Plasmonic applications, which are determined by the strong interaction between an electromagnetic wave and free electrons in nanostructures, are among the possible applications of silver nanoparticles. It appears that the frequency and intensity of the plasmon resonance depend on the polarization charge distribution determined by the shape and structure of a nanoparticle. Consequently, the control of the structure of nanoclusters allows varying the wavelengths of light that are scattered or absorbed on them. In this work, the limits of the thermal stability of the initial amorphous phase in silver clusters with sizes smaller than 2.0 nm with the number of atoms corresponding to the “magic” numbers of the fcc structure are studied by the molecular dynamics method with the modified TB-SMA tight-binding potential. The results are compared with the data for a similar set of particles with the initial fcc structure. It is shown that the characters of thermally induced structural transitions in the studied groups of nanoclusters are drastically different. This property can allow fabricating small silver clusters with the required internal structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
Silver is actively used in microelectronics primarily because of its high electric and thermal conductivities. However, nanoparticles of many even well-known metals can have surprising properties. In particular, recent experiments showed that small metal nanoclusters can behave as semiconductors because of the appearance of a band gap on the Fermi level [1]. It was found that the width of such band gap increases with a decrease in the size of the cluster and can exceed 2–3 eV in very small nanoparticles. For this reason, we focus on the analysis of some features of the behavior of silver nanoclusters in this most interesting size range.
Their studies are developed primarily in the direction of forecasting of various physicochemical properties and development of new synthesis methods and possible technological applications [2–4]. We describe the latter item in more detail and mention that the existing main applications of Ag nanoparticles expand from plasmonics, photoelectric devices, optical antennas, nanoscanning probes to various medical applications [5, 6], biosensors [7], etc. In optics, Ag clusters are the most popular candidates for the fabr-ication of two-dimensional quantum dots because such small clusters of atoms have interesting spectroscopic properties different from the properties of bulk analogs [8].
The extraordinary optical properties of Ag nanoclusters are determined primarily by quantum effects in them, which are most pronounced at sizes below 2.0 nm [9]. In this case, silver nanoclusters have molecular-like rather than bulk properties because their sizes are comparable with the wavelengths of Fermi electrons (~0.5 nm for Ag); as a result, small Ag clusters have discrete electron energy levels leading to, e.g., strong fluorescence [1].
Further studies showed that such quantum effects are strongly determined by sizes of nanoclusters, their shape, internal structure, and location and density of nanoparticles on a substrate. For example, large Ag nanoclusters can be used to apply absorption and scattering of light, whereas small silver nanoclusters are responsible for nonlinear optical properties. In particular, bulk silver is usually nonluminescent because of the metallic bond between atoms, single Ag atoms demonstrate only a narrow weak radiation band in the ultraviolet–blue spectral range, and Ag nanoclusters emit a broad luminescence band covering the entire visible spectral range [10, 11].
The processes of interaction between a metal and a light wave (plasmon effects) allow new engineering applications of silver because of the strong interaction between incident light and free electrons in nanostructures. Metal nanoparticles smaller than the wavelengths of visible light can strongly absorb light owing to surface plasmon resonance, which is caused by the collective oscillation of conduction electrons under the action of the light wave. It is currently clear that the size, shape, and structure of nanoparticles determine their optical properties, including resonant frequencies. In particular, the authors of [12] showed a typical correlation between the peak wavelength at surface plasmon resonance of silver nanoclusters and their size. For example, Ag nanoclusters demonstrate the surface plasmon resonance peak at 380 nm (diameter of the cluster is \(D = 1.6\) nm); at the diameters of the clusters D = 3.0 and 4.8 nm, the surface plasmon resonance peak is observed at 390 and 396 nm, respectively [12].
