The oxygen K-edge X-ray absorption spectra in EuBaCo2O5.52 ± 0.02 and EuBaCo2O5.24 ± 0.02 cobaltites are measured at temperatures of 300 and 440 K, which are below and above the metal–insulator transition temperature, respectively. According to these spectra, the substitution of Co2+ ions for some Co3+ ions with a decrease in the oxygen content in the chemical formula of a cobaltite and, hence, an increase in the relative fraction of CoO5 pyramids with respect to the number of CoO6 octahedra leads to an increase in the band gap by about 0.3 eV. The band structure of EuBaCo2O5.5 is calculated using the method of linearized muffin-tin orbitals in the local density approximation taking into account the local Coulomb interaction. It is found that the low-spin state of Co3+ ions occurs in CoO6 octahedra in EuBaCo2O5.5, whereas the high-spin state of Co3+ ions is typical of cobalt ions in pyramids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
LnBaCo2O5 + δ cobaltites (where Ln is a rare earth element and 0 ≤ δ ≤ 1) are characterized by magnetic and structural phase transitions and metal–insulator transitions and exhibit the magnetoresistance effect [1–3]. In cobalt oxides, cobalt ions can exhibit various charge and spin states depending on the temperature, oxygen nonstoichiometry, doping, etc. Oxidation states (charge states) and spin states of transition metal ions determine the physical, including magnetic, properties of these materials [4].
The low-spin (LS, \(t_{{2g \uparrow }}^{3}t_{{2g \downarrow }}^{3}e_{g}^{0}\)), intermediate-spin (IS, \(t_{{2g \uparrow }}^{3}t_{{2g \downarrow }}^{2}e_{{g \uparrow }}^{1}\)), and high-spin (HS, \(t_{{2g \uparrow }}^{3}t_{{2g \downarrow }}^{1}e_{{g \uparrow }}^{2}\)) configurations are possible for trivalent cobalt ions in the octahedral ligand field. The spin-state transition (crossover) temperature correlates with the metal–insulator transition temperature.
In layered LnBaCo2O5.5 cobaltites, Co3+ ions are located both in CoO6 octahedra and in CoO5 py-ramids. With a decrease in the oxygen content (LnBaCo2O5.0 cobaltites), pyramids with Co2+ and Co3+ ions remain the only structural elements. The problem of spin states of trivalent cobalt ions in octahedra and pyramids of cobaltites above and below the metal–insulator transition temperature is actively discussed. The results of the studies on spin states are reviewed in our works [5, 6]. The main contradiction concerns the spin states of Co3+ ions in pyramids. An almost unambiguous conclusion about the intermediate-spin character of Co3+ ions in the pyramids appearing in layered cobaltites is based on the magnetic measurements. However, the measurements of X-ray absorption spectra in GdBaCo2O5.5 suggest the high-spin character of Co3+ ions in pyramids [7].
In this work, we focus on the measurements of the oxygen K-edge X-ray absorption spectra for two cobaltites, EuBaCo2O5.52 ± 0.02 and EuBaCo2O5.24 ± 0.02, denoted as S1 and S2, respectively. The measurements were performed at temperatures of 300 and 440 K, which are below and above the metal–insulator transition temperature of 360 K for EuBaCo2O5.5 [8, 1]. Note that the spin crossover occurs in a certain temperature range. It is impossible to fix the exact temperature of the change in the spin state, but we can only speak about the change in the number of electrons in cobalt ions having the spin state different from the initial one.
