Abstract
The isoform of voltage-gated potassium channels KV1.2 is of interest because mutations in its gene are associated with various diseases, such as ataxia and epilepsy. Selective ligands are needed to study the function of KV1.2 in health and disease. In our work, we obtained such a ligand based on the known scorpion peptide toxin, charybdotoxin (ChTx, α-KTx1.1) from the venom of Leiurus hebraeus, by introducing a single amino acid substitution M29I into its structure. ChTx_M29I peptide was produced in a bacterial expression system. Its pharmacological characterization was carried out in Xenopus laevis frog oocytes expressing a panel of human KV1 channels. We found that, compared to the parent toxin, ChTx_M29I peptide showed lower affinity for KV1.1, 1.3, and 1.6 channels, while its activity against KV1.2 increased manifold. We attribute this effect to the interaction of the peptide with a specific channel residue (V381 in KV1.2). If there is a relatively small residue at this position, then an advantageous contact is formed that increases the affinity. ChTx_M29I peptide studied by us presents one of the highest affinity (with a half-maximal inhibitory concentration IC50 ≈ 6 pM) and selectivity among KV1.2 ligands (affinity for other isoforms is lower by 680 times or more).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Voltage-gated potassium channels (KV) are transmembrane proteins and contain four major α-subunits [1]. Each α-subunit consists of a cytoplasmic T1 domain and six transmembrane helices (S1–S6). The S1–S4 helices form the voltage-sensing domain, in which it is the S4 helix that operates as the voltage sensor. The amino acid residues K and R, which are not typical for the intramembrane space, are found in its structure. In response to changes in the transmembrane potential, they cause movement of the entire helix, which ultimately regulates the opening and closing of the channel. The S5 and S6 helices of all four α-subunits form the pore domain, in which the selectivity filter contains the conserved TVGYG sequence that interacts with K+ ions.
Forty genes encoding KV α-subunits have been found in the human genome, making this group the largest among ion channels. In particular, channels of the KV1 subfamily are widely represented in the mammalian brain, with KV1.1, 1.2, 1.4, and 1.6 being particularly common [2]. These channel isoforms can form both homo- and heteromers, which affects their characteristics [3, 4]. Homotetrameric channels are mostly available to study due to the limitations of experimental systems, in which it is difficult to control the expression of heteromeric channels.
The KV1.2 channel isoform is of particular interest because, firstly, it is relatively uniformly expressed in the central nervous system among other KV1 isoforms, and secondly, it can form both functional homomeric and heteromeric channels with other KV1 [3]. Several diseases, such as ataxia and epilepsy, are associated with mutations of this isoform [5, 6]. Selective and high-affinity ligands are needed to study KV1.2 function in normal and pathological conditions.
One of the rich sources of KV ligands is scorpion venom. Many peptide KV blockers (KTx) have been isolated from the venom of various scorpion species; according to the Kalium database, there are about 200 of them [7]. According to the amino acid sequence, they are divided into several families: α-, β-, γ-, δ-, ε-, κ-, and λ-KTx. The α-KTx family is the largest and most studied; it includes peptides containing ~20–40 residues that assume the cysteine-stabilized α-helix/β-sheet fold (CSα/β). They are also characterized by the so-called “functional dyad” of K and Y residues, where K physically blocks the channel pore and Y interacts with its outer vestibule [8].
Charybdotoxin (ChTx, α-KTx1.1) is a classic toxin from the venom of the scorpion Leiurus hebraeus that has been studied on many channels. It shows high affinity and selectivity to KV1.3 channels as well as the calcium-activated potassium channels KCa1.1 and KCa3.1 [9, 10]. A number of derivatives of ChTx were obtained in Chris Miller’s laboratory during the studies of its interaction of with various potassium channels. A peptide with an M29I substitution caught our attention [11]. It was shown that if the T449F replacement was introduced in the KV of Drosophila Shaker, the affinity of ChTx_M29I dropped by a factor of 1650 compared with that of wild-type ChTx. In human channels, various residues including large aromatic residues are found in an analogous position. Therefore, we decided to test how this mutation would affect the affinity of the toxin with respect to human channels.
MATERIALS AND METHODS
Recombinant protein production. ChTx_M29I was obtained according to the standard protocol that we used in previous studies [12]. The peptide was produced in a bacterial expression system as a fusion protein containing: the carrier protein thioredoxin (Trx) [13], the hydrolysis site of human enteropeptidase light chain, and a His-tag for protein purification by affinity chromatography.
