Abstract
The early postnatal development of the A-layers of the dorsal lateral geniculate nucleus (LGNd) was investigated in kittens aged 0–34 days by immunohistochemistry for the selective marker for neuronal differentiation (NeuN protein) and parvalbumin. We report two new facts about the LGNd development. First, there is a transient stratification of NeuN labelling in layer A, and to a lesser extent in layer A1, in kittens aged 0 and 4 days. Second, a transient population of large cells that are located between the LGNd A-layers (interlaminar cells) showed high expression levels of both NeuN and parvalbumin. These neurons possessed both the morphological and immunohistochemical features, similar to cells in the neighbouring perigeniculate nucleus. Both NeuN-stratification and double-stained interlaminar cells gradually disappeared during the second postnatal week, and almost completely vanished by the opening of the critical period. We discuss a possible linkage between these observed transitory networks and the ON-/OFF- and X-/Y-cells development and propose that the data obtained reflect the functioning of the early environmentally independent geniculate networks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The dorsal lateral geniculate nucleus (LGNd) is an important processing centre within the retinocortical pathway. The LGNd is not simply a principal relay station, but it also plays an essential role in the regulation and modulation of the information flow between the retina and the primary visual cortex (Hubel and Wiesel 1961; Sherman and Koch 1986; Mastronarde 1987; Mastronarde et al. 1991; Sherman and Guillery 2002, for review). In carnivores, the LGNd consists of six principal layers (A, A1, C, C1, C2, and C3); the most robust layers, A and A1, receive projections from distinct eyes: layer A from the contralateral side, and layer A1 from the ipsilateral side (Sanderson 1974; Stryker and Zahs 1983; Lee et al. 1999).
LGNd development begins in prenatal life. In cats, retinal afferents from the two eyes invade future layers A and A1 between days E32 and E37 (Shatz 1983), but the complete development of the two LGNd A-layers continues developing through the third postnatal week, when the experience-dependent stage (so called, critical period) begins (Crowley and Katz 2002; Huberman et al. 2005; Chalupa 2007; Speer et al. 2010). The segregation of retinal input from the two eyes into the LGNd A-layers has been used as a model system for investigating the mechanisms underlying afferent targeting during development (Huberman et al. 2005; Speer et al. 2010), particularly, for modelling the refinement of cortical ocular dominance columns during precritical and critical periods (Crowley and Katz 2002). There is evidence for developmental dissimilarity between layers A and A1, in favour of dominance by the contralateral retinal input (Linden et al. 1981; Shatz 1983; Chapman 2000); yet, many aspects of the developmental dynamic of the LGNd A-layers are poorly understood.
The aim of the present study was to document the temporal dynamic of the A-layers development during early postnatal ontogenesis, using NeuN immunostaining. NeuN is a neuron-specific nuclear protein, which is identified as the Fox-3 gene product (Kim et al. 2009) and is expressed in most mature neuronal cell types (except for certain cells, including cerebellar Purkinje cells, olfactory bulb mitral cells, retinal photoreceptor cells, Cajal-Retzius cells, inferior olivary neurons, dentate nucleus neurons, sympathetic ganglion cells, etc.) (Mullen et al. 1992; Sarnat et al. 1998). A heterochrony in NeuN expression was observed during the development of the CNS, and it was proposed that this protein “…may correspond to the state of differentiation or level of functioning of the neuron, rather than simply to withdrawal from the cell cycle” (Mullen et al. 1992). To date, NeuN has been widely used as a specific marker for the functional assessment of neurons (Alekseeva et al. 2015).
Materials and Methods
All experimental procedures were approved by the Ethics Commission of the Pavlov Institute of Physiology, and were performed in accordance with the requirements of Council Directive 2010/63EU of the European Parliament on the protection of animals used in experimental and other scientific purposes. Fourteen normal pigmented kittens, of either sex, aged 0 (n = 2), 4 (n = 2), 10 (n = 2), 14 (n = 2), 21 (n = 1), 28 (n = 2), and 34 (n = 3) postnatal days were used for this study. Under deep anaesthesia (a mixture of 2 mg/kg Zoletil and 20 mg/kg xylazine), all animals were perfused transcardially with 0.9% NaCl, followed by 4% paraformaldehyde. After it, brains were removed and stored in 20 and 30% sucrose, and subsequently cut into 50-μm sagittal sections using a freezing microtome.
