Abstract
A comparative analysis of the molecular and crystal structures is performed for 5-[(diphenylphosphoryl) methyl]-4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (1) in individual crystal (1a) and a crystal solvate with dimethylformamide (DMF) in the 1:1 ratio (1b). The crystals of both modifications have the identical geometries of the molecule of the key compound, and the crystals (despite their different crystal systems and unit cells parameters) are characterized by the formation of an identical one-dimensional supramolecular motif in them due to classical N–H...O hydrogen bonds and weaker noncovalent – C-H ... S interactions in crystal 1a and CH ... N in crystal 1b. A tetragonal packing of one-dimensional motifs oriented along the smallest unit cell parameter are observed in both cases. Solvate molecules are localized in zero-dimensional cavities in crystal 1b. Despite a denser molecular packing in crystal 1b, the solid-state phase transformation is observed for its polycrystalline sample, and the powder X-ray diffraction method shows that it partially transforms into form 1a with time. The latter form is characterized by a less dense molecular packing in the crystal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Russian Text © The Author(s), 2021, published in Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 3, pp. 481-489.
INTRODUCTION
The heterocyclic 1,2,4-triazole system is a unique heterocyclic platform [1—3] whose modification makes it possible to obtain a compound with a wide spectrum of the biological action, including antimicrobial, antibacterial, analgesic, anticancer, anti-inflammatory, etc., on its base [4—9]. Derivatives of 1,2,4-triazoles and 1,2,4-triazole-3-thiones are also used as insecticidal, herbicidal, antifungal, and plant growth regulating compounds [10, 11].
Such a wide spectrum of the biological activity stimulates the search for new synthetic approaches of obtaining polyfunctional compounds based on 1,2,4-triazoles in order to combine the pharmacological properties of separate fragments due to the synergetistic effect. Our scientific group has previously elaborated the synthesis of new phosphorylated derivatives of 1,2,4-triazole-3-thiones (Scheme 1) with substitution at the N atom by heterocyclization of respective diphenylphosphorylacetyl thiosemicarbazides and reported the results in [12, 13]. The occurrence of only a pair of proton-donor and proton-acceptor centers in a molecule of a derivative of this class of compounds substantially decreases the number of possible combinations of intermolecular interactions involving these centers. However, even when several acceptor centers are present in the molecule of the compound and there is a possible competition between them, the formation of the primary supramolecular motif in a crystal occurs with the participation of a phosphoryl group as a hydrogen bond acceptor [14, 15]. It may be assumed that for the crystalline derivatives of this series of compounds the formation of an identical supramolecular synthon involving these functionally active groups is observed. Note that crystal structure data for compounds whose molecules include a phosphorus-containing moiety and a planar triazole ring unsubstituted at the N2 position are absent in publications, which additionally actualizes structure studies of polyfunctional heterocyclic compounds of this type.
In this work, we consider the features of molecular and crystal structures of N-phenyl-2,4-dihydro-3Н-1,2,4-triazole-3-thione (1) presented as two crystalline modifications.
EXPERIMENTAL
Single crystal X-ray diffraction (XRD) experiments were performed on a Rigaku XtaLab Synergy S diffractometer [(λ(CuKα) = 1.54184 Å] at T = 100 K. Data collection, edition and refinement of unit cell parameters were carried out using the CrysAlisPro program. Absorption correction was applied using the ABSPACK program. The structures were solved by a direct method using SHELXT [16] and refined by the leas-squares technique in the isotropic, first, and then anisotropic approximation (for all non-hydrogen atoms) using SHELXL-2014 programs [17] in the Olex2 program package [18]. Coordinates of hydrogen atoms were calculated based on stereochemical criteria and refined in the respective riding models. Intermolecular interactions were analyzed and the figures were drawn using PLATON [19] and Mercury [20] programs. Crystallographic data for compounds 1a and 1b obtained in this work (Table 1) have been deposited with the Cambridge Crystallography Data Center and can be obtained by request at www.ccdc.cam.ac.uk/data_request/cif.
Powder XRD patterns were measured on an automated Bruker D8 Advance diffractometer equipped with a Vario attachment and a linear position-sensitive Vantec detector. CuKα1 radiation (λ = 1.54063 Å) monochromated by a curved Johansson monochromator was applied; the X-ray tube mode was 40 kV, 40 mA. Experiments were performed at room temperature in the Bragg–Brentano geometry with a planar sample. A polycrystalline sample for the XRD experiment was obtained by evaporating a DMF solution at room temperature. The ready powder was slightly mashed and without strong pressing deposited on a silicon plate decreasing the background scattering. XRD patterns were measured in a 2θ scattering angle range 3—50°, step 0.008°, point acquisition time 1 s. To remove the effect of the preferred sample orientation and data averaging the sample was rotated in its own plane with a speed of 15 rpm. The obtained data were processed using the EVA program package [21].