Cloning of the target gene. The DNA sequence encoding ChTx_M29I was obtained by two-step PCR using synthetic oligonucleotides. In the first step, four primers were used to obtain a full-length copy of the gene (Table 1). In the second step, the reaction mixture from the first step was used as a matrix for amplification with primers F1 and R1. The resulting DNA was cloned into the expression vector pET-32b (Novagen) at the KpnI and BamHI sites.
Expression and purification of the fusion protein. The expression strain Escherichia coli SHuffle T7 Express (New England Biolabs) was transformed with the vector carrying the ChTx_M29I gene; cell biomass growth in LB medium took place at 37°C until reaching the mid-exponential phase. Expression was induced by adding isopropyl-β-D-thiogalactopyranoside to a concentration of 0.4 mM. The cell biomass was then cultured at room temperature for 16 h. The cells were then disrupted by ultrasonication, and the cell lysate was applied to an HisPur Cobalt Resin column (Thermo Fisher Scientific). The hybrid protein was purified according to the resin manufacturer’s protocol.
Purification of the target peptide. The purified chimeric protein was dissolved in 50 mM Tris-HCl (pH 8.0) to a concentration of 1 mg/mL. Enzymatic hydrolysis was performed using human enteropeptidase light chain (1 U of enzyme per 1 mg of protein) at 37°C for 16 h [14]. The resulting hydrolysate was separated by reversed-phase high-performance liquid chromatography (RP-HPLC) in a linear gradient of acetonitrile concentration (0–60% for 60 min) on a Jupiter C5 column (4.6 × 250 mm, Phenomenex). Detection was based on the optical absorbance of the eluate, and the compound of interest was determined by comparing the calculated and experimentally obtained masses determined by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry.
Mass spectrometry. An Ultraflex TOF-TOF spectrometer (Bruker Daltonik) was used to measure the molecular masses as described previously [15]. 2,5-Dihydroxybenzoic acid (Sigma-Aldrich) was used as a matrix. The measurements were performed in the reflectron mode with a mass-accuracy error of no more than 100 ppm. Mass spectra were analyzed using Data Analysis 4.3 and Data AnalysisViewer 4.3 software (Bruker).
Electrophysiology. The experiments were performed according to the protocols published earlier [12]. Human voltage-gated potassium channels KV1.1 (GenBank ID: NM000217), 1.2 (NM004974), 1.3 (NM002232), and 1.6 (NM002235) were expressed in the oocytes of the frog Xenopus laevis. For this purpose, mRNAs encoding the channels were obtained using the mMESSAGE mMACHINE T7 kit (Thermo Fisher Scientific). The obtained mRNAs were then injected into the oocytes using a microinjector (Drummond Scientific).
Currents through the oocyte membrane were recorded at room temperature using the two-electrode voltage clamp method. Data were obtained using a GeneClamp 500 amplifier and Clampex 9 software (Molecular Devices). The holding potential was set at –90 mV, channel opening was induced by membrane depolarization to 0 mV for 500 ms, then the potential was maintained at –50 mV for another 500 ms and returned to the holding potential value.
To build the dose-response curve, the studied peptide was serially diluted and added to the oocyte chamber, where the final studied concentration was reached. The data obtained were analyzed using the Hill equation:
where y is the current inhibition in %, Cpeptide is the concentration of the peptide under study, IC50 is the half-maximum inhibitory concentration, and h is the Hill coefficient.
All data were obtained in at least three independent experiments (n ≥ 3). Results were processed using Origin software (OriginLab Corporation).
RESULTS
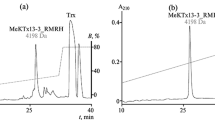
Obtaining the recombinant peptide ChTx_M29I. One of the main ways to obtain peptides with amino acid substitutions is to use bacterial expression systems. In order to make the peptide we want in bacteria, we need to obtain the genetic construct encoding it. We cloned the gene encoding ChTx_M29I into the expression vector pET-32b at the KpnI and BamHI restriction sites. We transformed the E. coli SHuffle T7 Express strain, which is designed to express disulfide-rich proteins [16], with the obtained construct. The target peptide was produced as a fusion protein with Trx, which was purified by immobilized metal ion affinity chromatography. The fusion protein was then subjected to enzymatic hydrolysis at the human enterokinase site, and the hydrolysate was separated by RP-HPLC (Fig. 1). The peak on the chromatogram corresponding to the ChTx_M29I peptide was determined by MALDI mass spectrometry. The yield of the target peptide was 4 mg from 1 liter of medium.