The symmetry of particles can also affect the magnitude of scattering and absorption a light wave. Without the formation of a strong dipole, scattering and absorption of light by nanoparticles will be insignificant. The strong dipole can be easily formed for highly symmetric objects such as spheres, cubes, and octahedra. In particular, the separation of the charge at vertices of the cube induces the dipole because vertices are located on opposite sides of the symmetry line. By analogy, the separation of the charge for spheres occurs in a completely isotropic medium. However, nanostructures that do not have such a symmetry, e.g., triangular plates, cannot form a strong dipole and they absorb light only slightly. It was found in the experimental work [13] that the plasmon resonance can be observed in the wavelength range from 300 to 1200 nm, depending on the structure of silver nanostructures. The main resonance peak for the spherical nanoparticle, cubic nanoparticle, decahedron of localized surface plasmon, and octahedron was in the ranges of 320–450, 400–480, 350–450, and 400–500 nm, respectively.
Consequently, the variation of the size, shape, and internal structure of the metal nanostructure allows controlling light with a very high accuracy. Consequently, it is not surprising that successes in the assembly of metal nanostructures open new capabilities for more accurate control of processes of interaction of metal nanoparticles with the light wave [13] and the determination of morphology of single silver nanoparticles is of great interest in this application. In our previous works [14, 15], we already analyzed the thermal stability of silver nanoclusters with the diameter smaller than 2.0 nm and with the initial fcc structure. It was shown that such form of clusters is thermally stable for nanoparticles containing more than 200 atoms. However, scenarios of the thermal evolution of the internal structure of smaller silver nanoclusters are much more complex. This work is also devoted to the analysis of the thermal stability of the structure of silver nanoclusters with the diameter smaller than 2.0 nm, but with the initial amorphous structure, which allows revealing the effect of the initial morphology of Ag nanoparticles on the character of structural transitions.
2 COMPUTER MODEL
In this work, we analyze the possible rearrangements of configurations of atoms in small silver clusters (\(D \leqslant 2.0\) nm) under thermal action. In addition to reasons indicated above, such small particles are chosen because the so-called “magic” numbers of different nature are of great importance in the stability of the structure of such particles [9, 16, 17]. Unfortunately, existing experimental methods cannot provide an adequate diffusion picture of displacements of atoms inside such small clusters; at best, only the image of the final stage of the evolution of chemically or physically synthesized nanoparticles will be available. However, electron microscopy data on the shape and internal structure of small metal clusters cannot be considered fully adequate.
In particular, the high-resolution electron microscopy study [18] of Cu3Au particles (\(D = 2.0{-} 20.0\) nm) showed that the electron microscope beam significantly changed the morphology of nanoparticles. At the first stage, nanoparticles of Cu3Au alloy did not have a clear shape, which varied from spherical to elliptical. After irradiation of particles by the electron beam for 2 min, their shape began to change and a new shape of particles was formed approximately after 15 min. Thus, irradiated particles are transformed to liquid drops, which were then crystallized. The shape and structure (fcc) of particles crystallized from the melt did not further change even if particles were again irradiated. It is obvious that the internal structure and shape of smaller metal nanoparticles exposed to the electron beam will change drastically; for this reason, we believe that computer simulation is more appropriate for the detailed study of processes of diffusion displacement of silver atoms under the thermal action.
The most convenient method for this study can be the molecular dynamics approach based on the calculation of classical (Newtonian) trajectories of an object in the phase space of coordinates and momenta of its atoms. This method makes it possible to quite accurately determine the structural and thermodynamic properties of clusters, as well as to trace the dynamics of atoms of nanoparticles under the variation of external parameters such as the temperature and pressure.
The correct choice of the interatomic interaction is also important for the successful simulation of real systems. Practice shows that the embedded atom method (EAM) potential cannot be applied to the simulation of small metal nanoparticles because of the basic features of the construction of such interaction. This method can give adequate results for sufficiently large particles, but simulation based on this method provides physically incorrect results for the small nanoclusters studied in this work. In particular, the melting temperatures of small nanoparticles determined with the EAM potential no longer depend on the size of the cluster; i.e., the binding energies of atoms are almost the same despite different numbers of coordination spheres in clusters with different sizes [19].
In this case, it can be more adequate to use the modified TB-SMA tight-binding potential [20] based on the assumption that numerous properties of transition metals can be completely determined from the density of states of outer d electrons. In our opinion, this method, where the ion–ion interaction is described taking into account the band character of bonds and the short-range repulsion pair potential, can quite correctly describe some features of small metal nanosystems. For this reason, the simulation of silver nanoparticles is performed in this work using this interatomic potential.
