Abstract
Spirocyclopentyl derivatives of cyanophenyl-substituted nitroxyl radicals (2-(4′-cyanophenyl)-4,5-bis(spirocyclopentyl)-4,5-dihydro-1H-imidazole-3-oxide-1-oxyl (NN) and the corresponding iminonitroxyl 2-(4′-cyanophenyl)-4,5-bis(spirocyclopentyl)-4,5-dihydro-1H-imidazole-1-oxyl (IN)) are synthesized; their structure and magnetic properties are studied. Interaction of IN and NN with bis(hexafluoroacetylacetonato) copper(II) [Cu(hfac)2] leads to the formation of heterospin chain polymeric {[Cu(hfac)2]2(IN)}, {[Cu(hfac)2]3(NN)2}, and layered {[Cu(hfac)2]3(NN)2}·C7H16 complexes. It is shown that the layered {[Cu(hfac)2]3(NN)2}·C7H16 is a soft ferromagnet that transforms into a magnetically ordered state at helium temperatures.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Nitroxyl radicals of the 2-imidazoline series are widely used in the design of magnetically active compounds based on coordination compounds of transition metal ions. Due to the presence of an organic component, the magnets exhibit practically important properties such as low density, elasticity, solubility in organic solvents, transparency in the visible spectrum of light, high resistance to electric current, biocompatibility. High kinetic stability and almost unlimited possibilities of chemical modification of nitroxyls allow tuning stereochemical and electronic characteristics of organic ligands to obtain heterospin compounds that are promising for various applications [1-3].
The presence of functional substituents in the nitroxyl radicals of the 2-imidazoline series facilitates the preparation of chained, layered, or framework coordination compounds demonstrating nontrivial magnetic properties. Thus, heterospin complexes with pyrazolyl- and pyridyl-substituted nitroxyls undergo structural transitions accompanied by magnetic anomalies [4-8]. It was earlier shown that the variation of substituents can be used to change donor properties of functional substituents, thereby influencing the type of the nitroxyl coordination [9, 10]. An alternative way to affect stereochemical and electronic characteristics of paramagnetic ligands is by modifying geminal substituents in the nitroxyl imidazoline ring, but magnetic properties of complexes are changed unpredictably within this approach [7, 11, 12].
To continue these investigations, we considered spin-labeled para cyanophenyl radicals NN and IN. This study was substantiated by the fact the previously synthesized 1D and 2D polymeric complexes of Co(II), Mn(II), and Cu(II) hexafluoroacetylacetonates with tetramethyl-substituted nitroxyls Me4-NN and Me4-IN exhibited nontrivial magnetic properties, while the Mn(II) complex with Me4-NN demonstrated a magnetic phase transition into the ferromagnetic state [13]. The present work is devoted to the synthesis of para cyanophenyl-substituted nitroxyl radicals NN and IN with spirocyclopentyl substituents at the 4th and 5th positions of the 2-imidazoline ring and the synthesis of Cu(hfac)2 polymer complexes with these radicals. Structures and magnetic properties of prepared compounds were studied (Scheme 1).
EXPERIMENTAL
Bis(hexafluoroacetylacetonato)copper(II) (Cu(hfac)2 [14] and 1,1´dihydroxylamino-bis(cyclopentyl) sulfate monohydrate [15] were synthesized by known procedures. Commercial reactants and solvents were used as received. Melting points were determined on a Stuart hot plate. Elemental analysis was performed on a EURO EA3000 CHNS analyzer.
Magnetic susceptibility of polycrystalline samples was measured on a MPMSXL SQUID magnetometer (Quantum Design) in the 2-300 K range in a magnetic field of 5 kOe. Paramagnetic components of magnetic susceptibility χ were determined while taking into account the diamagnetic contribution estimated from Pascal′s constants. The effective magnetic moment as a function of temperature was calculated as:
where N, k, β are the Avogadro constant, the Boltzmann constant, and the Bohr magneton, respectively. Experimental μeff(T) dependences were analyzed by the PHI software [16]. Since the {[Cu(hfac)2]3(NN)2}·C7H16 complex gradually loses solvate molecules, we conducted the magnetochemical measurements for a freshly prepared sample (20-30 min after the crystals were filtered). The elemental analysis for this compound was carried out 2-3 days after the magnetic measurements associated with a loss of solvate molecules. The elemental analysis data correspond to the partially desolvated {[Cu(hfac)2]3(NN)2}·0.3C7H16 complex.