The detailed procedure of immunohistochemical staining was described previously (Merkulyeva et al. 2016). After chemical unmasking and endogenous peroxidase activity blocking, sections were preincubated with 10% normal goat serum (NGS, Vector Laboratories, UK), followed by incubation for 70 h in a monoclonal mouse primary antibody to NeuN (Millipore, USA, PRID: AB_2298772, 1:5000 dilution) or in a polyclonal rabbit primary antibody to parvalbumin (Abcam, UC, PRID: AB_298032, 1:10000 dilution). Then, the slices were incubated in a biotinylated secondary antibody (horse anti-mouse IgG, Vector Laboratories, UK, 1:600 dilution) for 1 day or in an Alexa-conjugated secondary antibody (Alexa Fluor488 goat anti-mouse IgG (PRID: AB_2576208) or Alexa Fluor568 goat anti-rabbit IgG (PRID: AB_2576207), Thermo Fisher Scientific, USA, 1:200 dilution) for 2 h. In the former case, slices were subsequently processed using an avidin–biotin horseradish-peroxidase complex (ABC Elite system, Vector Laboratories) and diaminobenzidine (DAB)-NiCl-H2O2 reaction. The DAB-reacted slices were coverslipped in Canadian balsam and the Alexa-reacted slices were coverslipped in 50% glycerol.
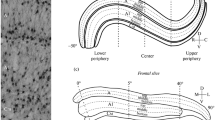
The DAB-reacted slices were analysed with an Olympus microscope (Olympus Corporation, Japan) using a Nikon camera (Nikon Corporation, Japan). The fluorescent Alexa-reacted slices were analysed with an inverted fluorescence microscope (Leica Camera, Germany). NeuN-positive cells were analysed in two regions of interest (ROI): in the visual centre (from − 15° to + 15°) and the upper peripheral representation areas (Fig. 1f), in accordance with previous work by K.J. Sanderson (1974). The number of neurons and their sizes were manually estimated using free software from Fiji (PRID: SCR_002285) (Schindelin et al. 2012). Approximately 100–120 labelled cells were analysed in each animal. Statistical differences were assessed using the Fisher-test.
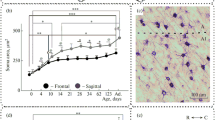
NeuN labelling distribution in the dorsal lateral geniculate nucleus (LGNd) and in the perigeniculate nucleus (PGN) of kittens aged 4 day (a), 14 days (b), and 34 days (c). Red countered boxes are enlarged regions of central (1, 3, and 5) and peripheral (2, 4, and 6) visual field representation. Red arrows indicate labelled stripes of NeuN+ cells in layers A and A1. Calibration marker is 500 µm. A diagram of interdependence between the size (in µm2) and optical density (in grey level) of NeuN+ cells located in the depth of layers A and A1 (A/A1), in the interlaminar area (INT), and in the PGN of newborn kittens (d). An example of NeuN+ cells in layers A and A1, in the interlaminar area, and in the PGN of newborn kittens (e). Red arrows indicate labelled stripes of NeuN+ cells in layers A and A1. Visual field representation in LGNd; (+) upper visual field, (−) lower visual field; A, P, D, V: anterior, posterior, dorsal, ventral. Red countered boxes are regions of interest (ROI) of central and peripheral visual field representation (f)
Results
Weak staining in the A-layers compared with the C-layers and with the neighbouring perigeniculate nucleus (PGN) is a common feature in newborn (aged 0 and 4 postnatal days) animals (Fig. 1a). In these kittens, we observed a stratification of the A-layers; specifically, NeuN expression is higher in the centre of the layer A (Fig. 1a, boxes 1 and 2, red arrows), and far fewer labelled cells were found within its dorsal and ventral regions. This stratification is clearly seen throughout the layer A, regardless of retinal eccentricity. For layer A1, we found only partial stratification in areas corresponding to the upper visual periphery representation (Fig. 1a, box 2, red arrows). Another common feature of NeuN staining in newborn kittens is the presence of large, dark NeuN-positive (NeuN+) cells in the interlaminar area between layers A and A1 (INT cells) (Fig. 1a, boxes 1 and 2,e). Unlike the cells at the depth of the A-layers, INT neurons exhibit prominent cytoplasmic labelling that spreads to the proximal dendrites. INT cells are larger than cells in the A-layers (223 ± 25 and 104 ± 6 µm2, respectively, F = 8.91, F crit = 1.95, p < 0.001) and darker than cells in the A-layers (grey level index, 56.1 ± 3.0 and 88.1 ± 2.6, respectively, F = 16.15, F crit = 1.95, p < 0.001) (Fig. 1d). Labelled INT cells are similar to labelled neurons within the PGN in size and in staining pattern (PGN cell size, 205 ± 13 µm2; grey level index, 52.6 ± 1.7) (see typical examples of NeuN+ cells in Fig. 1e).