The computer analysis of all processes occurring in Ag clusters was performed in the canonical NVT ensemble. The temperature was determined in terms of the average kinetic energy of atoms, which was calculated using the Verlet velocity algorithm with the time step h = 1 fs. Structural transitions were identified using imaging, as well as the radial distribution function and the temperature dependence of the potential energy.
The initial structure in the computer experiment was taken in the form of spherical silver clusters with sizes smaller than 2.0 nm. The initial amorphous structure of nanoclusters was obtained by sampling particles cut from the ideal fcc lattice, which were then heated until the complete destruction of the long-range order in them. Further, to fix the amorphous structure, clusters were “instantaneously” cooled to a temperature of 20 K. The sizes of nanoclusters were chosen such that the number of atoms in them corresponds to magic numbers of the fcc structure. Then, to estimate the thermal stability of the amorphous structure of small Ag clusters, simulated systems were smoothly heated from 20 to 1000 K using a Nosé thermostat. The upper limit of 1000 K is sufficient to melt simulated particles because the melting temperature for small Ag clusters is significantly lower than that for bulk silver (Tm = 1235.1 K). To determine the most stable cluster structure, we studied an ensemble of nanoparticles with the same size. In the process of heating, the temperature was changed stepwise generally with a step of 20 K and with a step of 5 K in the region of structural transitions; clusters were aged for 1.0 ns at each fixed temperature. The simulation was performed with the MDNTP software developed by Dr. Ralf Meyer (University of Duisburg, Germany).
3 RESULTS AND DISCUSSION
It is well known that silver atoms have a high oxidation ability [1]; for this reason, various stabilizers should be used to keep the chemical purity of Ag nanoclusters. However, these methods are too complex to be applied in industry [15, 21]. Another approach to this problem can be the synthesis of very pure particles with an ideal crystal structure. Only surface atoms in such single-crystal nanoparticles can be involved in chemical reactions because of a decrease in their coordination number. It is also noteworthy that the presence of defects of the crystal lattice and phase interfaces in a particle results in additional scattering of conductivity electrons, which leads to large optical losses reducing the plasmon efficiency. Thus, the ideal crystal structure is important for the long-term existence of the plasmon resonance.
This assumption has direct experimental evidence. In particular, using the method of deposition of a cluster beam based on magnetron sputtering, the authors of [21] obtained Ag single-crystal nanoparticles with D = (12.5 ± 1.1) nm and D = (24.0 ± 2.0) nm and an fcc single-crystal structure. They believed that just the single-crystal nature of Ag nanoparticles was a reason for the long-term stability of localized surface plasmon resonances. Silver nanoparticles synthesized in [21] demonstrated a high stability of the intensity of the plasmon resonance band, which decreased only by 20% after 30-day aging at room temperature in the environment.
We believe that an additional possibility of increasing the plasmon efficiency can be the use of silver nanoparticles with a size equal to magic numbers of various crystal structures as localized surface plasmon resonance structures. The experimental analysis of the size distribution of synthesized particles shows that clusters with a certain number of atoms corresponding to magic numbers are much more stable than clusters with different numbers. The degree of defectiveness of the surface of such magic clusters is minimal, whereas this degree in other clusters can be very high. Thus, the determination of boundaries of the thermal and size stabilities of the initial structure of nanoparticles seems very important for the use of silver nanoclusters in plasmonic applications.