Polycrystalline EuBaCo2O5 + δ, Sr2CoO3Cl, and EuCoO3 samples were synthesized using the solid-phase reaction method. The initial components for the synthesis of EuBaCo2O5 + δ were Eu2O3, BaCO3, and Co3O4. The samples were subjected to a stepwise annealing in the temperature range of 900−1150°C with the intermediate grinding. Upon finalizing the synthesis, the samples were slowly cooled in a furnace (at a rate of 1 K/min). The absolute oxygen content was determined by reducing the samples in the hydrogen atmosphere to the initial oxides Eu2O3, BaO, and metallic cobalt. The phase composition and crystal structure of the samples were determined using X-ray diffraction (DRON-2 diffractometer, Cr Kα radiation) at room temperature. The oxygen index of the EuBaCo2O5 + δ sample cooled simultaneously with the furnace was 5.52 ± 0.02 (EuBaCo2O5.52 ± 0.02). To prepare the EuBaCo2O5.24 ± 0.02 sample, the initial EuBaCo2O5.52 ± 0.02 powder was annealed at a temperature of 530°C for 6 h and then quenched in air.
According to the X-ray diffraction data, the synthesized EuBaCo2O5.52 ± 0.02 sample has the orthorhombic structure (space group Pmmm, no. 47); the lattice parameters of this compound are a = 3.880(1) Å, b = 7.824(1) Å, and c = 7.539(7) Å. The EuBaCo2O5.24 ± 0.02 sample has the tetragonal structure (space group P4/mmm, no. 123) and the lattice parameters a = 3.902(6) Å and c = 7.536(7) Å.
The Sr2CoO3Cl sample was prepared using SrCO3, Co3O4, and SrCl2 precursors at 830°C. According to the data of [9], Sr2CoO3Cl has the tetragonal structure (space group P4/nmm, no. 129) with the lattice parameters a = 3.901(2) Å and c = 14.341(3) Å.
The EuCoO3 sample was synthesized employing the Eu2O3 and Co3O4 compounds by the stepwise annealing in the range of 900–1140°C. The prepared sample has the orthorhombic structure (space group \(Pnma\), no. 62), with the lattice parameters \(a = \) 5.372(1) Å, \(b = 7.488(4)\) Å, and \(c = 5.259(6)\) Å.
The oxygen K-edge X-ray absorption spectra were measured at the Russian–German beamline of the BESSY-II storage ring in the total photoelectron yield mode at room temperature and at a temperature of 440 K. The spectral intensities were normalized to the electron beam current in the storage ring. To eliminate the influence of contamination of the parts of the spectrometer with oxygen-containing substances, the O K-edge spectra of the samples under study were normalized with respect to the oxygen spectrum obtained from a gold foil measured in the same energy range.
The band structure of EuBaCo2O5.5 was calculated by the linearized muffin-tin orbital method [10] using the local density approximation taking into account the local Coulomb interaction (LSDA + U) [11]. A similar calculation for GdBaCo2O5.5 was performed in [12]. The integration over the Brillouin zone was carried out using a 48-point mesh in the irreducible part of the zone. The set of basis functions included the following electronic states: Co (4s, 4p, 3d), O (2s, 2p, 3d), Ba (6s, 6p, 5d), and Eu (6s, 6p, 5d, 4f). The atomic positions for EuBaCo2O5.5 were taken from [13]. The local energy U of the Coulomb repulsion and the energy of the Hund’s rule intra-atomic exchange JH were the same as in [12], i.e., 7.00 and 0.99 eV, respectively.
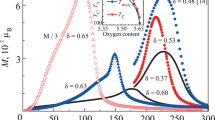
In Fig. 1, we show the oxygen K-edge X-ray absorption spectra of EuBaCo2O5.52 ± 0.02 (sample S1) and EuBaCo2O5.24 ± 0.02 (sample S2) cobaltite samples measured at room temperature and at 440 K, which far exceeds the metal–insulator transition temperature of 360 K for EuBaCo2O5.5 [1]. For comparison, we show the spectra of reference Sr2CoO3Cl, EuCoO3, and CoO compounds. The O K-edge X-ray absorption spectra are related to the O 1s → 2p electronic transition. Owing to the hybridization of 3d states of the transition element and 2p states of oxygen, the vacant 3d states of cobalt manifest themselves in the O K‑edge absorption spectrum.