Electrophysiological study of the obtained peptide. We characterized the ChTx_M29I peptide pharmacologically using the two-electrode voltage clamp technique on a panel of KV1 channels. Compared with ChTx, it presents much lower affinity for KV1.1 and 1.6 channels: at a concentration of 2 µM, it blocks these channels by 3.1 ± 2.7% and 9.6 ± 0.6%, respectively (Fig. 2, Table 2). Among KVs, ChTx exhibited selectivity toward the KV1.3 channel (Table 2), whereas the resulting mutant was 1500 times more active toward the KV1.2 channel (IC50 = 6 ± 0.4 pM) and 20 times less active against KV1.3 (IC50 = 4.1 ± 0.8 nM). Thus, the ratio between the IC50 values with respect to the KV1.2 and 1.3 channels is 680-fold, making ChTx_M29I one of the most selective KV1.2 ligands.
Previously, Miller et al. suggested that there is an interaction between the ChTx toxin residue M29 and the Shaker channel residue T449 (Table 3). This leads to a high-affinity complex (Kd ≈ 0.063 nM) [11]. Introducing substitutions into the toxin (M29I) and channel (T449F) produced a less stable complex (Kd ≈ 1100 nM). So, the introduction of a large aromatic residue to the 449 position of the channel leads to an extreme deterioration in the binding of the ChTx_M29I derivative. In the case of human KV1 isoforms, the deterioration of binding of this derivative to the KV1.1 and 1.6 channels is not unexpected because the corresponding position contains a large aromatic residue (Y379 and Y429, respectively; Table 3).
In the case of KV1.3 we also observe a decrease in affinity, which can similarly be attributed to the presence of a large residue (H451) in this position. In turn, the KV1.2 channel has a relatively small hydrophobic residue (V381) in this position; van der Waals interactions with it seem to explain the high affinity of ChTx_M29I to this channel. To interpret the observed effects, we can consider the already known structure of the ChTx complex with the KV1.2/2.1 chimera [18]. We assume that ChTx_M29I is located in the vestibule of the channel pore in the same way as ChTx itself. In Fig. 3, we can see that the residue M29 of the toxin and V381 of the channel are close together in space. Accordingly, if the channel has a large aromatic residue in this position, it leads to steric hindrance and decreased affinity.
A toxin from the venom of the scorpion Mesobuthus eupeus named MeKTx11-1 (α-KTx1.16) and showing high affinity to KV1.2 (IC50 ≈ 0.2 nM) was previously obtained and characterized in our laboratory [19]. However, this toxin is inferior in both affinity and selectivity to the ChTx_M29I peptide discussed in this article. MeKTx11-1 has a methionine residue in its structure at position 29, which suggests that a more selective ligand could be obtained by making the same substitution M29I.
In order to compare our results with the literature, a list of selective ligands of the KV1.2 channel was compiled using the Kalium database (Table 4). Most of them are scorpion toxins, but several toxins of sea anemones, cone snails, and snakes can also be noted. The general trend observed is that most of the ligands are not highly selective. In addition to MeKTx11-1, urotoxin [20] and mesomartoxin (MMTX) [21], which have comparable selectivity to ChTx_M29I, but whose affinity is significantly lower, are worth mentioning. Finally, Pi-4 from the venom of the scorpion Pandinus imperator [22] has similar affinity and even exceeds ChTx_M29I in selectivity, but the activity of this peptide has not been studied against KV1.6.
Mutations in the KV1.2 channel gene can lead to epileptic encephalopathy [6]. These are mainly loss-of-function mutations, in which the channel loses its ability to open in response to a depolarizing stimulus. However, pathogenic KV1.2 gain-of-function mutations are known to cause a shift in the threshold potential of channel activation into the negative direction. In such a case, selective blockers could just find application as a pharmacological agent correcting the function of the mutant channel.
(a) Chromatographic separation of products of the fusion protein hydrolysis by enteropeptidase. (b) Spectrum of purified ChTx_M29I obtained by MALDI mass spectrometry in the reflectron mode, showing the corresponding monoisotopic masses. The calculated monoisotopic mass [M+H]+ is 4293 Da, and the experimentally measured mass is also 4293 Da.