RESULTS AND DISCUSSION
The studied sample of the triazole derivative was obtained as a result of the previously described reaction [12] of 2-[(diphenylphosphoryl)acetyl]-N-phenylhydrazine-1-carbothiamide with phenyl isothiocyanate. Reaction product 5-[(diphenylphosphoryl)methyl]-4-phenyl-2,4-dihydro-3Н-1,2,4-triazole-3-thione (1) is white powder that according to the powder XRD data is crystalline. As is found, the sample is practically insoluble at room temperature in most standard solvents, except dimethyl sulfoxide (DMSO) and DMF. Thus, single crystals suitable for the XRD analysis were obtained by long crystallization from DMSO (compound 1a) and DMF (compound 1b) at room temperature.
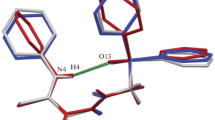
In the individual form, the phosphorylated derivative of 4-phenyl-2,4-dihydro-3Н-1,2,4-triazole-3-thione (compound 1a) forms monoclinic crystals with one molecule in the asymmetric unit of the unit cell whereas a solvate with DMF in the 1:1 ratio is presented as triclinic crystals (compound 1b), but also with one independent molecule of 1,2,4-triazole-3-thione in the unit cell. Molecular geometries in crystals 1a and 1b are depicted in Fig. 1. Selected bond lengths and bond angles of the molecules are listed in Table 2, and their values are in general within standard limits characteristic of organic compounds. In the crystals of compounds 1a and 1b, the planes of the phenyl substituent at the N position are turned relative to the triazole ring plane at dihedral angles with close values of 84.15° and 83.4° respectively (Fig. 1), while two phenyl rings of the diphenylphosphoryl moiety are turned relative to each other at dihedral angles of 72.36° in 1a and 80.47° in 1b.
On the example of several crystalline modifications of initial N1-(diphenylphosphoryl)acetyl-N4-phenyl-thiosemicarbaside, we have previously shown that in different crystals of this compound, despite the close geometry of their acyclic core, there is a noticeable distinction in the turns of phenyl rings in the diphenylphosphoryl moiety [15], up to their orthogonal arrangement. As we expected, it was related to the adjustment of supramolecular structures for the formation of the close molecular packing in the crystal. Nonetheless, in crystals 1a and 1b of 1,2,4-triazole-3-thione we do not observe this lability in the spatial arrangement of the planes of phenyl rings in the diphenylphosphoryl moiety, which may be due to their possible identical supramolecular morphology. Thus, two crystal modifications of 4-phenyl-2,4-dihydro-3Н-1,2,4-triazole-3-thione are characterized by minimum distinctions in the molecular geometry.
Asymmetric units of the unit cells of crystals 1a and 1b. Non-hydrogen atoms are represented by thermal vibrational ellipsoids (p = 50%); hydrogen atoms are shown by spheres of an arbitrary radius. Atomic numbering of the 1 molecule is identical in crystals 1a and 1b and is shown only for one of them.
It should be noted that the comparison of the experimental powder XRD pattern of the initial crystalline phase with XRD patterns (Fig. 2) calculated for crystals 1a and 1b indicates in favor of the fact that the starting reaction mass is either the pure unknown crystalline form of 4-phenyl-2,4-dihydro-3Н-1,2,4-triazole-3-thione or additionally contains a very small amount of its individual crystalline form whose single crystals were obtained by recrystallization from DMSO. The preliminary indexation of the powder XRD pattern for the determination of unit cell parameters did not unambiguously answer the question about the meaning of the non-individual character of crystallization of this compound.
As previously mentioned, the 4-phenyl-2,4-dihydro-3Н-1,2,4-triazole-3-thione molecule contains several functional group capable of participating in intermolecular interactions of different natures and strengths. Parameters of intermolecular interactions occurring in the crystals of compounds 1a and 1b are given in Table 3.
A linear H-chain formed by N–H…O interactions involving the O7 oxygen atom of the phosphoryl group (N2–H2…O7) and oriented along the least unit cell parameter b can be distinguished as the primary crystal-forming motif in crystal 1a (Fig. 3a). Similar one-dimensional H-chains are linked in pairs with each other by weaker – C–H…S interactions (С21–Н21…S3) involving a hydrogen atom of the diphenylphosphoryl moiety, forming a secondary supramolecular cylindrical motif so that aromatic hydrophobic fragments of molecules of two bound H-chains are located at the external surface of cylindrical structures (Fig. 3b).