O K-edge X-ray absorption spectra in (S1) EuBaCo2O5.52 ± 0.02 and (S2) EuBaCo2O5.24 ± 0.02 measured at room temperature and at about 440 K. For comparison, we present the spectra of the reference compounds Sr2CoO3Cl (high-spin Co3+ ions in pyramids), EuCoO3 (low-spin Co3+ ions in octahedra), and CoO (high-spin Co2+ ions in octahedra).
The Sr2CoO3Cl compound has the Ruddlesden–Popper structure [14]. The distorted CoO5Cl octahedra, because of relatively large Co–Cl distances (as compared to the Co–O distance), can be attributed to the CoO5 square pyramids [14]. The high-spin character of Co3+ ions in the Sr2CoO3Cl pyramids was revealed from the Co L2,3-edge and O K-edge X-ray absorption spectra [15, 16, 7]. Consequently, the structure of the O K-edge spectrum in the range from 528 to 533 eV is due to the transition of electrons from the inner O 1s orbitals to the O 2p orbitals, and the low energy peak a in the spectrum of Sr2CoO3Cl should be attributed to Co 3dxz, 3dyz, and 3dxy orbitals, which appear in the O K-edge spectra owing to their hybridization with O 2p orbitals. According to [15], Co3+ ions in octahedra in EuCoO3 cobaltite exhibit the low-spin state. Therefore, the first peak b in the O K-edge absorption spectrum should be related to unoccupied eg states (\(3{{d}_{{{{x}^{2}} - {{y}^{2}}}}},3{{d}_{{3{{z}^{2}} - {{r}^{2}}}}}\)) [15, 16, 7]. The CoO spectrum, which exhibits the high-spin states of Co2+ ions in octahedra, occupies the photon energy range which is located rather high relative to that of the O K-edge absorption in cobaltites.
Peak a in the spectrum of sample S1 coincides in energy with the corresponding peak in the spectrum of Sr2CoO3Cl. A decrease in the oxygen content in cobaltites (an increase in the relative fraction of CoO5 pyramids with respect to the CoO6 octahedra) manifests itself in the X-ray spectra: peak a' in the spectrum of sample S2 is shifted toward high energies by about 0.3 eV relative to that in the spectrum of sample S1. A similar shift of the peak in the O K-edge spectrum toward the higher energies with a decrease in the o-xygen content in cobaltites was found for the P-rBaCo2O5+δ system. According to [17], it occurs with the change in δ from 0.74 to 0.5 and, according to [18], with the change from δ = 0.802 to δ = 0.432. It was explained by a change in the degree of hybridization of the Co–O states [17].
We can suppose that the shift of the peak in the O K-edge absorption spectrum with a decrease in the relative oxygen content (appearance of Co2+ ions and an increase in the relative fraction of CoO5 structural fragments) is related to the change in the position of the bottom of the conduction band of cobaltites. A band gap of 0.05 eV was found from the optical experiments for EuBaCo2O5.5 cobaltite [19]. For related PrBaCo2O5.5 and GdBaCo2O5.5 cobaltites, the optical experiments give the gap values of 0.26 eV [12]. The authors of [12] suppose that the difference in the gap values is due to the different qualities of the single crystals used in the experiment. Taking into account the band gap Egap ≃ 0.05 eV and the shift of the peak a' in the O K-edge absorption spectrum of EuBaCo2O5.24±0.02 relative to the peak in the spectrum of EuBaCo2O5.52 ± 0.02 by 0.30 eV, we can expect the band gap in oxygen-deficient cobaltites to increase: Egap ≃ 0.35 ± 0.05 eV for EuBaCo2O5.25.