Since bulk silver has the standard fcc structure, we analyze the simulation results for silver clusters whose sizes are equal to magic numbers for this arrangement of atoms in the crystal lattice (N = 79, 135, and 201). First, we present the main data on the thermal stability of such clusters under the assumption of their initial fcc structure [14, 15]. At first glance, the fcc structure of clusters with a magic number of constituents should be completely stable, but the situation appears not so certain. Indeed, processes in clusters Ag79 and Ag201 proceeded according to the above hypothesis. Thus, the consideration of the geometric arrangement of atoms in an ensemble of clusters Ag79 and Ag201 revealed that their initial fcc structure held up to the melting temperature and was gradually destroyed beginning with the surface; i.e., the initial fcc phase of clusters with such a size was thermally stable, which is confirmed, in particular, in [17]. We detected no thermally induced structural transitions in nanoparticles with these sizes. Such a behavior of Ag clusters was expected and predicted, but this behavior was significantly violated in Ag135 clusters (\(D = 1.59\) nm), where the fcc → Ih transition to the icosahedral structure was revealed by the computer analysis. All molecular dynamics simulation experiments demonstrated the transition of the fcc structure of silver particles consisting of 135 atoms to the Ih structure. The resulting icosahedral phase further held up to the melting temperature of the cluster.
Since a similar effect was observed in our previous work [22] for 135-atom Ni and Cu clusters, we generally concluded that this number of atoms in a nanoparticle ensures the most favorable energy conditions for the formation of the Ih structure. Consequently, the thermally induced structure of the 135‑atom silver nanocluster was determined not only by the magic fcc number but also by other factors, e.g., electronic magic numbers [23]. The authors [24] also concluded that structural and electronic effects can simultaneously affect the stability of clusters. In our opinion, the closeness of the icosahedral magic number N = 147 and to the electronic magic number N = 138 could be responsible for the transition from one structure to the other in the cluster Ag135.
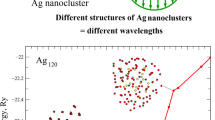
However, the properties revealed for silver nanoclusters with the same sizes N = 79, 135, and 201 atoms but with the initial amorphous structure indicate that the thermal stability of the amorphous phase for these particles is determined mostly by some other factors rather than by the correspondence to the magic numbers of the fcc structure. In particular, the analysis of the results of our molecular dynamics simulation shows that amorphous nanoparticles Ag79 in most cases (\( \approx \) 70%) completely hold the initial structure only in the initial heating stage (up to about 200–250 K). However, a weak rearrangement of the internal structure of clusters Ag79 begins already at room temperature and is enhanced at temperatures of about 400–450 K. All these properties are clearly confirmed by a stepwise drop of the potential energy of the cluster as a function of the temperature (Fig. 1). Although the depth of the appearing local energy minimum is very small, no more than 0.2–0.3% of the potential energy of the entire cluster at a given temperature, it is sufficient to form a pronounced nucleus of a five-particle structure, which holds up to the transition to the liquid state. It is clear that the region of such transition could be determined only conditionally, primarily from “instantaneous images” because the initial arrangement of atoms in the cluster corresponds to the amorphous phase and the structure is weakly rearranged in the process of heating. Nevertheless, an almost complete conservation of the initial amorphous structure in the process of heating can be considered as the basic variant of the thermal evolution of these Ag79 nanoparticles.
The simulation result can be explained as follows. The amorphous structure is not a close-packed structure and is characterized by a low average coordination; as a result, atoms in this structure can move significantly to form their most energetically favorable configuration. In the case of such small clusters, this configuration is characterized primarily by the minimum surface energy. Meanwhile, the fcc cluster does not have such surface energy value because of the features of its structure (presence of planar faces). Therefore, an amorphous structure can become more energetically favorable than the fcc structure.
We comparatively analyze the potential energies of Ag79 clusters with different initial structures because the potential energy can give a large amount of information on the features of the interatomic interaction. The cluster with the fcc structure at the temperature T = 50 K is expectedly more stable, having the energy Ep = –15.17 Ry, whereas the same cluster but with the initial amorphous structure had the energy Ep = ‒15.06 Ry (ΔEp ≈ 0.11 Ry ≈ 0.019 eV/atom). This situation held until the structural rearrangement in the amorphous cluster. In particular, the cluster with the initial fcc structure at T = 200 K still ensured a similar energy gain (Ep = –15.06 Ry versus Ep = –14.95 Ry for amorphous Ag79), but the energy difference at room temperature decreased sharply by almost a factor of 2 (ΔEp ≈ 0.06 Ry or ≈ 0.01 eV/atom). In our opinion, the reason is obvious: atoms in the cluster with the fcc structure and the size corresponding to the geometric magic number are quite rigidly fixed in the existing crystal lattice and hardly can undergo diffusion motion within the cluster to change its internal structure. At the same time, such restrictions are absent in the cluster with the initial amorphous structure, and as soon as the thermal energy kT becomes sufficient, the optimization of configuration of atoms begins in order to transfer the Ag79 cluster to a more energetically favorable state with the formation of an icosahedral nucleus. This transition occurs in two stages: the pr-eliminary stage in the temperature range of T = 250−350 K and the final stage in the temperature range of T = 350−450 K; as a result, the cluster with the initial amorphous structure at T = 500 K becomes even energetically slightly more stable (Ep = −14.78 Ry) than the “classical” fcc cluster (Ep = −14.8 Ry). This conclusion can certainly be valid only under several important conditions of the initial amorphous structure and a small size of the cluster, which was confirmed in our previous works [14, 15].