The sets of single-crystal XRD reflections were obtained on Bruker AXS SMART APEX II and APEX DUO diffractometers equipped with Helix and Cobra low-temperature accessories (Oxford Cryosystems). The absorption correction was performed by using the SADABS program (version 2.10) [17]. The structures were solved by direct methods and refined anisotropically by full-matrix least squares for all non-hydrogen atoms. The positions of H atoms were calculated geometrically and refined using a riding model. All calculations for structure determination and refinement were carried out using the SHELX package [18, 19]. The crystal data for the studied compounds and experiment details are summarized in Table 1; the bond lengths are listed in Table 2. Complete X-ray diffraction data were deposited at the Cambridge Crystallographic Data Centre (http://www.ccdc.cam.ac.uk/; CCDC 2245798-2245802).
2-(4′-Cyanophenyl)-4,5-bis(spirocyclopentyl)-4,5-dihydro-1H-imidazole-3-oxide-1-oxyl (NN). A solution of 4-cyanobenzaldehyde (0.1 g, 0.8 mmol) dissolved in ethanol (1 mL) was added at room temperature to a solution of 1,1´Dihydroxylamino-bis(cyclopentyl) sulfate monohydrate (0.25 g, 0.8 mmol) in water (4 mL). The reaction mixture was kept for a day at 4 °C and than neutralized by NaHCO3 (0.15 g) until the gas evolution ceased (pH = 7). The precipitate was filtered off (the mother liquor was separated for subsequent isolation of iminonitroxyl IN), washed with water on a filter, and dried in vacuum.
The dried precipitate (0.1 g, 0.33 mmol) was suspended in a mixture of CH2Cl2 (3 mL) and water (3 mL) and added to NaIO4 (0.1 g, 0.5 mmol) for 10 min at ~10 °C. The reaction mixture was stirred for 30 min, and then the organic phase was separated and the aqueous one was extracted CH2Cl2 (3·10 mL). The combined organic solutions were filtered through a 1.5·10 cm layer of Al2O3, and evaporated. The residue was recrystallized from a CH2Cl2–heptane mixture. Yield: 0.084 g (35%), dark blue prismatic crystals. Tm = 142-144 °C (diff.). Found (%): C 69.6, H 6.7, N 12.7. Calculated for C18H20N3O2 (%): C 69.7, H 6.5, N 13.5.
2-(4′-Cyanophenyl)-4,5-bis(spirocyclopentyl)-4,5-dihydro-1H-imidazole-1-oxyl (IN). NaIO4 (0.1 g, 0.5 mmol) was added under stirring (10 min, ~10 °C) to the mother liquor separated at the previous stage. After stirring the reaction mixture for 40 min, CH2Cl2 (3·10 mL) was extracted. The combined organic layer was dried over Na2SO4, filtered through a layer of Al2O3 (1.5·10 cm), and evaporated. The residue was recrystallized from CH2Cl2–hexane mixture; the resulting orange prismatic crystals were filtered off and washed with cold heptane. Yield: 0.023 g (10% with respect to the reagents of the initial condensation reaction). Tm = 94-96 °C. Found (%): C 73.6, H 6.5, N 14.2. Calculated for C18H20N3O (%): C 73.4, H 6.8, N 14.3.
{[Cu(hfac)2]2(IN)}. Heptane (1 mL) was added to a mixture of iminonitroxyl (0.02 g, 0.068 mmol) and Cu(hfac)2 (0.065 g, 0.136 mmol) in CH2Cl2 (0.5 mL). The resulting solution was kept in an open flask at 4 °C for 72 h. The obtained dark green plate-like crystals were filtered off, washed with cold heptane, and dried in air. Yield: 0.06 g (70%). Tm = 128-130 °C. Found (%): C 36.6, H 2.1, N 3.6, F 36.5. Calculated for C38H24Cu2F24N3O9 (%): C 36.5, H 1.9, N 3.4, F 36.5.