Pharmacological characterization of ChTx_M29I. (a–d) Recordings of currents through the oocyte membrane in control (black curves) and in the presence of the peptide (2 µM for KV1.1 and 1.6, 6 pM for KV1.2 and 4 nM for KV1.3; gray curves). (e, f) Dose-response curves for channels KV1.2 and 1.3, the dotted lines show IC50 values. The Hill coefficients (h) are 1.1 ± 0.1 and 0.35 ± 0.02, respectively.
CONCLUSIONS
In this work, we demonstrated that a single amino acid substitution can lead to a significant change in the affinity and selectivity of a potassium channel ligand using the well-studied charybdotoxin (ChTx) as an example. The M29I substitution led to a decrease in the affinity of the toxin with respect to KV1.1, 1.3, and 1.6 channels, and we observed an increase in affinity of more than 1500-fold (IC50 ≈ 6 pM) with respect to the KV1.2 channel. The resulting peptide ended up being a high-affinity and high-selectivity ligand of the KV1.2 channel.
REFERENCES
Hille B (2001) Ion Channels of Excitable Membranes, 3rd ed. Sinauer Associates; Inc., Sunderland; Mass.
Zeisel A, Hochgerner H, Lönnerberg P, Johnsson A, Memic F, van der Zwan J, Häring M, Braun E, Borm LE, La Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S (2018) Molecular Architecture of the Mouse Nervous System. Cell 174: 999–1014. e22. https://doi.org/10.1016/J.CELL.2018.06.021
Shamotienko OG, Parcej DN, Dolly JO (1997) Subunit combinations defined for K+ channel Kv1 subtypes in synaptic membranes from bovine brain. Biochemistry 36: 8195–8201. https://doi.org/10.1021/bi970237g
Dodson PD, Barker MC, Forsythe ID (2002) Two heteromeric Kv1 potassium channels differentially regulate action potential firing. J Neurosci 22: 6953–6961. https://doi.org/10.1523/jneurosci.22-16-06953.2002
Pena SDJ, Coimbra RLM (2015) Ataxia and myoclonic epilepsy due to a heterozygous new mutation in KCNA2: proposal for a new channelopathy. Clin Genet 87: e1–e3. https://doi.org/10.1111/CGE.12542
Syrbe S, Hedrich UBS, Riesch E, Djémié T, Müller S, Møller RS, Maher B, Hernandez-Hernandez L, Synofzik M, Caglayan HS, Arslan M, Serratosa JM, Nothnagel M, May P, Krause R, Löffler H, Detert K, Dorn T, Vogt H, Krämer G, Schöls L, Mullis PE, Linnankivi T, Lehesjoki AE, Sterbova K, Craiu DC, Hoffman-Zacharska D, Korff CM, Weber YG, Steinlin M, Gallati S, Bertsche A, Bernhard MK, Merkenschlager A, Kiess W, Gonzalez M, Züchner S, Palotie A, Suls A, De Jonghe P, Helbig I, Biskup S, Wolff M, Maljevic S, Schüle R, Sisodiya SM, Weckhuysen S, Lerche H, Lemke JR (2015) De novo loss- or gain-of-function mutations in KCNA2 cause epileptic encephalopathy. Nat Genet 474 (47): 393–399. https://doi.org/10.1038/ng.3239
Tabakmakher VM, Krylov NA, Kuzmenkov AI, Efremov RG, Vassilevski AA (2019) Kalium 2.0, a comprehensive database of polypeptide ligands of potassium channels. Sci Data 61 (6): 1–8. https://doi.org/10.1038/s41597-019-0074-x
Mouhat S, De Waard M, Sabatier JM (2005) Contribution of the functional dyad of animal toxins acting on voltage-gated Kv1-type channels. J Pept Sci 11: 65–68.