In kittens aged 10 days and older, the A-layers no longer exhibit pale staining; instead, they are similar in staining intensity to the C-layers (Fig. 1b, c). In kittens aged 14 days, stratification in the A-layers becomes less visible, and the weakly stained stripes are sometimes visualized only in the upper visual periphery (Fig. 1b, box 4). Darkly stained INT neurons are still visible in these animals, but they are distributed sparsely and no longer show labelling of the proximal dendrites.
In kittens aged 3 weeks and older, no striking differences were found in NeuN labelling between layers A and A1 or within either of the A-layers (Fig. 1c, boxes 5 and 6); NeuN+ cells are homogeneously distributed over both A-layers. Since the third postnatal week, the interlaminar space between layers A and A1 has become almost devoid of NeuN+ neurons.
We observed a clear similarity between the INT and PGN cells in newborn kittens. Since it was found that the interlaminar zones in the LGNd possess a cell type that is physiologically similar to neurons in the PGN, and that interlaminar and PGN cells are both parvalbumin-positive (Sanchez-Vives et al. 1996), we also performed parvalbumin staining in the animals aged 0–3 weeks. In all P0 and P4 kittens, parvalbumin staining was observed in the soma and proximal dendrites of most of the INT and PGN neurons; sparse cells were also found at the depth of the LGNd in layers A and A1 (Fig. 2a, b). All parvalbumin-positive INT cells were also NeuN+ (130 out of 130 cells analysed) (Fig. 2b, white arrows). Many cells within the PGN (approx. 60%, 342 out of 600 cells) were also double-labelled for parvalbumin and NeuN. This prominent parvalbumin staining in neuronal somas of INT cells disappears during maturation, as demonstrated by 14-day-old kittens expressing far fewer parvalbumin-positive neuronal somas compared with newborn kittens (7-11 cells per slice and 34-55 cells per slice, respectively). Additionally, in kittens older than 14 days, parvalbumin-positive cells within the interlaminar area were only sparsely observed.
NeuN and parvalbumin labelling in the dorsal lateral geniculate nucleus (LGNd) and in the perigeniculate nucleus (PGN) of newborn kittens (a, b). Calibration marker in a is 500 µm. Regions from a enlarged (in white dashed squares). Some double-labelled cells (merge) are marked by white arrows; A, A1, Cm—LGNd layers (b)
Discussion
NeuN is the Fox-3 gene product that was proposed to be a determinant factor of neural specificity during neuron-specific alternative splicing (Kim et al. 2009). Within a postmitotic neuron, the level of NeuN expression corresponds to its differentiation status and level of functioning (Mullen et al. 1992; Weyer and Schilling 2003; Kempermann et al. 2004; Alekseeva et al. 2015). It was found that immature neuronal cells are negative for NeuN (Lavezzi et al. 2013). In this study, three facts were obtained by using NeuN immunohistochemistry. First, we shown a clear stratification of the NeuN staining in the A-layers of kittens aged 0–2 postnatal weeks that disappeared gradually up to the second postnatal week; second, differences in this disappearance were found in favour to the layer A1. And third, a transient population of interlaminar cells expressed both NeuN and parvalbumin was obtained in kittens aged 0–1 postnatal weeks. Thereby, we propose that the data obtained here can be related to the gradual evolvement of different developing networks into the LGNd A-layers functioning.