{[Cu(hfac)2]3(NN)2}·C7H16. Heptane (5 mL) was added to a solution of nitronylnitroxyl (0.01 g, 0.032 mmol) and Cu(hfac)2 (0.023 g, 0.048 mmol) in CH2Cl2 (1 mL), and the mixture was kept in an open vessel at 4 °C for 72 h. The resulting elongated plate-like dark blue crystals were filtered off, washed with cold heptane, and dried in air. Yield: 0.024 g (66%). The elemental analysis was performed for a partially desolvated complex with the {[Cu(hfac)2]3(NN)2}·0.3C7H16 composition. Tm = 95-97 °C. Found (%): C 39.1, H 2.5, N 3.7, F 33.3. Calculated for C68.3H51.28Cu3F36N6O16 (%): C 39.3, H 2.5, N 4.0, F 33.1.
{[Cu(hfac)2]3(NN)2}. Heptane (5 mL) was added to a solution of nitronylnitroxyl (0.013 g, 0.04 mmol) and Cu(hfac)2 (0.038 g, 0.08 mmol) in CH2Cl2 (1 mL), and the solution was kept in an open vessel at 4 °C for 72 h. The resulting mixture of dark blue {[Cu(hfac)2]3NN2} plates and initial Cu(hfac)2 was separated mechanically; the isolated crystals of the complex were washed with cold heptane. The yield of the target product was 0.023 g (55%). Tm = 98-100 °C. Found (%): C 38.5, H 2.1, N 3.5, F 32.9. Calculated for C66H46Cu3F36N6O16 (%): C 38.6, H 2.3, N 4.1. F 33.3.
RESULTS AND DISCUSSION
The NN and IN nitronylnitroxyl radicals were prepared according to Ullman′s classical procedure [15] by the condensation of the corresponding aldehyde with bis(hydroxylamine) and subsequent oxidation of resulting hydroxyimidazolidines by the action of NaIO4 (Scheme 2). The peculiarity of this reaction is that the stage of neutralization of the NaHCO3 reaction mixture is accompanied by the formation of a precipitate from which nitronylnitroxyl NN is formed upon subsequent oxidation, while the IN iminonitroxyl radical is isolated from the remaining mother liquor as a result of its oxidation.
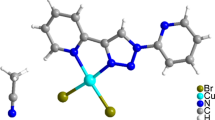
Structures of the radicals were established by single crystal XRD (Fig. 1a, b). The length of N–O bonds in nitroxyl groups are typical of nitroxyls [18] and are within 1.274(2)-1.285(2) Å. Note that the ONO atom in the structure of IN is disordered over two positions with the 94/06 ratio (in Fig. 1b, the O atom in the less occupied position is shown light gray). The benzene ring is rotated relative to the imidazoline fragment by 31.8° in IN and by 27.8° in NN. The C–C≡N fragment is linear: the CCN angles in NN and IN are similar and are equal to 179.4(2)° and 179.6(2)°, respectively.
Fig. 1c shows experimental μeff(T) dependences for IN and NN radicals. The μeff value for IN at 300 K is equal to 1.6 μB; it remains almost constant down to 25 K and then slightly decreases to 1.26 μB at 2 K. The high-temperature μeff value for IN is smaller than the theoretical pure spin value of 1.73 μB for the monoradical with the spin S = 1/2 for g = 2.