Let us now turn to the temperature effects in absorption spectra. It was found in [7] that the oxygen K-edge absorption spectrum in GdBaCo2O5.5 is only slightly shifted toward low energies by about 0.1 eV with increasing temperature from 300 to 400 K. In our experiments, the temperature changes in the spectra of samples S1 and S2 are absent, as follows from Fig. 1. This means that, in fact, there are no changes in the spin states of cobalt ions. This is not surprising, since only a small fraction of Co3+ ions are involved in the spin-state transition in this temperature range, and the changes in the spin states of the ions are too small to be detected in the measured spectra. Note that there is also a difference in the spin states of cobalt ions in PrCoO3 and EuCoO3, where Co3+ ions are located in CoO6 oxygen octahedra. At room temperature, Co3+ ions in PrCoO3 are in the high-spin state [20], while Co3+ ions in EuCoO3 have the low-spin character [15].
To interpret the experimental results, we use the ab-initio calculations of the electron density of states. In Fig. 2, we show the partial electron densities of states corresponding to Co 3d and O 2p for octahedra and pyramids in EuBaCo2O5.5. According to these calculations, the band gap is 0.04 eV. Note that the gap estimated in [12] for the related GdBaCo2O5.5 cobaltite using the LDA + U calculations is 0.24 eV. The main contribution near the bottom of the conduction band (unoccupied electron states) comes from hybridized O 2p–Co 3d states of the pyramids, whereas the contribution of the states corresponding to the octahedra is negligible. The spin magnetic moment of cobalt ions in the pyramids determined from the calculation of the density of states is 2.3μB, which is close to a value of 2.0μB expected for the high-spin state of the system involving Co3+ ions. The magnetic moment in octahedra is zero, which is a signature of the low-spin state.
Partial Co \(3d\) and O \(2p\) electron densities of states in EuBaCo2O5.5. Symbols Co\(\mathop {3d}\nolimits_ \uparrow \) and Co\(\mathop {3d}\nolimits_ \downarrow \) denote the densities of states for different spin orientations. These densities of states are shown for octahedra and pyramids. For comparison, we present the O K-edge X-ray absorption spectrum measured for EuBaCo2O5.52 ± 0.02 at room temperature. The spectrum is plotted using the common energy scale after fitting the energies of spectral peaks and the density of states for vacant O \(2p\) states.
To summarize, the electron states of cobalt ions in EuBaCo2O5.52 ± 0.02 and EuBaCo2O5.24 ± 0.02 have been studied using oxygen K-edge X-ray absorption spectra measured at temperatures of 300 and 440 K, which are below and above the metal–insulator transition temperature, respectively. The substitution of Co2+ ions for some Co3+ ions with a decrease in the oxygen content in the chemical formula of cobaltite and, therefore, an increase in the relative fraction of CoO5 pyramids with respect to the number of CoO6 octahedra determined from the spectra is accompanied by an increase in the band gap by about 0.3 eV. There is nearly no effect of temperature on the spectra. The band structure of EuBaCo2O5.5 has been calculated by the linearized muffin-tin orbital method in the framework of the local density approximation taking into account the local Coulomb interaction. It has been found that the low-spin state of Co3+ ions arises in CoO6 octahedra of EuBaCo2O5.5, while the high-spin state is characteristic of cobalt ions in the pyramids.
REFERENCES
A. Maignan, C. Martin, D. Pelloquin, N. Nguyen, and B. Raveau, J. Solid State Chem. 142, 247 (1999).
V. P. Plakhty, Y. P. Chernenkov, S. N. Barilo, A. Podlesnyak, E. Pomjakushina, E. V. Moskvin, and S. V. Gavrilov, Phys. Rev. B 71, 214407 (2005).
S. Roy, I. S. Dubenko, M. Khan, E. M. Condon, J. Craig, N. Ali, W. Liu, and B. S. Mitchell, Phys. Rev. B 71, 024419 (2005).
E.-L. Rautama and M. Karppinen, J. Solid State Chem. 183, 1102 (2010).