The finding of the A-layers stratification possibly can mean that neurons within the central parts of the A-layers are developed earlier than the cells within the border parts of the A-layers. What differences between these parts are known? Sublamination in the A-layers has been observed in some carnivores such as ferrets, minks, weasels, and coatis, where ON- and OFF-systems are functionally segregated (Sanderson 1974; Stryker and Zahs 1983). Although a similar, clear sublamination pattern of the A-layers into leaflets has not been described in cats, electrophysiological studies report that ON-cells are predominant at the tops of the A-layers, and OFF-cells at the bottoms, and both types are balanced in the centres of the A-layers (Bowling and Wieniawa-Narkiewicz 1986). Moreover, the steepest gradients and the maximum differences in proportions of ON- and OFF-cells were obtained in layer A, not in layer A1. If regions with a mixed ON-/OFF-neuronal population are mature earlier than ones with only ON or OFF population, we can expect different dynamics of the NeuN staining in these regions.
Another possible explanation of the present data is related to the properties and development of different types of the principal neurons in layers A and A1. In cats, the pathway from the retina to the LGNd comprises at least three morphologically and physiologically distinct neuronal pathways, referred to as X, Y, and W, which are thought to analyse different aspects of the visual scene (Enroth-Cugell and Robson 1966; Sherman and Spear 1982; Wilson et al. 1984; Sur 1988). There are several evidences for X and Y neurons have different distribution patterns through the LGNd layers, a tendency most prominently observed in layer A; Y cells are concentrated at the laminae borders and X cells are concentrated mainly in the centres of the layers (Mitzdorf and Singer 1977; Bowling and Wieniawa-Narkiewicz 1986]. In accordance with the fact that X and Y retinogeniculate axons invade the LGNd non-simultaneously in favour to the X cells (Sur 1988; Garraghty et al. 1998), and in accordance to the fact that Y cells complete their physiological maturation later than X cells do (Norman et al. 1977; Mangel et al. 1983; Sherman 1985; Hockfield and Sur 1990), we may expect different developmental profiles for central and border parts of the A-layers and for NeuN staining as well. We also should note that a heterogeneity in geniculate populations of both Y and X cells was obtained previously (X and Y populations were divided into the lagged and unlagged cells; Humphrey and Weller 1988; Mastronarde et al. 1991); thus, stratification observed here possibly can be related not to the distribution and development of total X and Y populations, but to the distribution and development of these subpopulations.
We also obtained a different disappearance of the A-layers stratification in regions correspondent to visual centre and visual periphery representation (4-th out of 14-th postnatal day, respectively). This fact is in good agreement with the data about the late maturation of the entire peripheral visual system compared with the visual centre (see Burnat 2015). Since in relative terms the Y cells are most frequent in peripheral visual field representation than X cells (Fukuda and Stone 1974; So and Shapley 1979; Watanabe and Fukuda 2002), we again can suppose that A-layers sublamination can be related to the different timings of X and Y cells development.
The disappearance of the A-layers stratification was faster in layer A1 compared with layer A. Developmental studies in cats and ferrets have reported that contralateral eye afferents invade the LGNd before ipsilateral eye afferents do, and during the subsequent period in which two retinal inputs overlap, a contralateral dominance has been detected (Daniels et al. 1978; Linden et al. 1981; Shatz 1983; Sretavan and Shatz 1986). Optical imaging and single-unit recordings have demonstrated a contralateral eye dominance in driving cortical mapping for orientation and ocular dominance, before eye opening (Crair et al. 1998). Therefore, an earlier maturation of the contralateral input was expected. Present data illustrate the opposite effect; thus, it is unlikely related to retinal dominance per se.
Our third most important result presented here is a transient neuronal population of large INT cells double-stained for NeuN and parvalbumin that were revealed in kittens aged 0–4 days, and these neurons gradually disappear up to the critical period beginning. These neurons share similar morphological features with PGN neurons, and it was shown that the LGNd interlaminar neurons and PGN cells are anatomically and functionally similar (Montero 1989; Sanchez-Vives et al. 1996; Bickford et al. 2008), and both are parvalbumin-positive (Demeulemeester et al. 1991; Sanchez-Vives et al. 1996). Since the PGN modulates transmission through the LGNd and between the dorsal thalamus and the cortex (Sherman and Koch 1986; Cucchiaro et al. 1991; Uhlrich et al. 1991; Fitzgibbon 2002), we can propose that the population of double-stained INT cells observed here can be related to the early functioning of these networks before the critical period beginning.
