Abstract
One of the new applications of silver nanoparticles is their use in plasmonic applications determined by the strong interaction of the electromagnetic wave and free electrons in nanostructures. Silver particles of a size smaller than the visible light wavelength can strongly absorb light due to the surface plasmonic resonance caused by the collective oscillation of the conduction electrons. The frequency and intensity of the plasmonic resonance depends on the distribution of the nanostructure polarized charge, which is determined by the shape and structure of the nanoparticle. But the rapid oxidation/sulfidation due to the ambient atmosphere dramatically reduces all the advantages of silver and causes difficulties from the view point of practical applications. Possible solution to this problem could be the formation of very pure particles of a perfect crystal structure, which should be more resistant to the abovementioned phenomena. We believe that unaccounted possibility of increasing the plasmon efficiency can be the usage of silver nanoparticles with a size equal to the magic numbers of various structures. To test this hypothesis, computer simulation was performed to determine the stability of the structure of silver clusters with a size of up to 2.0 nm. It shows that the use of small silver clusters in plasmonic applications strongly requires considering the problem of the thermal stability of their cluster structure with consideration of various kinds of “magic” numbers.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Today, nano-phase engineering is one of the most dynamically developed fields of the high technology that creates structural and functional materials combining the required chemical, mechanical, electrical, and optical properties (Mirguet et al. 2008). The nanoclusters and nanoparticles of various chemical composition and shape are the basis for this area. Small nanoclusters of noble metals consisting of tens or hundreds of atoms are of a special interest due to their unique physical and chemical properties (Wilcoxon and Abrams 2006). These properties make these nanoparticles widely demanded in many fields of physics, chemistry, medicine, and electronics (Cuenya 2010; Rycenga et al. 2011).
Currently, silver and gold nanoparticles are likely to be the two most studied metal nanoparticles (Aikens 2011; Horta-Fraijo et al. 2018). The investigations are aimed at forecasting various physical and chemical properties of these nanoparticles and the development of new methods of synthesis and design of potential technological applications (Horta-Fraijo et al. 2018; Guo and Irudayaraj 2011). The developed applications of Ag or Au nanoparticles vary within plasmonics, photovoltaic devices, optical antennas, nano-energy probes, medical and biological sensors, and many other fields. In addition, Au and Ag clusters are the most popular elements for the construction of two-dimensional quantum dots. Such small combinations of atoms have promising spectroscopic properties that are different from the properties of their high-mass analogues. Moreover, they can also be used to immobilize large molecules, such as proteins (Alkis et al. 2009).
Beyond that, Ag or Au nanoclusters show improved optical properties if compared with other chemical elements, which is mainly due to the available quantum effects apparent mostly with the sizes from 1.0 to 2.0 nm (Akbarzadeh and Yaghoubi 2014). Thus, Ag nanoclusters of a diameter equal to or smaller than 2.0 nm have clear molecular-like properties, for their small size is comparable with the wavelength of Fermi electrons (~ 0.5 nm for Ag). This indicates that Ag small clusters have discrete energy levels of electrons that cause intense fluorescence (Hua and Hongtao 2015). These effects depend on the size of the nanoclusters, their shape, location, and the nanoparticle density on the substrate. So, the large nanoclusters are primarily responsible for the light absorption and diffusion, while the small nanoclusters are responsible for the nonlinear optical properties (Kuznetsov et al. 2012). In particular, while the bulk silver is generally not luminescent due to that the metallic nature of the atomic binding and single atoms of Ag shows only weak and narrow band radiation in the ultraviolet-blue part of the spectrum, the Ag nanoclusters emit broad band luminescence covering the entire visible spectral range (Velázquez et al. 2012).
The type of a chemical element and the nanoparticle size and shape are also known to clearly correlate with the efficiency and wavelength of the defused electromagnetic waves (EMs) in the optical applications. Specifically, the visible spectrum of EM radiation can penetrate up to ~ 7 μm (if λ = 700 nm) in crystalline Si (c-Si), while in the amorphous silicon (α-Si), it penetrates only up to ~ 5 μm with the same wavelength (Dhoubhadel et al. 2014).