S. V. Naumov, V. I. Voronin, I. F. Berger, M. S. Udintseva, V. V. Mesilov, B. A. Gizhevskii, S. V. Telegin, and V. R. Galakhov, J. Alloys Compd. 817, 152775 (2020).
V. R. Galakhov, Phys. Met. Metallogr. 122, 83 (2021).
Z. Hu, H. Wu, T. C. Koethe, S. N. Barilo, et al., New J. Phys. 14, 123025 (2012).
C. Martin, A. Maignan, D. Pelloquin, N. Nguyen, and B. Raveau, Appl. Phys. Lett. 71, 1421 (1997).
S. M. Loureiro, C. Felser, Q. Huang, and R. J. Cava, Chem. Mater. 12, 3181 (2000).
O. K. Andersen and O. Jepsen, Phys. Rev. Lett. 53, 2571 (1984).
V. I. Anisimov, J. Zaanen, and O. K. Andersen, Phys. Rev. B 44, 943 (1991).
A. A. Makhnev, L. V. Nomerovannaya, S. V. Strel’tsov, V. I. Anisimov, S. N. Barilo, and S. V. Shiryaev, Phys. Solid State 51, 525 (2009).
Z. Shi, T. Xia, F. Meng, J. Wang, J. Lian, H. Zhao, J.‑M. Bassat, J.-C. Grenier, and J. Meng, Fuel Cells 14, 979 (2014).
N. McGlothlin, D. Ho, and R. J. Cava, Mater. Res. Bull. 35, 1035 (2000).
Z. Hu, H. Wu, M. W. Haverkort, H. H. Hsieh, H. J. Lin, T. Lorenz, J. Baier, A. Reichl, I. Bonn, C. Felser, A. Tanaka, C. T. Chen, and L. H. Tjeng, Phys. Rev. Lett. 92, 207402 (2004).
C. F. Chang, Z. Hu, H. Wu, T. Burnus, N. Hollmann, M. Benomar, T. Lorenz, A. Tanaka, H.-J. Lin, H. H. Hsieh, C. T. Chen, and L. H. Tjeng, Phys. Rev. Lett. 102, 116401 (2009).
P. Miao, X. Lin, S. Lee, Y. Ishikawa, S. Torii, M. Yonemura, T. Ueno, N. Inami, K. Ono, Y. Wang, and T. Kamiyama, Phys. Rev. B 95, 125123 (2017).
E. Marelli, J. Gazquez, E. Poghosyan, E. Müller, D. J. Gawryluk, E. Pomjakushina, D. Sheptyakov, C. Piamonteze, D. Aegerter, T. J. Schmidt, M. Medarde, and E. Fabbri, Angew. Chem. Int. Ed. 60, 14609 (2021).
A. A. Makhnev, L. V. Nomerovannaya, A. O. Tashlykov, S. N. Barilo, and S. V. Shiryaev, Phys. Solid State 49, 894 (2007).
Y. Ren, J.-Q. Yan, J.-S. Zhou, J. B. Goodenough, J. D. Jorgensen, S. Short, H. Kim, T. Proffen, S. Chang, and R. J. McQueeney, Phys. Rev. B 84, 214409 (2011).
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (state assignment no. АААА-А18-118020190098-5, project Electron, and state assignment no. AAAA-A18-118020290104-2, project Spin) and partially by the Russian Foundation for Basic Research (project no. 20-02-00461). The measurements of X-ray spectra were partially supported by the bilateral Russian–German Laboratory at BESSY. M.S. Udintseva acknowledges the support of the Mikheev Institute of Metal Physics, Ural Branch, Russian Academy of Sciences (project no. M 8-21).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by K. Kugel
Rights and permissions
About this article
Cite this article
Udintseva, M.S., Efremov, A.V., Smirnov, D. et al. Electronic States of Cobalt Ions in EuBaCo2O5 + δ Layered Cobaltites. Jetp Lett. 114, 475–478 (2021). https://doi.org/10.1134/S002136402120011X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002136402120011X