Apparently, this is due to the small amount of the material available for measurements and due to the possible presence of diamagnetic impurities, even though the microanalysis data indicated purity of the IN sample. The high-temperature μeff value for NN is equal to 1.71 μB and agrees well with the theoretical value for the monoradical. At first, μeff gradually decreases with decreasing temperature, more rapidly below 50 K, and reaches 0.38 μB at 2 K, thus indicating the presence of quite strong antiferromagnetic exchange interactions. The experimental μeff(T) dependence for IN is well described by the dimer model (the spin Hamiltonian H = –2J·S1S2), whereas that for NN is described by the model of exchange-coupled chains (H = –2J·∑SiSi+1). The optimal values of exchange interaction parameters J are equal to –0.94±0.02 cm–1 and –5.48±0.07 cm–1 for IN and NN, respectively. The difference between magnetic behaviors of the radicals agrees with the XRD data and is related to the features of their molecular packings in crystals. Thus, the intermolecular distances between ONO atoms of paramagnetic fragments in the structure of IN exceed 4.5 Å, while ONO…ONO and ONO…NNO distances in the structure of NN are significantly shorter (3.693(2) Å and 3.486(2) Å, respectively).
A series of coordination polymers of various structures was prepared by the interaction of IN and NN radicals with Cu(hfac)2. The complex with the {[Cu(hfac)2]2(IN)} composition (Cu(hfac)2:IN = 2:1) is a chain polymer (Fig. 2) formed by the bridging coordination of the radical by ONO atoms of the nitroxyl group (Cu2–ONO 2.703(2) Å) and NCN atoms of the nitrile group (Cu2′–NCN 2.514(3) Å). The N atoms of iminonitroxyls are additionally connected to the Cu(hfac)2 terminal fragments (Cu1–N 1.999(3) Å). The Cu1 coordination environment is a distorted trigonal bipyramid (τ = 0.657 [20]); axial Cu–Ohfac distances are 1.940(3) Å and 1.941(3) Å; equatorial Cu–Ohfac distances are 2.037(3) Å and 2.077(3) Å. The Cu2 coordination polyhedron is an elongated square bipyramid (equatorial Cu–Ohfac distances are 1.917(3)-1.934(3) Å). The angle of rotation of the benzene ring {C6} relative to the imidazoline fragment {C2N2O} in the coordinated IN is larger by 12° than that in the free IN (Table 2).
The polymer chain of the {[Cu(hfac)2]2(IN)} complex has a close structure to that of a polymorphic modification of the complex with the tetramethyl analogue Me4–IN [21]. The {[Cu(hfac)2]2(Me4–IN)} complex also realizes the tritope function of the paramagnetic ligand; the chain polymer is formed due to the bridging coordination of the radical by ONO atoms of the nitroxyl group and NCN atoms of the nitrile group with additional coordination of the iminonitroxyl N atom by the terminal Cu(hfac)2 fragment. But Cu2–ONO distances in the complex with a tetramethyl analogue are shorter (2.621 Å) while Cu2′–NCN and Cu1–N distances are longer (2.557 Å and 2.026 Å, respectively) than in {[Cu(hfac)2]2(IN)}.
Fig. 3 shows results of the study of magnetic properties of {[Cu(hfac)2]2(IN)}. The μeff value at 300 K is equal to 3.38 μB; it increases with decreasing temperature up to 3.91 μB at 15 K and then decreases to 3.54 μB at 2 K. The experimental μeff(T) dependence is well described by the trimer model (spin Hamiltonian H = –2J1·SCu1SR – 2J2·SRSCu2) taking into account intercluster exchange interactions zJ′. Optimal values of the g-factor of Cu(II) ions and exchange interaction parameters J1, J2, and zJ′ are equal to 2.09±0.01 cm–1, 130±3 cm–1, 26.6±0.5 cm–1, and –0.06±0.01 cm–1, respectively.
The reaction of Cu(hfac)2 with NN (Cu(hfac)2:NN = 2:3) yielded a complex with the {[Cu(hfac)2]3(NN)2}·C7H16 composition. After increasing the ratio of reactants up to 2:1, unsolvated {[Cu(hfac)2]3(NN)2} was isolated into the solid phase from a mixture with Cu(hfac)2. The crystals of this compound were separated mechanically. Even though the ratio of NN and Cu(hfac)2 in {[Cu(hfac)2]3(NN)2} is the same as in and Cu(hfac)2]3(NN)2}·C7H16, these complexes have different structures and magnetic properties.