The process of segregation of the contralateral and ipsilateral inputs in the LGNd is virtually complete near the time of birth; but laminar development is lasted for several weeks (from 2 to 8) (Kalil 1978; Shatz 1983). In this study, we shown that NeuN staining within LGNd A-layers changed significantly up to the second postnatal week, the time point when transient population of large NeuN/parvalbumin-positive cells is disappeared. We propose that data obtained here reflect the functioning of early environment-independent geniculate networks during precritical period of LGNd development.
References
Alekseeva OS, Gusel VV, Beznin GV, Korzhevskii DE (2015) Prospects for the application of NeuN nuclear protein as a marker of the functional state of nerve cells in vertebrates. J Evol Biochem Physiol 51:357–369. https://doi.org/10.1134/S0022093015050014
Bickford ME, Wei H, Eisenback MA et al (2008) Synaptic organization of thalamocortical axon collaterals in the perigeniculate nucleus and dorsal lateral geniculate nucleus. J Comp Neurol 508:264–285. https://doi.org/10.1002/cne.21671
Bowling DB, Wieniawa-Narkiewicz E (1986) The distribution of on- and off-centre X- and Y-like cells in the A layers of the cat’s lateral geniculate nucleus. J Physiol 375:561–572. https://doi.org/10.1113/jphysiol.1986.sp016133
Burnat K (2015) Are visual peripheries forever young? Neural Plast 2015:1–13. https://doi.org/10.1155/2015/307929
Chalupa LM (2007) A reassessment of the role of activity in the formation of eye-specific retinogeniculate projections. Brain Res Rev 55:228–236. https://doi.org/10.1016/j.brainresrev.2007.03.003
Chapman B (2000) Necessity for afferent activity to maintain eye-specific segregation in ferret lateral geniculate nucleus. Science 287:2479–2482. https://doi.org/10.1126/science.287.5462.2479
Crair MC, Gillespie DC, Stryker MP (1998) The role of visual experience in the development of columns in cat visual cortex. Science 279:566–570. https://doi.org/10.1126/science.279.5350.566
Crowley JC, Katz LC (2002) Ocular dominance development revisited. Curr Opin Neurobiol 12:104–109. https://doi.org/10.1016/S0959-4388(02)00297-0
Cucchiaro JB, Uhlrich DJ, Sherman SM (1991) Electron-microscopic analysis of synaptic input from the perigeniculate nucleus to the A-laminae of the lateral geniculate nucleus in cats. J Comp Neurol 310:316–336. https://doi.org/10.1002/cne.903100304
Daniels JD, Pettigrew JD, Norman JL (1978) Development of single-neuron responses in kitten’s lateral geniculate nucleus. J Neurophysiol 41:1373–1393. https://doi.org/10.1152/jn.1978.41.6.1373
Demeulemeester H, Arckens L, Vandesande F et al (1991) Calcium binding proteins as molecular markers for cat geniculate neurons. Exp Brain Res 83:513–520. https://doi.org/10.1007/BF00229828
Enroth-cugell C, Robson JG (1966) The contrast sensitivity of retinal ganglion cells of the cat. J Physiol 187:517–552. https://doi.org/10.1113/jphysiol.1966.sp008107
Fitzgibbon T (2002) Organization of reciprocal connections between the perigeniculate nucleus and dorsal lateral geniculate nucleus in the cat: a transneuronal transport study. Vis Neurosci 19:511–520. https://doi.org/10.1017/S0952523802194120
Fukuda Y, Stone J (1974) Retinal distribution and central projections of Y-, X-, and W-cells of the cat’s retina. J Neurophysiol 37:749–772. https://doi.org/10.1152/jn.1974.37.4.749
Garraghty PE, Roe A, Sur M (1998) Specification of retinogeniculate X and Y axon arbors in cats: fundamental differences in developmental programs. Brain Res Dev Brain Res 107:227–231. https://doi.org/10.1016/S0165-3806(97)00223-X
Hockfield S, Sur M (1990) Monoclonal antibody Cat-301 identifies Y-cells in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol 300:320–330. https://doi.org/10.1002/cne.903000305