The transition from the amorphous structure to the fairly well formed decahedral structure was observed in the other 30% of experiments. However, the rearrangement of the cluster structure occurred differently. The analysis of the energies of amorphous Ag79 clusters of the first and second types indicates that the transition to the five-particle structure nucleus in the second variant of thermal evolution occurred already at the stage of initial relaxation of the cluster. In particular, this cluster at T = 50 K has the energy Ep = ‒15.21 Ry; the same clusters but with the initial amorphous and fcc structures have the energies Ep = ‒15.06 and –15.17 Ry, respectively. Further, the decahedral structure is completely formed at temperatures of about 450–500 K and holds up to the melting temperature of the cluster. In particular, the analysis of the thermal stability of the initial amorphous structure of Ag79 clusters indicates that the magic number of the fcc structure cannot affect this process. The initial ideal fcc structure of the Ag79 cluster is thermally stable up to the melting temperature [14, 15], whereas the initial amorphous structure is stable in most of the performed model experiments.
As mentioned above, bulk silver has a cubic lattice, but the competition between the bulk and surface energies in the nanometer range can result in the formation of several different isomers because the energies of different structures are very close to each other. In particular, the molecular dynamics simulation with the Gupta potential performed in [25] for 38-atom gold clusters close to silver clusters in structure formation gives the energies of –3.4405, –3.44, and 3.431 eV/atom for the clusters in the amorphous, fcc, and icosahedral ground states, respectively. These small differences between the energies of all three isomers hold up to room temperature. The thermal energy at T > 250 K is already sufficient to overcome the potential barrier between different structures; as a result, all three structures are randomly observed. The main reason for this behavior can be that almost all atoms in such nanoparticles are located on the surface and an infinitesimal change in their positions caused by thermal diffusion can be sufficient to spontaneously form another structural modification.
In [26], the authors of [25] performed a similar molecular dynamics simulation with the Gupta potential for larger gold clusters. In particular, the difference in binding energy between the amorphous and Ih structures with N = 55 is 9.4 meV/atom, and the five-particle Dh structure with \(N = 75\) is more stable than the amorphous structure by only 5.7 meV/atom. To test the result, the authors of [26] carried out an additional ab initio study of the relative stability of gold clusters with the crystal and amorphous structures. It was found that the difference in binding energy per atom between the most stable amorphous structure and crystal structures with the above sizes is less than 0.01 eV/atom.
Further, we analyze features of the behavior of the Ag135 cluster. As mentioned above, in this case, the transition of the initial fcc structure to the icosahedral structure under smooth heating necessarily occurs at a temperature of about 400 K. We consider the thermal evolution of the structure of such nanoparticle with the initial amorphous arrangement of atoms. Unlike the classical fcc case, the rearrangement of this cluster structure can occur through three scenarios. At the first stage, we analyze the most probable scenario implemented in about 70% of the model cases (Fig. 2). First, we note that the Ag135 cluster with the fcc structure at T = 50 K had the typical potential energy Ep = –26.32 Ry, whereas the same cluster with the initial amorphous structure expectedly had the higher a-verage energy Ep = –26.21 Ry (ΔEp ≈ 0.11 Ry or ≈0.011 eV/atom). However, these energies at room temperature became almost equal to each other owing to the small rearrangement of the amorphous phase at T = 250 K. The second structural transition occurred also at the temperature T = 400 K and led to the formation of the almost ideal icosahedron. The more energetically stable structure with the energy Ep = ‒25.88 Ry was formed at T = 500 K, whereas the energy in the classical fcc case was –25.8 Ry. Correspondingly, the melting temperature of the Ag135 cluster with the initial amorphous state is higher. Consequently, the evolution of the internal structure of the Ag135 cluster beginning with a temperature of about 400 K is almost the same for any initial structure of the cluster. Possible reasons for the fcc → Ih transition in the silver cluster with this size were considered in detail in our previous works [14, 15].
