., Kai, Y., Yasuoka, N., and Kasai, N., Bull. Chem. Soc. Jpn., 1977, vol. 50, no. 11, p. 2888.
Zatolochnaya, O.V., Gordeev, E.G., Jahier, C., Ananikov, V.P., and Gevorgyan, V., Chem. Eur. J., 2014, vol. 20, p. 9578.
Chen, T., Guo, C., Goto, M., and Han, L.-B., Chem. Commun., 2013, vol. 49, p. 7498.
Chen, R., Chen, T., Zhao, Y., Qiu, R., Zhou, Y., Yin, S., Wang, X., Goto, M., and Han, L.-B., J. Am. Chem. Soc., 2011, vol. 133, p. 17037.
Kawata, A., Kuninobu, Y., and Takai, K., Chem. Lett., 2009, vol. 38, p. 836.
Hagihara, N., Tamura, M., Yamazaki, H., and Fujiwara, M., Bull. Chem. Soc. Jpn., 1961, vol. 34, p. 892.
Akita, N., Yasuda, H., and Nakamura, A., Bull. Chem. Soc. Jpn., 1984, vol. 57, p. 480.
Horton, A.D., J. Chem. Soc. Chem. Commun., 1992, p. 185.
Platel, R.H. and Schafer, L.L., Chem. Commun., 2012, vol. 48, p. 10609.
Thompson, M.E., Baxter, S.M., Bulls, A.R., Burger, B.J., Nolan, M.C., Santarsiero, B.D., Schaefer, W.P., and Bercaw, J.E., J. Am. Chem. Soc., 1987, vol. 109, p. 203.
St. Clair, M., Schaefer, W.P., and Bercaw, J.E., Organometallics, 1991, vol. 10, p. 525.
Den Haan, K.H., Wielstra, Y., and Teuben, J.H., Organometallics, 1987, vol. 6, p. 2053.
Heeres, H.J. and Teuben, J.H., Organometallics, 1991, vol. 10, p. 1980.
Evans, W.J., Keyer, R.A., and Ziller, J.W., Organometallics, 1990, vol. 9, p. 2628.
Evans, W.J., Keyer, R.A., and Ziller, J.W., Organometallics, 1993, vol. 12, p. 2618.
Nishiura, M., Hou, Z., Wakatsuki, Y., Yamaki, T., and Miyamoto, T., J. Am. Chem. Soc., 2003, vol. 125, p. 1184.
Nishiura, M. and Hou, Z., J. Mol. Catal. A: Chem., 2004, vol. 213, p. 101.
Ge, S., Quiroga Narambuena, V.F., and Hessen, B., Organometallics, 2007, vol. 26, p. 6508.
Haskel, A., Straub, T., Dash, A.K. and Eisen, M.S., J. Am. Chem. Soc., 1999, vol. 121, p. 3014.
Haskel, A., Wang, J.Q., Straub, T., Neyroud, T.G., and Eisen, M.S., J. Am. Chem. Soc., 1999, vol. 121, p. 3025.
Dash, A.K., Wang, J.S., Berthet, J.C., Ephritikhine, M., and Eisen, M.S., J. Organomet. Chem., 2000, vol. 604, p. 83.
Dash, A.K., Gourevich, I., Wang, J.Q., Wang, J., Kapon, M., and Eisen, M., Organometallics, 2001, vol. 20, p. 5084.
Dash, A. and Eisen, M.S., Org. Lett., 2000, vol. 2, p. 737.
Schmidbaur, H. and Schier, A., Organometallics, 2010, vol. 20, p. 2.
Yakobson, V.V., Geskin, V.M., Klimenko, N.M., Bozhenko, K.V., and Temkin, O.N., Teor. Eksp. Khim., 1985, no. 3, p. 303.
Klimenko, N.M., Bozhenko, K.V., Strumina, E.V., Rykova, E.A., and Temkin, O.N., J. Mol. Struct. (THEOCHEM), 1999, vol. 490, p. 233.
Temkin, O.N., Kinet. Catal., 2014, vol. 55, no. 2, p. 172.
McDade, Ch. and Bercaw, J.E., J. Organomet. Chem., 1985, vol. 279, p. 281.
Frohnapfel, D.S. and Templeton, J.L., Coord. Chem. Rev., 2000, vols. 206–207, p. 199.
Antonova, A.B., Kolobova, N.E., Petrovsky, P.V., Lokshin, B.V., and Obezyuk, N.S., J. Organomet. Chem., 1977, vol. 137, p. 55.
Kolobova, N.E., Antonova, A.B., Khitrova, O.M., Antipin, M.Yu., and Struchkov, Yu.T., J. Organomet. Chem., 1977, vol. 137, p. 69.
Antonova, A.B. and Ioganson, A.A., Usp. Khim., 1989, vol. 58, p. 1197.
De Angelis, F. and Sgamellotti, A., Organometallics, 2002, vol. 21, no. 13, p. 2715.
De Angelis, F., Sgamellotti, A., and Re, N., Organometallics, 2002, vol. 21, no. 26, p. 5944.
Grotjahn, D.B., Zeng, Xi., Cooksy, A.L., Scott Kassel, W., DiPasquale, A.G., Zakharov, L.N., and Rheingold, A.L., Organometallics, 2007, vol. 26, no. 14, p. 3385.
De Angelis, F., Sgamellotti, A., and Re, N., Organometallics, 2007, vol. 26, no. 14, p. 5285.
Vastine, B.A. and Hall, M., Organometallics, 2008, vol. 27, p. 4325.
Tenorio, M.J., Puerta, M.C., Valegra, P., Ortuno, M.A., Ujaque, G., and Llendos, A., Inorg. Chem., 2013, vol. 52, p. 8919.
Olivan, M., Clot, E., Eisenstein, O., and Caulton, K.G., Organometallics, 1998, vol. 17, no. 14, p. 3091.
Buil, M.L., Eisenstein, O., Esteruelas, M.A., Garsia-Yebra, C., Gutierrez-Puebla, E., Olivan, M., Onate, E., Ruiz, N., and Tajada, M., Organometallics, 1999, vol. 18, p. 4949.
Bassetti, M., Cadierno, V., Gimeno, J., and Pasquini, C., Organometallics, 2008, vol. 27, no. 19, p. 5009.
Muthoh, Y., Imai, K., Kimura, Y., Ikeda, Y., and Ishii, Y., Organometallics, 2011, vol. 30, p. 204.
Salvio, R., Julia-Hernandez, F., Pisciottni, L., Mendoza-Merono, R., Garcia-Granda, S., and Bassetti, M., Organometallics, 2017, vol. 36, no. 19, p. 3830.
ACKNOWLEDGMENTS
The author is grateful to Karen Egiazaryan, student of the MIREA – Russian Technological University (Moscow), for his help in graphical presentation of this review.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to “Kinetika i Kataliz” journal on its 60 Years Edition Anniversary, and to academician I.I. Moiseev, one of the creators of modern catalysis with metal complexes, on his 90th birthday
Translated by P. Pozdeev
Rights and permissions
About this article
Cite this article
Temkin, O.N. “Golden Age” of Homogeneous Catalysis Chemistry of Alkynes: Dimerization and Oligomerization of Alkynes. Kinet Catal 60, 689–732 (2019). https://doi.org/10.1134/S0023158419060120
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158419060120