Hubel DH, Wiesel TN (1961) Integrative action in the cat’s lateral geniculate body. J Physiol 155:385–398. https://doi.org/10.1113/jphysiol.1961.sp006635
Huberman AD, Dehay C, Berland M et al (2005) Early and rapid targeting of eye-specific axonal projections to the dorsal lateral geniculate nucleus in the fetal macaque. J Neurosci 25:4014–4023. https://doi.org/10.1523/JNEUROSCI.4292-04.2005
Humphrey AL, Weller RE (1988) Structural correlates of functionally distinct X-cells in the lateral geniculate nucleus of the cat. J Comp Neurol 268:448–468. https://doi.org/10.1002/cne.902680312
Kalil R (1978) Development of the dorsal lateral geniculate nucleus in the cat. J Comp Neurol 182:265–291. https://doi.org/10.1002/cne.901820206
Kempermann G, Jessberger S, Steiner B, Kronenberg G (2004) Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27:447–452. https://doi.org/10.1016/j.tins.2004.05.013
Kim KK, Adelstein RS, Kawamoto S (2009) Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. J Biol Chem 284:31052–31061. https://doi.org/10.1074/jbc.M109.052969
Lavezzi AM, Corna MF, Matturri L (2013) Neuronal nuclear antigen (NeuN): a useful marker of neuronal immaturity in sudden unexplained perinatal death. J Neurol Sci 329:45–50. https://doi.org/10.1016/j.jns.2013.03.012
Lee I, Kim J, Lee C (1999) Anatomical characteristics and three-dimensional model of the dog dorsal lateral geniculate body. Anat Rec 256:29–39
Linden DC, Guillery RW, Cucchiaro JB (1981) The dorsal lateral geniculate nucleus of the normal ferret and its postnatal development. J Comp Neurol 203:189–211. https://doi.org/10.1002/cne.902030204
Mangel SC, Wilson JR, Sherman SM (1983) Development of neuronal response properties in the cat dorsal lateral geniculate nucleus during monocular deprivation. J Neurophysiol 50:240–264. https://doi.org/10.1152/jn.1983.50.1.240
Mastronarde DN (1987) Two classes of single-input X-cells in cat lateral geniculate nucleus. I. Receptive-field properties and classification of cells. J Neurophysiol 57:357–380. https://doi.org/10.1152/jn.1987.57.2.357
Mastronarde DN, Humphrey AL, Saul AB (1991) Lagged Y cells in the cat lateral geniculate nucleus. Vis Neurosci 7:191–200. https://doi.org/10.1017/S0952523800004028
Merkulyeva N, Veshchitskii A, Makarov F et al (2016) Distribution of 28 kDa calbindin-immunopositive neurons in the cat spinal cord. Front Neuroanat 9:166. https://doi.org/10.3389/fnana.2015.00166
Mitzdorf U, Singer W (1977) Laminar segregation of afferents to lateral geniculate nucleus of the cat: an analysis of current source density. J Neurophysiol 40:1227–1244. https://doi.org/10.1152/jn.1977.40.6.1227
Montero VM (1989) The GABA-immunoreactive neurons in the interlaminar regions of the cat lateral geniculate nucleus: light and electron microscopic observations. Exp Brain Res 75:497–512. https://doi.org/10.1007/BF00249901
Mullen RJ, Buck CR, Smith AM (1992) NeuN, a neuronal specific nuclear protein in vertebrates. Development 116:201–211
Norman JL, Pettigrew JD, Daniels JD (1977) Early development of X-cells in kitten lateral geniculate nucleus. Science 198:202–204. https://doi.org/10.1126/science.905824
Sanchez-Vives MV, Bal T, Kim U et al (1996) Are the interlaminar zones of the ferret dorsal lateral geniculate nucleus actually part of the perigeniculate nucleus? J Neurosci 16:5923–5941. https://doi.org/10.1523/JNEUROSCI.16-19-05923.1996
Sanderson KJ (1974) Lamination of the dorsal lateral geniculate nucleus in carnivores of the weasel (Mustelidae), raccoon (Procyonidae) and fox (Canidae) families. J Comp Neurol 153:238–266. https://doi.org/10.1002/cne.901530303
Sarnat HB, Nochlin D, Born DE (1998) Neuronal nuclear antigen (NeuN): a marker of neuronal maturation in early human fetal nervous system. Brain Dev 20:88–94. https://doi.org/10.1016/S0387-7604(97)00111-3