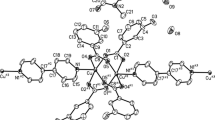
The structure of unsolvated {[Cu(hfac)2]3(NN)2} is formed by polymer chains where ONO atoms of the nitronyl nitroxyl fragment NN connect Cu(hfac)2 fragments (atoms Cu1 and Cu3 in Fig. 4a). Two neighboring chains are oriented towards each other by their cyanophenyl groups (all cyanophenyl fragments are located on the same side of the chain), and additional Cu(hfac)2 fragments (atoms Cu2 and Cu4 in Fig. 4a), coordinating NCN atoms, combine the chains into ribbons. The coordination environment of Cu2 and Cu4 is a centrosymmetric square bipyramid with the lengths of equatorial Cu–Ohfac bonds equal to 1.940(1)-1.960(1) Å and the lengths of axial Cu–NCN bonds equal to 2.421(5) Å and 2.461(6) Å (Table 2). The elongated square bipyramids of Cu1 and Cu3 with cis coordinated hfac differ by the lengths of axial Cu–ONO bonds and the angles at ONO atoms (1.950(3) Å, 2.477(4) Å and 124.0(3)°, 124.2(3)° for Cu1 and 2.395(4) Å, 2.685(4) Å and 130.3(3)°, 156.9(3)° for Cu3); the lengths of equatorial Cu–Ohfac bonds range from 1.907(4) to 1.968(3) Å. The intramolecular distance between coordinated ONO atoms is 2.978(5) Å. The lengths of N–O bonds in the nitroxyl groups are different (1.260(6)-1.278(5) Å and 1.302(4) Å), so that the shorter Cu–ONO distance corresponds to the longer N–O.
The temperature dependence μeff for {[Cu(hfac)2]3NN2} has a complex character (Fig. 4c). At 300 K, μeff is equal to 3.37 μB, which is much lower than the theoretical pure spin value 4.06 μB for five paramagnetic centers with spins S = 1/2 (three Cu(II) ions with gCu = 2.16 and two nitroxyls with gR = 2.0) and is due to strong antiferromagnetic exchange interactions typical for equatorial nitroxyls in a square-bipyramidal environment of the Cu(II) ion (the Cu1–O1R distance is 1.950 Å). As the temperature decreases, the μeff value slightly decreases down to 3.3 μB at 150 K, increases to 3.45 μB at 15 K, and then sharply decreases. The μeff increase in the temperature range 150-15 K indicates a presence of ferromagnetic exchange interactions typical for axial nitroxyls (the Cu3–O1S distance is 2.395 Å). When conducting analysis of the experimental μeff(T) dependence based on the XRD data, it is reasonable to use a model that would take into account strong antiferromagnetic exchange interactions JAF for the equatorial coordination of NN by the Cu1 ion, ferromagnetic exchange interactions JF for the axial coordination of NN by the Cu3 ion, weak exchange interactions JW between the spins of the Cu4 ion and the coordinated nitrile groups of nitroxyls, and quasi-isolated spins of Cu2 ions (Fig. 4b). Optimal values of exchange interaction parameters JAF, JF, and JW are equal to 325±5 cm–1, 36±1 cm–1, and –0.41±0.02 cm–1 (Fig. 4b, red curve) at fixed values gCu = 2.16 and gR = 2.0. The employed model slightly overestimates the JF value, since the theoretical curve is higher than the experimental data in the region of 25-130 K.
The solvated {[Cu(hfac)2]3(NN)2}·C7H16 complex is a layered polymer (Fig. 5). Similarly to the unsolvated complex, its structure contains polymer chains formed by bridging O,O-coordination of NN, but all Cu atoms occur in a centrosymmetric square-bipyramidal environment with axial Cu1–ONO and Cu2–ONO distances equal to 2.521(3) Å and 2.455(3) Å, respectively, and equatorial Cu–O and Cu–N distances ranging from 1.913(1) Å to 1.934(1) Å. The centrosymmetric character of the environment of Cu1 and Cu2 ions makes that the cyanophenyl fragments of neighboring NNs orient in different directions relative to the chain, while the coordination of NCN atoms by additional Cu(hfac)2 fragments (Cu3–NCN distances are 2.381(1) Å) connects the chains into a layer.