Thus, the clear vision of the real structure of the cluster is the starting point for understanding many of its features. It is well known that metal clusters of the nanometer size can have both crystalline and non-crystalline structures. The latter are very common with a smaller size; in the case of noble and transition metals, the structures acquire icosahedron (Ih) or decahedrion (Dh) forms (Baletto et al. 2002; Aikens 2011; Akbarzadeh and Yaghoubi 2014). On the other hand, new researches have reported about developing and potential use of layered and flat nanoparticles for the production of sensors. It is possible due to the measurement of the refractive indices on the basis of the absorption band dependence on the spectrum of the incident electromagnetic wave caused by the interactions with the localized surface plasmons (Horta-Fraijo et al. 2018).
Our research is focused on the study of structural properties of small clusters of silver. Mainly, chemical methods of synthesis are used to develop and control the size and structure of Ag nanoclusters, with the synthesis of anisotropic Ag nanostructures having been one of the most important achievements of colloid chemistry for recent decades (Attia et al. 2014). Particularly, the liquid precursors (aqueous or organic) are often used to obtain an ordered nanoscale assembly. Moreover, it is the chemical synthesis of nanoparticles from the solution that turned out to be the most effective in the large-scale production of nanostructures with controlled shapes and properties (Wiley et al. 2007; Wilcoxon and Abrams 2006; Lu et al. 2005; Engemann et al. 2016), although physical methods of synthesis are also possible (Numazawa et al. 2011; Manninen et al. 2014).
New fast synthesis methods facilitate the development of technical applications based on silver nanoparticles and make these applications available to the scientific and technical community. The operation, at least, of a part of these applications is based on the nanoparticle morphology. Thus, silver nanoparticles of a triangular shape appeared to be the most sensitive to SERS (surface-enhanced Raman spectroscopy) effects, while silver hexagonal nanoparticles can be used for targeted delivery of drugs, such as dextran molecules (Horta-Fraijo et al. 2018).
Especially, due to their optical activity and antimicrobial effect, the silver cluster properties expand the field of application. For example, the antibacterial activity of silver materials can be applied in medicine to treat ambustion and to prevent bacteria colonization on prostheses, catheters, vascular grafts, dental materials, stainless steel items, and so on. The silver-containing materials can also be used to eliminate microorganisms on the textile fabrics or to purify the water (Engemann et al. 2016).
The silver nanoparticles have highly intensive cytoprotective activity towards HIV-infected cells. The antimicrobial activity of the colloidal silver is strongly dependent on the particle size as distinguished from the bactericidal effect of the silver ions: the smaller the particle is, the greater its antibacterial effect is. Therefore, the development of the synthesis methods for silver nanoparticles is focused on their size monitoring (Panáček et al. 2006).
Today, silver is widely used in electronics, catalysis of various chemical reactions (Akbarzadeh and Yaghoubi 2014; Hua and Hongtao 2015; Cuenya 2010), and as structural material in a number of cases. Very high electrical and thermal conductivity of silver makes it an ideal component for electrical connections. However, the recent experiments have shown that stable subnanometer clusters of metals behave like semiconductors due to the wide band gap developing at the Fermi level, which leads to new properties, such as photoluminescence and magnetism (Attia et al. 2014). The band gap was found to increase when the cluster size is decreasing, with the value exceeding ~ 2–3 eV for the smallest clusters recorded so far (Attia et al. 2014). Mainly, when exposed to air, Ag forms a silver sulfide film on its surface (up to 60 Å through its thickness) that is more or less transparent for visible light (Rycenga et al. 2011). So, the interaction of silver nanoparticles and light is considered further in more details.
Silver has been an important material throughout the mankind history. Although in ancient cultures people admired primarily its property to reflect light, the contemporary use of silver in nanometer optical structures makes potential usage of Ag completely different. These new applications are beyond simple reflection of light. They are based on understanding the interaction of the metal and the light wave, which is studied by plasmonics. The most successful possible applications of plasmonics are creation of superlens, non-reflective clothes, and quantum computing devices. But plasmonic nanostructures also allow to achieve fast operation speed in such technologies as microprocessors and photovoltaic devices (Rycenga et al. 2011).
These applications become possible due to the strong interaction of the impinging light and free electrons in nanostructures (Fig. 1). Figure 1a shows that it is the case of the plasmon resonance that is typical of certain nanoclusters. Silver particles of a size smaller than the visible light wavelength can intensively absorb light due to the surface plasmonic resonance caused by the collective oscillation of the conduction electrons which is induced by the impinging light (Rycenga et al. 2011). The frequency and intensity of the plasmonic resonance depends on the distribution of the nanostructure polarized charge, which is determined by the nanoparticle shape. Thus, the monitoring of the metal nanoparticle structure allows to regulate the length of the light waves that the nanoparticle defuses and absorbs (Tamaru et al. 2002).