However, we revealed two other possible scenarios of the rearrangement of the internal structure of the Ag135 cluster. A situation similar to the above case of the Ag79 cluster was observed in 20% of model experiments. Thus, an icosahedral (decahedral) structure nucleus was formed owing to stochastic diffusion processes already at the initial stage of thermal relaxation, which led to the reduction of the potential energy to the value Ep = –26.25 Ry (T = 50 K), meaning an energy gain of about 0.004 eV/atom. The energies of the Ag135 clusters evolving through the first and second scenarios become equal to each other at room temperature owing to the formation of approximately identical icosahedral structures. However, at higher temperatures about T = 500 K, the second scenario results in the formation of a lower quality icosahedron with the energy Ep = –25.8 Ry (cf. Ep = –25.88 Ry in the first scenario of thermal evolution), which almost coincides with the energy of the classical fcc Ag135 cluster at the same temperature; correspondingly, the melting temperatures are also close to each other.
The rarest scenario (≈10%) of thermal evolution of the Ag135 cluster involved two successive structural transitions: amorphous → Dh → Ih. We analyze the energy characteristic of the internal rearrangement in these clusters. The potential energy at T = 50 K was on average higher, Ep = –26.2 Ry, than that in the above scenarios. Further, the energies of clusters evolving through the first and second scenarios usually became equal to each other at T = 300 K, but this was not the case in the third scenario, where an energy difference of 0.1 Ry still remained. The real structural transition was observed only at T = 400 K, but it led only to a weak local minimum owing to the formation of a low-quality decahedral structure with the energy Ep = ‒25.82 Ry. The Dh → Ih transition begins at T = 550 K, which leads to a further decrease in the energy to the value Ep = –25.84 Ry comparable with that in the most probable first scenario. However, the most significant difference from the other 90% of model experiments is a smooth temperature dependence of the potential energy in the range up to 700–750 K, which is comparable with the typical behavior of the Ag79 cluster (Fig. 1).
Consequently, the analysis of the thermal stability of the initial amorphous structure Ag135 clusters indicates that the magic number of the fcc structure also does not affect this process. The transition of the initial ideal fcc structure of the Ag135 cluster to the Ih structure [14, 15] was observed at temperatures of about 400 K; in the considered initial amorphous structure, this transition was observed in all performed model experiments but with some variations.
The last of the considered magic fcc silver clusters contained 201 atoms. As determined above, the initial ideal fcc structure held up to the melting temperature, gradually decaying beginning with the surface, which indicated its thermal stability. In turn, the behavior of Ag201 nanoclusters with the initial amorphous arrangement of atoms changed significantly compared to this case. The transition to the icosahedral structure becomes favorable (in approximately 80% of the experiments) at temperatures T ≈ 350–400 K (Fig. 3). Such amorphous → Ih transition gives an energy gain of about 0.3 Ry (0.02 eV/atom), which makes the icosahedral structure of Ag201 nanoclusters very stable.