Fig. 6 shows results of the study of magnetic properties of {[Cu(hfac)2]3(NN)2}·C7H16. The μeff value at room temperature is equal to 4.25 μB, in good agreement with the theoretical pure spin value 4.06 μB for five paramagnetic centers with spins S = 1/2 (three Cu(II) ions (gCu = 2.16) and two nitroxyls (gR = 2.0)). The μeff value increases with decreasing temperature, thus indicating that exchange interactions between the spins of paramagnetic centers are ferromagnetic in character (Fig. 6a). A sharp increase of μeff below 20 K indicates a transition to the magnetically ordered state. The energy of exchange interactions can be estimated by analyzing the experimental μeff(T) dependence within the exchange-coupled chain model (spin Hamiltonian H = –2J·∑SiSi+1) while considering magnetic susceptibility of Cu(II) ions of CuO4N2 coordination sites according to Curie′s law. Optimal gCu and J values are 2.161±0.007 cm–1 and 19.1±0.3 cm–1, respectively. The dependence of magnetization M on the strength of external magnetic field for {[Cu(hfac)2]3(NN)2}·C7H16 is non-linear (Fig. 6b). Above 20 kOe, the magnetization reaches saturation of ~5 μB corresponding to ferromagnetic spin ordering. The M(H) dependence shows no hysteresis, meaning that {[Cu(hfac)2]3(NN)2}·C7H16 is a soft ferromagnet at helium temperatures.
Coordination polymers {[Cu(hfac)2]3(NN)2} and {[Cu(hfac)2]3(NN)2}·C7H16 are built by chains with nitroxyl fragments acting as –Cu–{O–N–C–N–O}–Cu–O– bridges, and the difference between magnetic properties is related primarily to the structure of coordination sites. The polymer chains of {[Cu(hfac)2]3(NN)2} contain alternating coordination sites CuO6 with cis- and trans-coordinated nitroxyl groups so that a half of these sites participate in strong antiferromagnetic exchange interactions. In {[Cu(hfac)2]3(NN)2}·C7H16, all CuO6 coordination sites are centrosymmetric, i.e. ONO atoms occur in trans positions, thus ensuring ferromagnetic exchange interaction between paramagnetic centers inside the chains. In the complexes with the tetramethyl analogue Me4–NN [13, 21], ONO atoms in the polymer chains also occur in the axial positions of Cu bipyramids, but their Cu–ONO distances are much longer (2.488 Å and 2.697 Å for the α-modification, 2.414 Å and 2.638 Å for β-[Cu(hfac)2(Me4–NN)], and 2.530 Å and 2.637 Å for the {[Cu(hfac)2]2(Me4–NN)} complex). The increase of Cu–ONO distances in Cu(II) complexes with the Me4–NN tetramethyl nitroxyl decreases the energy of ferromagnetic exchange interactions and hinders the transition to the magnetically ordered state.
CONCLUSIONS
In this study, we prepared cyanophenyl-substituted nitroxyl radicals NN and IN containing spirocyclopentyl substituents in the imidazoline fragment. It was established that the interaction of NN and IN nitroxyls with Cu(hfac)2 leads to the formation of chain or layered polymeric heterospin complexes due to the realization of a tritopic function of paramagnetic ligands. The conducted magnetochemical studies showed that the formation of exchange-coupled chains due to the bridge coordination of the nitronylnitroxyl fragment with ONO atoms in axial positions of [CuO6] coordination sites provides ferromagnetic exchange interactions in {[Cu(hfac)2]3(NN)2}·C7H16 and transition to the magnetically ordered state at helium temperatures.
ACKNOWLEDGMENTS
This work was funded by the Russian Science Foundation (grant No. 22-73-00315, synthesis of compounds) and by the State Assignment of the Ministry of Science and Higher Education of the Russian Federation (theme No. 121012290037-2, structural and magnetic studies).