Schematic representation of two types of plasmonic nanostructures: a LPSR—plasmon resonance localized on the surface and b PSR—propagating surface plasmon resonance. E0 the light wave intensity, k the wave vector. According to Velázquez et al. (2012)
In plasmonics, it is very important to choose the metal that can support strong plasmonic effect with the required resonant wavelength. Of all the metals, silver is likely to have taken the most significant part in the plasmonic development; unique properties of Ag make it the most suitable material for major number of contemporary plasmonic technologies (Rycenga et al. 2011). Silver has many advantages over other metals used to support surface plasmonic effect (SP) in the optical and near-infrared region, such as Au, Cu, Li, and Al. An important aspect of metal use in plasmonic applications is its cost. Silver is relatively inexpensive, if compared with the metals that support plasmonic effect. In addition, it is very important that these metals are easy to transform into nanostructures of a controlled size and shape (Rycenga et al. 2011).
Over the last decade, chemical methods allowed to achieve a sustained synthesis of various nanostructures for only Au, Ag, and Pd. Taking into account that Pd is unsuitable for plasmonic applications (Rycenga et al. 2011), these are Au and Ag that are the most promising materials for plasmonics, but Au is almost 50 times more expensive than Ag. Moreover, if compared with the gold nanoparticles, silver nanoparticles of a diameter less than 60 nm defuse light with twice the efficiency and amplify the Raman scattering signals of the adsorbed molecules better by two orders of magnitude (Rycenga et al. 2011). It means that sufficiently small silver nanoclusters have a very strong optical absorption and emission property, which makes them nearly perfect fluorophor for molecular spectroscopy. Consequently, if compared with other metals, silver is quite unique due to its excellent plasmonic properties as well as due to the economic value.
Today, it is obvious that the size, shape, and structure of nanoparticles determine their optical properties, including the resonant frequency. So, work (Padmos et al. 2015) shows typical correlation of peak wavelength in the surface plasmon resonance (SPR) of silver nanoclusters and their size. For example, Ag nanoclusters showed SPR peaks at 380 nm (the cluster diameter D = 1.6 nm); when the cluster diameter D was 3.0 nm, SPR peak was already at 390 nm; at D = 4.8 nm, it was at 396 nm (Padmos et al. 2015). Moreover, the involvement of Ag nanoparticles into the dielectric matrix changes the light transmission based on SPR as well. Therefore, in order to achieve the required functional properties, it is necessary to precisely monitor the structure and morphology of the substrates and Ag nanoclusters (Manninen et al. 2014).
Bulk silver is of a highly symmetric FCC structure, but it is possible to grow various anisotropic forms when monitoring the assembly of the metal atoms in the solution (Becerril and Noguez 2015). Ag nanostructures synthesized with chemical methods tend to have one of four possible forms: an ideal nanoparticle, a nanoparticle with single twinning, a nanoparticle with multiple twinning (often with the symmetry axis of the fifth order), and interconnected nanoparticles with a packing defect when stacked (Wiley et al. 2007). The use of these different structures has a significant impact on the plasmonic properties, as the defects on the boundaries of twins can serve as defusing centers for the conduction electrons in the metal (Tamaru et al. 2002). But even in the case of one and the same type of the synthesized nanostructure, the peak and shape of the plasmon resonance can be highly variable (Rycenga et al. 2011).
To conclude, we cite the experimental data (Rycenga et al. 2011). The plasmon resonance can be observed in the range from 300 to 1200 nm; it depends on the type of the silver nanostructure. To add, the main peak of the resonance in the spherical nanoparticle is recorded in the range from 320 to 450 nm; in the cubic one, it is from 400 to 480 nm; LSP resonance in the decahedron is at 350–450 nm; in the octahedron, it is at 400–500 nm. Consequently, the use of silver nanoclusters with different internal structures gives the possibility to directly monitor the main characteristics of the plasmonic effect.