We now analyze the thermal stability of the structure of Ag201 clusters with different initial arrangements of atoms in terms of the potential (binding) energy. The energy of the fcc structure at the lowest considered temperature T = 50 K is Ep = –39.98 Ry, which is noticeably lower than the energy of the cluster with the amorphous structure Ep = –39.45 Ry; i.e., the energy gain is very large, 0.037 eV/atom. This tendency holds at room temperature, at which the difference between the corresponding binding energies is 0.034 eV/atom. It is clear that the Ag201 cluster with the initial amorphous structure is much less energetically favorable than the Ag201 cluster with the initial fcc structure. Consequently, the former silver cluster cannot be thermodynamically stable and the amorphous structure is transformed at T ≈ 350–400 K to the icosahedral structure, though it is incompletely ideal. Now, the energy difference between two types of nanoparticles almost vanishes and the potential energy of the initially amorphous cluster at T = 400 K is Ep = –39.2 Ry, which is comparable with the energy Ep = –39.3 Ry of the cluster with the fcc structure. With the further increase in the temperature, this difference in potential energy approximately holds until the beginning of melting.
However, another branch of the thermal evolution of Ag201 clusters with the initial amorphous structure was observed in 20% of our computer experiments. Thermal evolution began at a temperature T = 50 K with the same potential energy Ep = –39.45 Ry. Then, up to temperatures T ≈ 300–350 K, no significant differences in energy and structure were detected. However, the structural transition in this case began at T ≈ 300–350 K rather than at T ≈ 350–400 K, as in the basic scenario for the Ag201 cluster. This transition gave only half the energy gain, about 0.15 Ry (0.01 eV/atom). The structural rearrangement resulted in a decahedron, but the energies of the Ag201 clusters with the icosahedral and decahedral structures at T = 500 K became the same, about –38.9 Ry. Thus, structural magic numbers of the fcc structure also could not promote the formation of this configuration in this case.
4 CONCLUSIONS
Silver nanoclusters are widely used in various engineering, medical, and plasmonic applications. Investigations reveal that their physicochemical properties are determined mostly by their sizes, shape, and internal structure. Crystal structures corresponding to the so-called structural magic numbers should be the most stable. Meanwhile, the mechanisms of the formation and stability of these structures are still unknown.
The continuous density of states in the conduction band of a bulk metal particle changes qualitatively to a set of discrete levels when the size of the particle decreases to several hundred or several tens of atoms; the intervals between these levels can be larger than the thermal energy kBT, which results in the formation of a band gap. Clusters with different sizes and different internal structures have different electronic structures and, correspondingly, different distances between levels; this property can be used to design nanomaterials and to fabricate various technical devices. In particular, light-induced transitions between energy levels determine the color of a material, which is widely used in plasmonic applications. The capability of a cluster to react with other materials also depends on the size and structure of the cluster.
In this work, the thermal stability of small silver nanoclusters with diameters smaller than 2.0 nm and the number of atoms corresponding to some magic numbers of the fcc structure have been studied by molecular dynamics simulation in the case of the initial amorphous structure. The results have been compared to previous data for a similar set of particles with the initial fcc structure. It has been shown that the character of thermally induced structural transitions in the studied nanoclusters is drastically different from that in the previously observed transitions: fcc and hcp structures are absent against the prevailing Ih structures. Consequently, the use of different initial structures of small silver nanoclusters (\(N < 200\) atoms) allows one to form clusters with a required internal structure through thermal evolution, which can be unachievable through usual chemical or physical synthesis. This circumstance can be used in plasmonic applications, for which it is necessary to study in detail the thermal stability of the cluster structure with allowance for effect of various magic numbers.
REFERENCES
D. Hua and Y. Hongtao, Adv. Nat. Sci. 8, 1 (2015).
J. Natsuki, T. Natsuki, and Y. Hashimoto, Int. J. Mater. Sci. Appl. 4, 325 (2015).
P. Horta-Fraijo, M. Cortez-Valadez, R. Britto Hurtado, R. A. Vargas-Ortiz, A. Perez-Rodriguez, and M. Flores-Acosta, Phys. E (Amsterdam, Neth.) 97, 111 (2018).
C. Guo and J. Irudayaraj, Anal. Chem. 83, 2883 (2011).
T. C. Dakal, A. Kumar, R. S. Majumdar, and V. Yadav, Front. Microbiol. 7, 1831 (2016).
A.-C. Burdusel, O. Gherasim, A. M. Grumezescu, L. Mogoanta, A. Ficai, and E. Andronescu, Nanomaterials 8, 681 (2018).