Schindelin J, Arganda-Carreras I, Frise E et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Shatz CJ (1983) The prenatal development of the cat’s retinogeniculate pathway. J Neurosci 3:482–499. https://doi.org/10.1523/JNEUROSCI.03-03-00482.1983
Sherman SM (1985) Development of retinal projections to the cat’s lateral geniculate nucleus. Trends Neurosci 8:350–355. https://doi.org/10.1016/0166-2236(85)90121-3
Sherman SM, Guillery RW (2002) The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci 357:1695–1708. https://doi.org/10.1098/rstb.2002.1161
Sherman SM, Koch C (1986) The control of retinogeniculate transmission in the mammalian lateral geniculate nucleus. Exp Brain Res 63:1–20. https://doi.org/10.1007/BF00235642
Sherman SM, Spear PD (1982) Organization of visual pathways in normal and visually deprived cats. Physiol Rev 62:738–855. https://doi.org/10.1152/physrev.1982.62.2.738
So YT, Shapley R (1979) Spatial properties of X and Y cells in the lateral geniculate nucleus of the cat and conduction veolcities of their inputs. Exp Brain Res 36:533–550. https://doi.org/10.1007/BF00238521
Speer CM, Mikula S, Huberman AD, Chapman B (2010) The developmental remodeling of eye-specific afferents in the ferret dorsal lateral geniculate nucleus. Anat Rec 293:1–24. https://doi.org/10.1002/ar.21001
Sretavan DW, Shatz CJ (1986) Prenatal development of retinal ganglion cell axons: segregation into eye-specific layers within the cat’s lateral geniculate nucleus. J Neurosci 6:234–251. https://doi.org/10.1523/JNEUROSCI.06-01-00234.1986
Stryker MP, Zahs KR (1983) On and off sublaminae in the lateral geniculate nucleus of the ferret. J Neurosci 3:1943–1951. https://doi.org/10.1523/JNEUROSCI.03-10-01943.1983
Sur M (1988) Development and plasticity of retinal X and Y axon terminations in the cat’s lateral geniculate nucleus. Brain Behav Evol 31:243–251. https://doi.org/10.1159/000116592
Uhlrich DJ, Cucchiaro JB, Humphrey AL, Sherman SM (1991) Morphology and axonal projection patterns of individual neurons in the cat perigeniculate nucleus. J Neurophysiol 65:1528–1541. https://doi.org/10.1152/jn.1991.65.6.1528
Watanabe M, Fukuda Y (2002) Survival and axonal regeneration of retinal ganglion cells in adult cats. Prog Retin Eye Res 21:529–553. https://doi.org/10.1016/S1350-9462(02)00037-X
Weyer A, Schilling K (2003) Developmental and cell type-specific expression of the neuronal marker NeuN in the murine cerebellum. J Neurosci Res 73:400–409. https://doi.org/10.1002/jnr.10655
Wilson JR, Friedlander MJ, Sherman SM (1984) Fine structural morphology of identified X- and Y-cells in the cat’s lateral geniculate nucleus. Proc R Soc London Ser B 221:411–436. https://doi.org/10.1098/rspb.1984.0042
Acknowledgements
The study was supported by the Russian Foundation for Basic Research (RFBR Grant No. 16-04-01791). The authors thank the Centre for Molecular and Cell Technologies, Research Park, Saint Petersburg State University, for supporting this research. The authors also thank Nikitina Nina for animal care and Schkorbatova Polina for help with immunostaining.
Author information
Authors and Affiliations
Contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: MN. Acquisition of data: MN, MA. Analysis and interpretation of data: MN, MA. Drafting of the manuscript: MN, MA. Critical revision of the manuscript for important intellectual content: MN, MA. Statistical analysis: MN, MA. Obtained funding: MN, MA. Administrative, technical, and material support: MN, MA, ZP. Study supervision: MN.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Merkulyeva, N., Mikhalkin, A. & Zykin, P. Early Postnatal Development of the Lamination in the Lateral Geniculate Nucleus A-Layers in Cats. Cell Mol Neurobiol 38, 1137–1143 (2018). https://doi.org/10.1007/s10571-018-0585-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-018-0585-6