Computer model
Computer program MDNTP developed by Dr. Ralf Meyer from University Duisburg (Germany) was used for simulations with molecular dynamic (MD) processes of structure formation of silver nanoclusters. For molecular-dynamic consideration, Newton’s equations of motion are numerically solved for each atom in the force field of the remaining atoms. To calculate the forces acting between them, an interaction potential of a particular form should be used. The choice of a potential is determined by the character of the formulated problem and by the properties that should be studied. By our MD simulations, the interatomic interaction was calculated with the modified tight-binding potential TB-SMA with the cutoff radius relevant to the fifth coordination sphere inclusive (Cleri and Rosato 1993). These potentials have been widely used in different types of computer simulation of macroscopic materials and clusters and have been thoroughly verified to advantage for many parameters. So, the results of the simulation of a number of parameters of structural point defects (vacancies, interstices, and their small complexes), thermodynamic properties of metals (melting temperatures, transition temperatures, heat capacity, thermal expansion coefficient, Gruneisen parameter, etc.), and phonon spectra were compared with experimental data. The discrepancies in various thermodynamic properties were estimated on average 5–10% (Cleri and Rosato 1993).
In our simulation, the temperature of the system was found with the average kinetic energy of atoms, being calculated according to Velocity Verlet algorithm (Pang 2006) with a time step of h = 1 fs. The structural transitions of clusters were determined from the jumps in the potential energy as a function of the temperature, which resulted in an increase in the heat capacity in a very narrow temperature range around the transition point. Moreover, the radial distribution functions g(r), which allow one to analyze the structures of the simulated nanoparticles and to determine the character of structural transitions, were additionally calculated at different temperatures.
The simulated systems were gradually heated from 20 to 1000 K with the Nosé–Hoover thermostat (Pang 2006). All the clusters were thermally relaxed at the temperature T = 100 K to optimize the internal structure and shape. The upper limit of 1000 K is sufficient to melt the simulated particles, for the melting point for Ag clusters decreases significantly when the particle size decreases, if compared with the bulk material (Tm = 1235.1 K). An ensemble of nanoparticles of the same size was taken to determine the most stable cluster structure (Liu et al. 2011). In the process of the thermal energy supply, the temperature was being stepwise changed with a step of 20 K, in the structural transition zone with a step of 1 K; at each fixed value, the clusters were kept for 1.0 ns.
Results and discussion
If compared with the gold atoms, the silver atoms have higher reactivity, which consists in their relatively easy oxidation (Hua and Hongtao 2015); it results in the fact that it is necessary to use stabilizers to keep Ag nanoclusters chemically pure (Roese et al. 2016). In addition, for the purpose of maintaining properties that depend on the cluster size, the clusters are to be embedded in certain inert environment to keep them from aggregation (Roese et al. 2016). The rapid oxidation/sulfidation due to the ambient atmosphere dramatically reduces all the advantages of silver and causes difficulties from the view point of practical applications (Novikov et al. 2017; Copp et al. 2016). For example, sensors with Ag nanoparticles usually require the inert gases for their storage, while the operating time of such devices in the ambient atmosphere is rather limited. One of the contemporary solutions is to create a protective layer which can prevent the Ag structure destruction caused by the environment. However, these methods are not always applicable or rather complicated to use them in industries (Novikov et al. 2017).
The first type of stabilizers is a conventional inorganic substrate (silicon, carbon, etc.). However, due to the strong cohesive energy between metal atoms and between metal and inorganic content, the metal clusters on such surfaces should be passivated with organic molecules or be split up with the inactive matrix. In the case of silver, the molecules with thiol groups are frequently used for the passivation of the cluster surface. Another alternative is the total passivation of the entire surface (Alkis et al. 2009). Several types of Ag nanoparticles have been experimentally synthesized by using different protective ligands (Aikens 2011), such as protein, peptide, and dendrimer. However, the most successful experiments were thoseconducted for thiol groups (Becerril and Noguez 2015), because they showed high stability, small size of Ag clusters, and their narrow distribution according to the size (Padmos et al. 2015; Copp et al. 2016).
Although most of the main materials used to stabilize Ag nanoclusters are liquid, polymer, or organic materials, it has recently been discovered that some types of bulk oxyfluoride glass can also be applied for these purposes. The obvious advantages of the glassy state open up new perspectives for the application of Ag nanoparticles, such as possibilities of manufacturing fibers, films, and objects of any arbitrary shape (Velázquez et al. 2012).