A similar one-dimensional H-chain is formed also along the least unit cell parameter a in crystal 1b due to the N2–H2…O7 interaction. However, the formation of the secondary supramolecular structure (a one-dimensional cylindrical motif similar to that observed in crystal 1a) occurs already due to C–H…N interactions (C9–H9…N1). Here the solvate molecules are weakly bound with the molecules of the main compound only by non-classical intermolecular С–H…O interactions and are located in island voids (Table 3, Fig. 4, 1b).
The DMF molecule contains an oxygen atom that can participate in competing intermolecular interactions with molecules of the main compound. The inclusion of this molecule into the crystal structure seems to affect both molecular structure of the compound and supramolecular organization of the crystal as a whole. Meanwhile, on the example of 4-phenyl-2,4-dihydro-3Н-1,2,4-triazole-3-thione it is shown that two crystal modifications 1a and 1b are characterized by not only the identical geometry of molecules and the supramolecular organization in crystals, but also the maintenance of the tetragonal type of packings of one-dimensional cylindrical motifs in the crystals (Fig. 5) and the orientation of these supramolecular structures along the least cell parameter. We have previously shown that the observed phenomenon is universal and was noted in crystals of benzodiazepine derivatives [22], derivatives of enantiopure and racemic terminal ethers of glycerin [23] and a series of other compounds. The absence of significant interactions in directions appears to imply that they are least stable directions in the crystal along which its destruction should be expected at the initial stages of the process.
The inclusion of solvate molecules into the crystal structure of 1b resulted in the filling of voids present in the crystal and a corresponding increase in the Kitaigorodskii packing coefficient [24] of 68.9% – the middle part of the range characteristic of crystals of organic compounds (0.65—0.75).
At the same time, the packing coefficient is substantially lower in crystal 1a and is 65.7%, although its analysis indicates the absence of vacant cavities in the crystal, which have a volume enough to accommodate solvate molecules. However, the features of the arrangement hydrophobic fragments of molecules in crystal 1a and the occurrence of small cavities in the hydrophobic regions (Fig. 4, 1a) do not reject the possible formation of solvated forms of crystals with embedding solvent molecules just into these crystal parts.
Despite a closer molecular packing in crystal 1b and the presence of a weak intermolecular binding between solvent molecules and molecules of the main compound, in the polycrystalline form, the compound is subject to solid-phase modifications, which we detected by powder XRD. As seen from Fig. 5, the first experimental powder XRD pattern (3) of the polycrystalline sample in general corresponds to the theoretical powder XRD pattern of crystal 1b (1) calculated based on single crystal data. In first XRD pattern (3), the appearance of the diffraction peak can be noted at 2θ = 8.3°, and in subsequent XRD patterns (4—8), there is an increase in the intensity of this peak. It is quite probable that first experimental curve (3) in Fig. 5 is obtained already during a change in this crystalline phase. One of the reasons for the observed solid-phase transition may be the removal of DMF solvent molecules from the crystal lattice and a transition of a part of the crystal solvate into the solvate-free form. However, the possibility that rapid crystallization from DMF results in the formation of the crystal solvat with it and a minor part of the initial sample crystallizes as the individual form of phosphorylated 4-phenyl-2,4-dihydro-3Н-1,2,4-triazole-3-thione cannot be excluded. In Fig. 5 the position of a new diffraction peak corresponds to the position of the most intense diffraction peak of the theoretical powder XRD pattern of crystal 1a (2). Moreover, despite the similarity of the crystal structures, their rearrangement turned out to be long enough.
CONCLUSIONS
Thus, by the single crystal XRD analysis it is found that two crystalline modifications of 4-phenyl-2,4-dihydro-3Н-1,2,4-triazole-3-thione, obtained by crystallization from DMSO (crystal 1a) and DMF (crystal 1b), despite different crystal systems and cell parameters, are characterized by the identical molecular structure, the supramolecular organization, and the tetragonal packing type of secondary supramolecular structures – one-dimensional cylindrical motifs. Solvent molecules are located in crystal 1b in island voids formed by aromatic fragments of the molecules and are weakly bound with the molecules of the main compound. At a closer molecular packing in crystal solvate 1b, its polycrystalline sample is subject to solid-phase modifications, and as is found by powder XRD, because of the identity of the supramolecular organization in the modifications, it partially transforms into solvate-free form 1a with time, which is characterized by a less close molecular packing.
REFERENCES
S. Maddila, R. Pagadala, and S. B. Jonnalagadda. Lett. Org. Chem., 2013, 10, 693–714.
J. K. Shneine and Y. H. Alaraji. Int. J. Recent Sci. Res., 2016, 5, 1411–1423.
B. Namratha and S. L. Gaonkar. Int. J. Pharm. Pharmaceut. Sci., 2014, 6, 73–80.
D. Ji, J. R. Lu, B. W. Lu, C. W. Xin, J. B. Mu, J. F. Li, C. Y. Peng, and X. R. Bao. Bioorg. Med. Chem. Lett., 2013, 23, 1997–2000.