MacKinnon R, Miller C (1988) Mechanism of charybdotoxin block of the high-conductance, Ca2+-activated K+ channel. J Gen Physiol 91: 335. https://doi.org/10.1085/JGP.91.3.335
Garcia ML, Garcia-Calvo M, Hidalgo P, Lee A, MacKinnon R (1994) Purification and characterization of three inhibitors of voltage-dependent K+ channels from Leiurus quinquestriatus var. hebraeus venom. Biochemistry 33: 6834–6839. https://doi.org/10.1021/bi00188a012
Naranjo D, Miller C (1996) A strongly interacting pair of residues on the contact surface of charybdotoxin and a Shaker K+ channel. Neuron 16: 123–130. https://doi.org/10.1016/S0896-6273(00)80029-X
Berkut AA, Usmanova DR, Peigneur S, Oparin PB, Konstantin S, Odintsova TI, Tytgat J, Arseniev AS, Grishin E V., Vassilevski AA, Mineev KS, Odintsova TI, Tytgat J, Arseniev AS, Grishin EV, Vassilevski AA (2014) Structural similarity between defense peptide from wheat and scorpion neurotoxin permits rational functional design. J Biol Chem 289: 14331–14340. https://doi.org/10.1074/jbc.M113.530477
McCoy J, LaVallie E (2001) Expression and Purification of Thioredoxin Fusion Proteins. In: Current Protocols in Molecular Biology. John Wiley & Sons Inc. Hoboken NJ USA. 16.8.1–16.8.14
Gasparian ME, Ostapchenko VG, Schulga AA, Dolgikh DA, Kirpichnikov MP (2003) Expression, purification, and characterization of human enteropeptidase catalytic subunit in Escherichia coli. Protein Expr Purif 31(1):133–139. https://doi.org/10.1016/S1046-5928(03)00159-1
Kuzmenkov AI, Sachkova MY, Kovalchuk SI, Grishin EV, Vassilevski AA (2016) Lachesana tarabaevi, an expert in membrane-active toxins. Biochem J 473: 2495–506. https://doi.org/10.1042/BCJ20160436
Lobstein J, Emrich CA, Jeans C, Faulkner M, Riggs P, Berkmen M (2012) SHuffle, a novel Escherichia coli protein expression strain capable of correctly folding disulfide bonded proteins in its cytoplasm. Microb Cell Fact 11: 56. https://doi.org/10.1186/1475-2859-11-56
Takacs Z, Toups M, Kollewe A, Johnson E, Cuello LG, Driessens G, Biancalana M, Koide A, Ponte CG, Perozo E, Gajewski TF, Suarez-Kurtz G, Koide S, Goldstein SAN (2009) A designer ligand specific for Kv1.3 channels from a scorpion neurotoxin-based library. Proc Natl Acad Sci USA 106: 22211–22216. https://doi.org/10.1073/PNAS.0910123106
Banerjee A, Lee A, Campbell E, MacKinnon R (2013) Structure of a pore-blocking toxin in complex with a eukaryotic voltage-dependent K+ channel. Elife 21(2): e00594. https://doi.org/10.7554/eLife.00594
Kuzmenkov AI, Nekrasova O V., Peigneur S, Tabakmakher VM, Gigolaev AM, Fradkov AF, Kudryashova KS, Chugunov AO, Efremov RG, Tytgat J, Feofanov A V., Vassilevski AA (2018) KV1.2 channel-specific blocker from Mesobuthus eupeus scorpion venom: Structural basis of selectivity. Neuropharmacology 143: 228–238. https://doi.org/10.1016/j.neuropharm.2018.09.030
Luna-Ramírez K, Bartok A, Restano-Cassulini R, Quintero-Hernández V, Coronas FIV, Christensen J, Wright CE, Panyi G, Possani LD (2014) Structure, molecular modeling, and function of the novel potassium channel blocker urotoxin isolated from the venom of the Australian scorpion Urodacus yaschenkoi. Mol Pharmacol 86: 28–41. https://doi.org/10.1124/MOL.113.090183
Wang X, Umetsu Y, Gao B, Ohki S, Zhu S (2015) Mesomartoxin, a new K(v)1.2-selective scorpion toxin interacting with the channel selectivity filter. Biochem Pharmacol 93: 232–239. https://doi.org/10.1016/J.BCP.2014.12.002
M’Barek S, Mosbah A, Sandoz G, Fajloun Z, Olamendi-Portugal T, Rochat H, Sampieri F, Guijarro JI, Mansuelle P, Delepierre M, De Waard M, Sabatier JM (2003) Synthesis and characterization of Pi4, a scorpion toxin from Pandinus imperator that acts on K+ channels. Eur J Biochem 270: 3583–3592. https://doi.org/10.1046/J.1432-1033.2003.03743.X