Another possible approach to solving the problem of oxidation/sulfidation of silver nanoclusters could be the formation of very pure particles of a perfect crystal structure (Novikov et al. 2017), which should be more resistant to the abovementioned phenomena. A very promising approach to the preparation of such nanoparticles is aggregation of metal atoms into the clusters of very clean sources in vacuum. These clusters can be collimated into beams to be deposited or implanted on different substrates, which provides good opportunities to control the structure and properties of such nanomaterials.
According to the authors (Novikov et al. 2017), it is the single-crystal nature (FCC) of Ag nanoparticles that leads to the long-term stability of the localized surface plasmon resonances (LSPR). So, Ag nanoparticles synthesized in Novikov et al. (2017) showed excellent stability of the intensity of the band of plasmon resonance that decreased only by 20% after 30 days at room temperature in the ambient atmosphere. It is also worth noting that the lattice defects and defective phase boundaries in the particle lead to additional diffusion of conduction electrons, resulting in large optical losses that reduce the plasmon efficiency. Thus, the ideal crystal structure is an important factor of the continued life of the plasmon resonance.
We believe that another unaccounted possibility of increasing the plasmon efficiency in silver can be the usage of nanoparticles with a size equal to the structural “magic” numbers of various crystalline types. The structural magic numbers are called so since they arise when minimizing the volume with a maximum possible density of the nanoparticle with close to spherical shape (Demtröder 2000). This concept is confirmed by the fact that the degree of defectiveness of the surface of clusters with magic numbers of atoms is minimal, while for the others, it can be very significant (Liu et al. 2011). However, as studies showed, stable clusters with the number of atoms different from structural magic numbers are quite often formed during synthesis. Simulation showed that these numbers correspond to clusters with the most stable electronic configuration. Therefore, these numbers were called the electronic magic numbers (Yang et al. 1987).
In the aspect of using silver nanoclusters in plasmonic applications, it is very important to define limits of thermal and dimensional stability for the initial crystal structure of nanoparticles, as different internal structures of metal nanoparticles mean different physico-chemical properties. To do this, the paper considers possible changes of silver cluster configuration in the process of heating to the melting temperature and makes an attempt to set the dimension limits for clusters where the structural transition can occur. During the computer experiment, spherical FCC clusters of silver up to 2.0 nm were used as initial structure. The choice of clusters of this size was determined by the fact that at these sizes, the greatest role in the structural stability belongs to the magic numbers (Akbarzadeh and Yaghoubi 2014; Chiu et al. 2008; Liu et al. 2011). In the present paper, we will focus only on the study of the influence of structural magic numbers on the thermal stability of Ag nanoclusters.
Silver clusters with the magic numbers of FCC structure
The first modeling experiments to study the thermal stability of FCC phase were carried out for silver clusters of the size equal to the magic numbers of FCC structure (N = 79, 135, and 201 atoms). The situation turned out to be quite ambiguous. Considering the internal structure of Ag79 clusters, we found that the initial FCC structure was preserved up to the melting temperature being gradually destroyed on the surface; it is shown by the smooth rise of the potential energy of the particle during its heating and by “instant” snapshots of Ag79 nanocluster. Thus, the obtained data about dependence of the internal structure of 79 atom silver nanoclusters on the temperature showed that the spontaneous structural transition is not peculiar of Ag nanoparticles of this size; in this case, the initial FCC phase is thermally stable, which is confirmed, in particular, by work (Liu et al. 2011).
Further, increasing gradually the radius of the sphere cut out of the ideal face-centered lattice of Ag, we receive spherical FCC silver cluster of 135 atoms, with the diameter being D = 1.59 nm. Unlike Ag79, the particles of this size when heated showed a sharp decrease of the cluster potential energy accompanied with the crystal structure transition from FCC phase to the icosahedral modification. At the first stage, the temperature increase of Ag135 cluster from 100 to about 400 K causes only a slight change of the atoms position in the particle, with the preserved FCC structure. It means that atoms in the nanoparticle gradually change their location, which generally does not really influence the final cluster configuration. The real structural phases transition FCC → Ih took place at T > 400 K. The further increase in the nanoparticle temperature results in the gradual increase in the nanoparticle energy and deformation of the icosahedral structure. The complete destruction of the crystal lattice of the cluster is observed at Tm = 631 K.