REFERENCES
V. Ovcharenko. Metal–Nitroxide Complexes: Synthesis and Magnetostructural Correlations. In: Stable Radicals / Ed. R. Hicks. Chichester, UK: John Wiley & Sons, 2010, 461. https://doi.org/10.1002/9780470666975.ch13
V. Ovcharenko and E. Bagryanskaya. Breathing Crystals from Copper Nitroxyl Complexes. In: Spin-Crossover Mater / Ed. M.A. Halcrow. Oxford, UK: John Wiley & Sons, 2013, 239. https://doi.org/10.1002/9781118519301.ch9
P. Rey and V. I. Ovcharenko. Copper(II) nitroxide molecular spin-transition complexes. ChemInform, 2003, 34(32), 41. https://doi.org/10.1002/chin.200332201
V. I. Ovcharenko, S. V. Fokin, G. V. Romanenko, Y. G. Shvedenkov, V. N. Ikorskii, E. V. Tretyakov, and S. F. Vasilevskii. Nonclassical spin transitions. J. Struct. Chem., 2002, 43(1), 153. https://doi.org/10.1023/A:1016094421024
V. I. Ovcharenko, S. V. Fokin, E. T. Kostina, G. V. Romanenko, A. S. Bogomyakov, and E. V. Tretyakov. First example of a reversible single-crystal-to-single-crystal polymerization–depolymerization accompanied by a magnetic anomaly for a transition-metal complex with an organic radical. Inorg. Chem., 2012, 51, 12188. https://doi.org/10.1021/ic301328x
V. I. Ovcharenko, G. V. Romanenko, K. Y. Maryunina, A. S. Bogomyakov, and E. V. Gorelik. Thermally induced magnetic anomalies in solvates of the bis(hexafluoroacetylacetonate)copper(II) complex with pyrazolyl-substituted nitronyl nitroxide. Inorg. Chem., 2008, 47, 9537. https://doi.org/10.1021/ic8011074
N. A. Artiukhova, G. V. Romanenko, G. A. Letyagin, A. S. Bogomyakov, S. L. Veber, O. V. Minakova, M. P. Petrova, V. A. Morozov, and V. I. Ovcharenko. Spin transition in the Cu(hfac)2 complex with (4-ethylpyridin-3-yl)-substituted nitronyl nitroxide caused by the “asymmetric” structural rearrangement of exchange clusters in the heterospin molecule. Crystals, 2019, 9, 285. https://doi.org/10.3390/cryst9060285
M. A. Agafonov, E. V. Alexandrov, N. A. Artyukhova, G. E. Bekmukhamedov, V. A. Blatov, V. V. Butova, Y. M. Gayfulin, A. A. Garibyan, Z. N. Gafurov, Y. G. Gorbunova, L. G. Gordeeva, M. S. Gruzdev, A. N. Gusev, G. L. Denisov, D. N. Dybtsev, Y. Y. Enakieva, A. A. Kagilev, A. O. Kantyukov, M. A. Kiskin, K. A. Kovalenko, A. M. Kolker, D. I. Kolokolov, Y. M. Litvinova, A. A. Lysova, N. V. Maksimchuk, Y. V. Mironov, Y. V. Nelyubina, V. V. Novikov, V. I. Ovcharenko, A. V. Piskunov, D. M. Polyukhov, V. A. Polyakov, V. G. Ponomareva, A. S. Poryvaev, G. V. Romanenko, A. V. Soldatov, M. V. Solovyeva, A. G. Stepanov, I. V. Terekhova, O. Y. Trofimova, V. P. Fedin, M. V. Fedin, O. A. Kholdeeva, A. Yu. Tsivadze, U. V. Chervonova, A. I. Cherevko, V. F. Shul′gin, E. S. Shutova, and D. G. Yakhvarov. Metal-organic frameworks in Russia: From the synthesis and structure to functional properties and materials. J. Struct. Chem., 2022, 63(5), 671-843. https://doi.org/10.1134/s0022476622050018
S. V. Fokin, S. E. Tolstikov, E. V. Tretyakov, G. V. Romanenko, A. S. Bogomyakov, S. L. Veber, R. Z. Sagdeev, and V. I. Ovcharenko. Molecular magnets based on chain polymer complexes of copper(II) bis(hexafluoroacetylacetonate) with isoxazolyl-substituted nitronyl nitroxides. Russ. Chem. Bull., 2011, 60(12), 2470-2484. https://doi.org/10.1007/s11172-011-0382-6