Corzo G, Papp F, Varga Z, Barraza O, Espino-Solis PG, Rodríguez de la Vega RC, Gaspar R, Panyi G, Possani LD (2008) A selective blocker of Kv1.2 and Kv1.3 potassium channels from the venom of the scorpion Centruroides suffusus suffusus. Biochem Pharmacol 76: 1142–1154. https://doi.org/10.1016/J.BCP.2008.08.018
Olamendi-Portugal T, Bartok A, Zamudio-Zuñiga F, Balajthy A, Becerril B, Panyi G, Possani LD (2016) Isolation, chemical and functional characterization of several new K(+)-channel blocking peptides from the venom of the scorpion Centruroides tecomanus. Toxicon 115: 1–12. https://doi.org/10.1016/J.TOXICON.2016.02.017
Cerni FA, Pucca MB, Peigneur S, Cremonez CM, Bordon KCF, Tytgat J, Arantes EC (2014) Electrophysiological characterization of Ts6 and Ts7, K+ channel toxins isolated through an improved Tityus serrulatus venom purification procedure. Toxins (Basel) 6: 892–913. https://doi.org/10.3390/TOXINS6030892
Possani LD, Selisko B, Gurrola GB (1999) Structure and function of scorpion toxins affecting K+-channels. Perspect Drug Discov Des 150 (15):15–40. https://doi.org/10.1023/A:1017062613503
Papp F, Batista CVF, Varga Z, Herceg M, Román-González SA, Gaspar R, Possani LD, Panyi G (2009) Tst26, a novel peptide blocker of Kv1.2 and Kv1.3 channels from the venom of Tityus stigmurus. Toxicon 54: 379–389. https://doi.org/10.1016/J.TOXICON.2009.05.023
Fajloun Z, Carlier E, Lecomte C, Geib S, Di Luccio E, Bichet D, Mabrouk K, Rochat H, De Waard M, Sabatier JM (2000) Chemical synthesis and characterization of Pi1, a scorpion toxin from Pandinus imperator active on K+ channels. Eur J Biochem 267: 5149–5155. https://doi.org/10.1046/J.1432-1327.2000.01577.X
Péter M, Varga Z, Panyi G, Bene L, Damjanovich S, Pieri C, Possani LD, Gáspár R (1998) Pandinus imperator scorpion venom blocks voltage-gated K+ channels in human lymphocytes. Biochem Biophys Res Commun 242: 621–625. https://doi.org/10.1006/BBRC.1997.8018
Kharrat R, Mansuelle P, Sampieri F, Crest M, Oughideni R, Van Rietschoten J, Martin-Eauclaire MF, Rochat H, El Ayeb M (1997) Maurotoxin, a four disulfide bridge toxin from Scorpio maurus venom: purification, structure and action on potassium channels. FEBS Lett 406: 284–290. https://doi.org/10.1016/S0014-5793(97)00285-8
Abdel-Mottaleb Y, Clynen E, Jalali A, Bosmans F, Vatanpour H, Schoofs L, Tytgat J (2006) The first potassium channel toxin from the venom of the Iranian scorpion Odonthobuthus doriae. FEBS Lett 580: 6254–6258. https://doi.org/10.1016/J.FEBSLET.2006.10.029
Mahjoubi-Boubaker B, Crest M, Khalifa R Ben, El Ayeb M, Kharrat R (2004) Kbot1, a three disulfide bridges toxin from Buthus occitanus tunetanus venom highly active on both SK and Kv channels. Peptides 25: 637–645. https://doi.org/10.1016/j.peptides.2004.02.017
Jouirou B, Mosbah A, Visan V, Grissmer S, M’Barek S, Fajloun Z, Van Rietschoten J, Devaux C, Rochat H, Lippens G, El Ayeb M, De Waard M, Mabrouk K, Sabatier JM (2004) Cobatoxin 1 from Centruroides noxius scorpion venom: chemical synthesis, three-dimensional structure in solution, pharmacology and docking on K+ channels. Biochem J 377: 37–49. https://doi.org/10.1042/BJ20030977
Dudina EE, Korolkova YV, Bocharova NE, Koshelev SG, Egorov TA, Huys I, Tytgat J, Grishin EV (2001) OsK2, a new selective inhibitor of Kv1.2 potassium channels purified from the venom of the scorpion Orthochirus scrobiculosus. Biochem Biophys Res Commun 286: 841–847. https://doi.org/10.1006/BBRC.2001.5492
Cologna CT, Peigneur S, Rosa JC, Selistre-de-Araujo HS, Varanda WA, Tytgat J, Arantes EC (2011) Purification and characterization of Ts15, the first member of a new α-KTX subfamily from the venom of the Brazilian scorpion Tityus serrulatus. Toxicon 58: 54–61. https://doi.org/10.1016/J.TOXICON.2011.05.001