In the process of MD simulation, such a spontaneous transition to Ih structure was detected in 100% of experiments with FCC silver particles of 135 atoms. However, the temperature of the structural transition FCC → Ih for different clusters of Ag135 of the same size was in the temperature range from 375 to 443 K, which is associated with a varying trajectory of the thermal evolution of different Ag135clusters. The obtained icosahedral phase was lasting till the melting temperature of the cluster.
We previously established a similar effect for nickel and copper clusters of 135 atoms (Gafner et al. 2009). Since the spontaneous formation of icosahedron is also observed with other metal clusters with 135 atoms in size, it is obvious that such number of atoms in the nanoparticle creates favorable energy conditions to develop Ih structure. Therefore, we can conclude that formed under the thermal effect, the structure of the silver nanoclusters with size N = 135 atoms is determined not only by magic FCC number but also by some other factors as well.
It was experimentally shown that the peak intensity of the mass spectrum for the clusters of alkaline and rare earth metals arises from the development of closed shells. For the clusters that have completely full energy levels, these are the electronic magic numbers that have the greatest influence on the formation of the internal structure. Therefore, for these metal clusters, the equilibrium state may be associated not only with the correct geometric position of atoms but also with the filled shell as well (Demtröder 2000).
In our opinion, the structure in Ag135 may be determined by both structural and electronic aspects. This is indicated by the fact that in Ag135 nanoparticle, the increasing temperature caused the reduction of the surface, which is controlled with the structural magic numbers. It is clear that the greatest minimization of the volume is determined by the Mackay icosahedron formation, its surface consisting of 20 equilateral triangles. The Mackay icosahedra of a larger size are formed by adding more Ih shells, which leads to the minimization of the cluster surface area, consequently, to the gain in the surface energy (Becerril and Noguez 2015).
The value of the energy barrier between FCC and icosahedral structures is usually not large; therefore, the influence of such supplementary factors as structural defects, temperature, and electronic magic numbers can be crucial. As the simulation used the silver clusters of a defect-free structure and the temperature was equally growing in all the clusters, the first two factors can largely be excluded to conclude that not only geometry of atoms but electronic configuration as well is responsible for the structural stability of Ag135 particles. To confirm this conclusion, we can cite work (Starace et al. 2008) that studied the structure of small aluminum clusters of 25–84 atoms, using the SIESTA computer code. Starace et al. (2008) also came to the conclusion that structural and electronic effects can simultaneously influence the cluster stability.
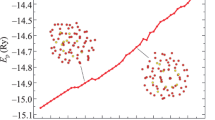
Further, the heating process for silver nanoclusters of 201 atoms (D = 1.9 nm) is considered to study the structural change. Typical calorithmical curve with the structural configurations gained is shown in Fig. 2. The figure shows that the potential energy of Ag201 is gradually increasing in the process of the nanoparticle heating. The nanocluster energy increase was accompanied with the destruction of the long-range order of the atomic arrangement in the particle, while FCC structure of the cluster was preserved up to the melting temperature Tm = 711 K. Thus, studying the internal structure of the silver nanoclusters of 201 atoms while heating, we determined that a spontaneous structural transition is not typical of this size of the nanoparticle.
Hence, according to the modeling data, even the silver cluster with “magical” structural number corresponding to the FCC structure does not guarantee complete stability of this structure. So the FCC structure of Ag135 cluster kept stable only up to T ≈ 370 K, followed by the structural transition FCC → Ih. However, Ag79 and Ag201 clusters did not show the similar transition to the icosahedral structure. In our opinion, the main reason for this was that magic icosahedral numbers (N = 13, 55, 147, 309...) being the nearest to the ideal FCC size were too far, if compared with the situation with Ag135 cluster. In the latter case, a very near electronic magic number N = 138 could also cause transition from one structure to another.
Silver clusters with the magic numbers of Dh structure
Further, we will examine the function of geometric magic decahedral numbers by the example of Ag75 and Ag101 clusters (Liu et al. 2011). Similar to the cases discussed previously, the initial structures were silver clusters cut of a perfect FCC lattice. All Ag75 and Ag101 clusters were thermally relaxed at the temperature T = 100 K to optimize the internal structure and shape.
The analysis starts with Ag75. The analysis of the data obtained during the simulation showed unexpectedly a low impact of decahedral magic number with this size of the silver cluster. The entire simulated ensemble of Ag75 clusters behaved identically at the thermal energy input. Particularly, the original FCC structure of the clusters remained unchanged throughout the heating process up to the melting temperature, being gradually destroyed on its surface. Only at temperature T = 560 K, there was a slight decrease in the potential energy associated with some optimization of the surface of Ag75 clusters.