C. Li, J. C. Liu, Y. R. Li, C. Gou, M. L. Zhang, H. Y. Liu, X. Z. Li, C. J. Zheng, and H. R. Piao. Med. Chem. Lett., 2015, 25, 3052–3056.
G. Lv, D.-L. Zhang, D. Wang, L. Pan, and Y. Liu. J. Struct. Chem., 2019, 60(7), 1173–1179.
B. Miroslaw, T. Plech, and M. Wujec. J. Mol. Struct., 2015, 1083, 187–193.
D. Sitaram, G. Celik, P. Khloya, D. Vullo, C. T. Supuran, and P. K. Sharma. Bioorg. Med. Chem., 2014, 22, 1873–1882.
A. Almasirad, S. A. Tabatabai, M. Faizi, A. Kebriaeezadeh, N. Mehrabi, A. Dalvandi, and A. Shafiee. Bioorg. Med. Chem. Lett., 2004, 14, 6057–6059.
M. Haidukowski, A. Visconti, G. Perrone, S. Vanadia, D. Pancaldi, L. Covarelli, R. Balestrazzi, and M. Pascale. Phytopathol. Mediterr., 2012, 51, 236–246.
Z. Zhiwen, W. Qiao, S. Zhonghua, T. Chengxia, W. Jianquan, and L. Xinghai. Chin. J. Org. Chem., 2017, 37, 232–236.
I. A. Krutov, E. L. Gavrilova, R. N. Burangulova, S. S. Kornilov, A. A. Valieva, A. I. Samigullina, A. T. Gubaidullin, O. G. Sinyashin, I. I. Semina, D. O. Nikitin, and A. V. Plotnikova. Russ. J. Gen. Chem., 2017, 87, 2794–2800.
E. L. Gavrilova, I. A. Krutov, A. A. Valieva, Kh. R. Khayarov, A. I. Samigullina, A. T. Gubaidullin, N. I. Shatalova, R. N. Burangulova, and O. G. Sinyashin. Russ. J. Gen. Chem., 2018, 88, 2269–2275.
I. A. Litvinov, S. V. Bukharov, F. A. Karamov, R. A. Khabibullina, N. I. Akylbekov, A. R. Burilov, R. G. Tagasheva, and E. L. Gavrilova. J. Struct. Chem., 2019, 60(11), 1804–1811.
A. I. Samigullina, I. A. Krutov, E. L. Gavrilova, and A. T. Gubaidullin. Phosphorus, Sulfur Silicon Relat. Elem., 2019, 194, 300–301.
G. M. Sheldrick. Acta Crystallogr., Sect. A, 2015, 71, 3.
G. M. Sheldrick. Acta Crystallogr., Sect. A, 2007, 64, 112.
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. J. Puschmann. Appl. Crystallogr., 2009, 42, 339.
A. L. Spek. J. Appl. Crystallogr., 2003, 36, 7.
C. F. Macrae, P. R. Edgington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler, and J. van de Streek. J. Appl. Crystallogr., 2006, 39, 453.
DIFFRAC Plus Evaluation package EVA (Version 11). Bruker AXS: Karlsruhe, Germany, 2005.
A. I. Samigullina, A. T. Gubaidullin, L. V. Mustakimova, and V. A. Mamedov. Russ. Chem. Bull., 2014, 63, 1444–1450.
A. T. Gubaidullin, A. I. Samigullina, Z. A. Bredikhina, and A. A. Bredikhin. CrystEngComm, 2014, 16, 6716–6729.
A. I. Kitaigorodskii. Molecular Crystals and Molecules. Academic Press: N.Y., 1973.
Funding
Single crystal XRD studies were supported by RFBR within scientific project No. 19-33-60032. Powder XRD experiments were performed within the State Contract of the Federal Scientific Kazan Research Center, Russian Academy of Sciences (A. T. Gubaidullin). The synthesis of 4-phenyl-2,4-dihydro-3Н-1,2,4-triazole-3-thione was supported by the Russian Scientific Foundation (grant No. 14-23-00073-p).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Russian Text © The Author(s), 2021, published in Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 3, pp. 481-489.https://doi.org/10.26902/JSC_id69917
Rights and permissions
About this article
Cite this article
Samigullina, A.I., Krutov, I.A., Gavrilova, E.L. et al. A SUPRAMOLECULAR STRUCTURE OF PHOSPHORYLATED N-PHENYL-1,2,4-TRIAZOLE- 3-THIONE AND ITS CRYSTAL SOLVATE. J Struct Chem 62, 452–459 (2021). https://doi.org/10.1134/S0022476621030124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621030124