A. S. Bogomyakov, G. V. Romanenko, S. V. Fokin, E. T. Chubakova, E. V. Tretyakov, and V. I. Ovcharenko. Copper(II) complexes with CF3-substituted spin-labeled pyrazoles. Russ. J. Coord. Chem., 2022, 48(12), 772-783. https://doi.org/10.1134/s1070328422700014
C. Hirel, L. Li, P. Brough, K. Vostrikova, J. Pécaut, B. Mehdaoui, M. Bernard, P. Turek, and P. Rey. New spin-transition-like copper(II)–nitroxide species. Inorg. Chem., 2007, 46, 7545. https://doi.org/10.1021/ic700851x
N. A. Artiukhova, K. Y. Maryunina, S. V. Fokin, E. V. Tretyakov, G. V. Romanenko, A. V. Polushkin, A. S. Bogomyakov, R. Z. Sagdeev, and V. I. Ovcharenko. Spirocyclic derivatives of nitronyl nitroxides in the design of heterospin Cu(II) complexes manifesting spin transitions. Russ. Chem. Bull., 2013, 62, 2132. https://doi.org/10.1007/s11172-013-0312-x
O. V. Koreneva, G. V. Romanenko, Y. G. Shvedenkov, V. N. Ikorskii, and V. I. Ovcharenko. Molecular magnets based on M(hfac)2 and spin-labeled nitrile. Polyhedron, 2003, 22, 2487. https://doi.org/10.1016/S0277-5387(03)00229-8
J. A. Bertrand and R. I. Kaplan. A study of bis(hexafluoroacetylacetonato) copper(II). Inorg. Chem., 1966, 5, 489. https://doi.org/10.1021/ic50037a039
J. H. Osiecki and E. F. Ullman. Studies of free radicals. I. α-Nitronyl nitroxides, a new class of stable radicals. J. Am. Chem. Soc., 1968, 90, 1078. https://doi.org/10.1021/ja01006a053
N. F. Chilton, R. P. Anderson, L. D. Turner, A. Soncini, and K. S. Murray. PHI: a powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem., 2013, 34, 1164-1175. https://doi.org/10.1002/jcc.23234
L. Krause, R. Herbst-Irmer, G. M. Sheldrick, and D. J. Stalke. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. Appl. Crystallogr., 2015, 48, 3. https://doi.org/10.1107/S1600576714022985
G. M. Sheldrick. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3-8. https://doi.org/10.1107/S2053273314026370
G. M. Sheldrick. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3-8. https://doi.org/10.1107/S2053229614024218
A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn, and G. C. Verschoor. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua [1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate J. Chem. Soc., Dalton Trans., 1984, 7, 1349. https://doi.org/10.1039/DT9840001349
O. V. Koreneva, G. V. Romanenko, and V. I. Ovcharenko. Polymorphs of Cu(hfac)2 complexes with iminonitroxides. J. Struct. Chem., 2004, 45(2), 340-343. https://doi.org/10.1023/B:JORY.0000048889.84289.58
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2023, published in Zhurnal Strukturnoi Khimii, 2023, Vol. 64, No. 6, 111320.https://doi.org/10.26902/JSC_id111320
Rights and permissions
About this article
Cite this article
Artyukhova, N.A., Zargarova, L.V., Romanenko, G.V. et al. Synthesis, Structure, and Magnetic Properties of Cu(hfac)2 Polymeric Complexes with Cyanophenyl-Substituted Nitroxyls. J Struct Chem 64, 963–973 (2023). https://doi.org/10.1134/S002247662306001X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002247662306001X