Thus, the obtained data about dependence of the internal structure of silver nanoclusters of 75 atoms on the temperature showed that the structural transition is not peculiar of Ag nanoparticles of this size; the initial FCC phase is thermally stable. This result appeared to be rather unexpected, since the influence of the structural magic numbers is normally quite significant in a small cluster size. The reason for the gained result is explained below.
As already mentioned, silver in a bulk state has a face-centered cubic lattice, but the competition of bulk and surface energies in the nanometer range can lead to the formation of several different isomers. One of the reasons for the development of various structural modifications is very close values of energy calculated from different structures. For example, the MD simulation of the gold clusters that close to the silver clusters according to the structure formation processes was held in work (Garzón et al. 1998) with the usage of Gupta potential, with N = 38 atoms. It has given the following values of the free energy of the cluster in the ground state: amorphous (− 3.4405 eV/atom), FCC (− 3.44 eV/atom), and icosahedral (− 3.431 eV/atom). Moreover, such a weak difference in all the three isomers remained unchanged down to the room temperature. At T > 250 K the thermal energy is sufficient to overcome the potential barrier between different structures; as a result, all the three isomers start coming across in a random way. The main reason for this behavior can be considered the situation that at such a cluster size, almost all the atoms are on the surface and the slightest change in their position due to the thermal diffusion may be sufficient for another structural modification formed spontaneously.
In another work by the same authors (Garzón et al. 1999), the gold clusters of a larger size were similarly estimated with MD simulations by means of Gupta potential as well. Thus, the difference in binding energy of the amorphous and Ih structures at N = 55 made up 9.4 meV/atom, while at N = 75, the five-part Dh modification proved to be more stable than the amorphous one by only 5.7 meV/atom. To verify the obtained result, Garzón et al. (1999) carried out ab initio study of the relative stability of the gold clusters of crystalline and amorphous structure. It was defined that the difference in the energy of the atom of the most stable amorphous and crystalline modifications with the abovementioned dimensions is less than 0.01 eV/atom.
As shown during DFB modeling (Liu et al. 2011), Dh cluster of Ag75 is known to have the energy value close to the icosahedron energy at very low temperature, which is slightly lower than the energy value for the cluster with FCC structure. As it was shown above, this small difference in energy appears to be kept for Ih and Dh clusters of silver throughout the entire simulated temperature range, i.e., almost to the melting temperature of the cluster. The magic Ih size of Ag55 cluster causes a very rapid transition of FCC structure into an icosahedral one, while in the course of heating, the next size magic Ih of Ag147 cluster could form icosahedral as well as decahedral modifications with about the same proportion.
That is, under proper conditions, five-part symmetry is quite easily implemented in silver clusters of a small size. However, this transition was not recorded by us for Ag75 FCC cluster. As it was shown in Garzón et al. (1998, 1999), and Liu et al. (2011), the difference in energies between Dh structure and even the amorphous one can be very small. Accordingly, the energy gap between Dh structure and FCC structure will be even less. Therefore, even minor impact can suppress theoretically expected structural transition FCC → Dh. In our view, such an impact consisted in the influence of FCC geometric magic number observed at N = 79 atoms. Indeed, as it was mentioned above, Ag79 cluster kept its original FCC structure unchanged up to the melting temperature; the original FCC structure and the proximity to FCC the magic number that obviously stabilizes this type of the atomic arrangement appear not to allow Ag75 cluster to make transition FCC → Dh. The energy estimates for Ag75 (Dh) and Ag79 (FCC), obtained by DFB calculations (Liu et al. 2011), give almost identical energy values for both internal and surface atoms. Therefore, the structural stability of these Ag particles was determined (Liu et al. 2011) to be approximately the same, which is consistent with our result.
This assumption is confirmed by the following simulated structural magic Dh size. The data about Ag101 obtained during the simulation allow to conclude that at the stage of the preliminary thermal relaxation, there was a polytypic transition FCC → Ih, similar to Ag55 cluster. But Ag55 cluster acquired a perfect icosahedral structure, while the structures of Ag101 cluster at the relaxation stage were icosahedral, but obviously not perfect. The increase in the cluster temperature caused correction of the Ih structure; at the temperature of about T = 450 K, the Ih structure of Ag101 cluster was already quite correct, but the full completion of the third Ih shell lacks 46 atoms. It does not allow Ag101 cluster to develop minimum potential energy that guarantees the stability of the current atomic structure. That is why at T = 470 K, Ag101 cluster rearranged atoms to form a structure close to a decahedral one that remained till the melting temperature (Fig. 3).