Orts DJB, Peigneur S, Madio B, Cassoli JS, Montandon GG, Pimenta AMC, Bicudo JEPW, Freitas JC, Zaharenko AJ, Tytgat J (2013) Biochemical and electrophysiological characterization of two sea anemone type 1 potassium toxins from a geographically distant population of bunodosoma caissarum. Mar Drugs 11: 655–679. https://doi.org/10.3390/md11030655
Chen P, Dendorfer A, Finol-Urdaneta RK, Terlau H, Olivera BM (2010) Biochemical characterization of kappaM-RIIIJ, a Kv1.2 channel blocker: evaluation of cardioprotective effects of kappaM-conotoxins. J Biol Chem 285: 14882–14889. https://doi.org/10.1074/JBC.M109.068486
Ferber M, Sporning A, Jeserich G, DeLaCruz R, Watkins M, Olivera BM, Terlau H (2003) A novel conus peptide ligand for K+ channels. J Biol Chem 278: 2177–2183. https://doi.org/10.1074/JBC.M205953200
Ferber M, Al-Sabi A, Stocker M, Olivera BM, Terlau H (2004) Identification of a mammalian target of κM-conotoxin RIIIK. Toxicon 43: 915–921. https://doi.org/10.1016/j.toxicon.2003.12.010
Tytgat J, Debont T, Carmeliet E, Daenens P (1995) The alpha-dendrotoxin footprint on a mammalian potassium channel. J Biol Chem 270: 24776–24781. https://doi.org/10.1074/JBC.270.42.24776
Swanson R, Marshall J, Smith JS, Williams JB, Boyle MB, Folander K, Luneau CJ, Antanavage J, Oliva C, Buhrow SA, Bennet C, Stein RB, Kaczmarek LK (1990) Cloning and expression of cDNA and genomic clones encoding three delayed rectifier potassium channels in rat brain. Neuron 4: 929–939. https://doi.org/10.1016/0896-6273(90)90146-7
Hurst RS, Busch AE, Kavanaugh MP, Osborne PB, North RA, Adelman JP (1991) Identification of amino acid residues involved in dendrotoxin block of rat voltage-dependent potassium channels. Mol Pharmacol 40.
Robertson B, Owen D, Stow J, Butler C, Newland C (1996) Novel effects of dendrotoxin homologues on subtypes of mammalian Kv1 potassium channels expressed in Xenopus oocytes. FEBS Lett 383: 26–30. https://doi.org/10.1016/0014-5793(96)00211-6
Hopkins WF, Demas V, Tempel BL (1994) Both N- and C-terminal regions contribute to the assembly and functional expression of homo- and heteromultimeric voltage-gated K+ channels. J Neurosci 14: 1385–1393. https://doi.org/10.1523/JNEUROSCI.14-03-01385.1994
Hopkins WF, Allen ML, Houamed KM, Tempel BL (1994) Properties of voltage-gated K+ currents expressed in Xenopus oocytes by mKv1.1, mKv1.2 and their heteromultimers as revealed by mutagenesis of the dendrotoxin-binding site in mKv1.1. Pflugers Arch 428: 382–390. https://doi.org/10.1007/BF00724522
Funding
This work was supported by the Russian Science Foundation (project no. 20-44-01015, electrophysiology) and the Russian Foundation for Basic Research (project no. 20-34-90158, peptide production).
Author information
Authors and Affiliations
Contributions
A.M.G. and A.A.V. planned the study. A.M.G. performed biochemical experiments and obtained recombinant peptides. S.P. and E.L.P.-J. performed electrophysiological experiments. A.A.V. supervised the biochemical experiments. J.T. supervised the electrophysiological experiments. A.M.G. and A.A.V. wrote the article.
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Dyomina
Russian Text © The Author(s), 2022, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2022, Vol. 108, No. 12, pp. 1627–1638https://doi.org/10.31857/S0869813922120056.
Rights and permissions
About this article
Cite this article
Gigolaev, A.M., Pinheiro-Junior, E.L., Peigneur, S. et al. KV1.2-Selective Peptide with High Affinity. J Evol Biochem Phys 58, 2048–2057 (2022). https://doi.org/10.1134/S002209302206031X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002209302206031X