I. Ghiuta and D. Cristea, Nanoeng. Biomater. Adv. Drug Deliv., 347 (2020). https://doi.org/10.1016/B978-0-08-102985-5.00015-2
S. Alkis, J. L. Krause, J. N. Fry, and H.-P. Cheng, Phys. Rev. B 79, 121402(R) (2009).
H. Akbarzadeh and H. Yaghoubi, J. Colloid Interface Sci. 418, 178 (2014).
A. S. Kuznetsov, N. T. Cuong, V. K. Tikhomirov, M. Jivanescu, A. Stesmans, L. F. Chibotaru, J. J. Velázquez, V. D. Rodríguez, D. Kirilenko, G. van Tendeloo, and V. V. Moshchalkov, Opt. Mater. 34, 616 (2012).
J. J. Velázquez, V. K. Tikhomirov, L. F. Chibotaru, N. T. Cuong, A. S. Kuznetsov, V. D. Rodríguez, M. T. Nguyen, and V. V. Moshchalkov, Opt. Express 20, 13582 (2012).
J. D. Padmos, R. T. M. Boudreau, D. F. Weaver, and P. Zhang, J. Phys. Chem. C 119, 24627 (2015).
M. Rycenga, C. M. Cobley, J. Zeng, W. Li, Ch. H. Moran, Q. Zhang, D. Qin, and Y. Xia, Chem. Rev. 111, 3669 (2011).
L. V. Redel’, Yu. Ya. Gafner, and S. L. Gafner, Phys. Solid State 57, 2117 (2015).
Y. Gafner, S. Gafner, and D. Bashkova, J. Nanopart. Res. 21, 243 (2019).
Y.-P. Chiu, Ch.-M. Wei, and Ch.-S. Chang, Phys. Rev. B 78, 115402 (2008).
D. Liu, Z. Wen, and Q. Jiang, Curr. Nanosci. 7, 463 (2011).
D. T. Tran, I. P. Jones, R. L. Johnston, J. A. Preece, and C. R. van den Brom, J. Phys.: Conf. Ser. 241, 012086 (2010).
V. M. Samsonov, S. A. Vasilyev, K. K. Nebyvalova, V. Talyzin, N. Y. Sdobnyakov, D. N. Sokolov, and M. I. Alimov, J. Nanopart. Res. 22, 247 (2020).
F. Cleri and V. Rosato, Phys. Rev. B 48, 22 (1993).
S. M. Novikov, V. N. Popok, A. B. Evlyukhin, M. Hanif, P. Morgen, J. Fiutowski, J. Beermann, H.-G. Rubahn, and S. I. Bozhevolnyi, Langmuir 33, 6062 (2017).
S. L. Gafner, L. V. Redel, Zh. V. Golovenko, Yu. Ya. Gafner, V. M. Samsonov, and S. S. Kharechkin, JETP Lett. 89, 364 (2009).
W. Demtröder, Molekülphysik: Theoretische Grundlagen und experimentelle Methoden (Oldenburg, Heidelberg, 2000).
A. K. Starace, C. M. Neal, B. Cao, M. F. Jarrold, A. Aguado, and J. M. Lopez, J. Chem. Phys. 129, 144702 (2008).
I. L. Garzon, K. Michaelian, M. R. Beltan, A. Posada-Amarillas, P. Ordejon, E. Artacho, D. Sanchez-Portal, and J. M. Soler, Eur. Phys. J. D 9, 211 (1999).
I. L. Garzon, K. Michaelian, M. R. Beltrán, A. Posada-Amarillas, P. Ordejón, E. Artacho, D. Sánchez-Portal, and J. M. Soler, Phys. Rev. Lett. 81, 1600 (1998).
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 19-48-190002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by R. Tyapaev
Rights and permissions
About this article
Cite this article
Ryzhkova, D.A., Gafner, S.L. & Gafner, Y.Y. Effect of “Magic” fcc Numbers on the Stability of the Structure of Small Silver Nanoclusters. Jetp Lett. 113, 638–645 (2021). https://doi.org/10.1134/S002136402110009X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002136402110009X