In order to understand why the Ag101 cluster turned out to be subject to the processes of thermal restructuring of its structure, in contrast to the Ag75 cluster, it is necessary to consider their structure more carefully. When Ag75 and Ag101 clusters separately have the standard (2, 2, 2) and (2, 3, 2) Dh structures where the three numbers in the brackets are separately denotes the atoms on the width and height direction of the rectangular (100) facets and depth direction of reentrance (Liu et al. 2011). That is, the structure of Ag101 clusters is less stable due to distortion in one of the crystallographic directions, which leads to an increase in surface energy. In this case, what the initial structure was no longer important, the Ag101 cluster will always be less stable with respect to Ag75 (Liu et al. 2011), as our calculations showed.
Therefore, according to the results of the thermal stability analysis of FCC silver clusters, we can conclude that despite the fact that Ag101 clusters were in strict conformity with the structural magic numbers of Dh structures, at high temperature, icosahedral as well as decahedral modification (with equal proportion) could be formed in the course of heating, which again indicates the close values of energy of these five-part structures.
The DFB calculations performed in Liu et al. (2011) showed that at sizes 75 and 101 atoms, energy minima corresponding to the Dh structure are observed. Those from an energetic point of view, at given sizes, these structures will be the most stable. But if the Ag cluster at the initial stage had a different structure, in our case, FCC, then it is not always possible to transfer it to an energetically more favorable Dh structure by the heating method.
Thus, at low temperature, the influence of the geometric magic numbers can be crucial, while at higher temperature, this effect may be suppressed by various kinetic factors, in particular the random nature of diffusion and exceeding magnitude of thermal energy kBT over the energy gap separating different structural modifications.
Silver clusters with the magic numbers of HCP structure
Further, we consider configuration changes of Ag clusters of 89 and 153 atoms that correspond to HCP structural magic numbers as the last typical example of the magic number participation in the thermal stability of the silver cluster structure. The simulation data analysis shows that heating of Ag89 cluster (D = 1.48 nm) sharply decreases its potential energy; at already T = 73 K, the nanoparticle is trying to make transition from the initial FCC phase to the icosahedral modification (Fig. 4). However, the thermal atomic energy of Ag89 nanocluster is not enough to make the transition completely—the atoms in the particle form a mixed configuration of FCC and Ih structures. Further increase in the nanoparticle temperature leads to a gradual increase in the energy cluster and the destruction of its crystal lattice at the temperature Tm = 549 K.
Such a structural transition with a partial change in the cluster structure was observed in 80% of cases. Some of the simulated particles of Ag89 have only a seed of an icosahedral phase (as shown in Fig. 4); the icosahedron structure of other particles was more fully formed. In the remaining 20% of the experiments, the initial FCC structure of clusters transformed into a mixture of FCC and HCP phases. This transition was also accompanied with a sudden decrease of potential energy of clusters in the temperature range from 66 to 78 K.
Consequently, even if the cluster is fairly small, the geometric magic number of HCP structure cannot guarantee its functioning in the course of heating the cluster of a different internal structure. This conclusion is consistent with the data of Liu et al. (2011), where it was shown that even at very low temperature, Ag89 cluster is energetically much less stable, if compared with clusters with icosahedral or decahedral structure. It means that the transition to these structures during the thermal energy input can be energetically more favorable, which was demonstrated in our simulations. However, the large energy gap between the structures of Ih, Dh, and FCC and HCP types in Ag89 cluster in most cases does not allow to generate an ideal five-part structure during such a configurational transition.
The next geometric magic number at N = 153 atoms gives a perfect HCP structure of atoms in the cluster. Here, the situation is quite similar to the earlier case of Ag89. Since Ag153 cluster has a larger size, the initial FCC configuration is retained in the course of heating much longer, up to about 380–400 K. At this temperature, the process of nuclear restructuring begins in the HCP structure, but at the same time, the icosahedral structure is partially formed. As the result of sharp competition between them a clearly defined structure fails to be formed, which shows a mixed cluster structure up to the melting point.
The DFB analysis of the energy stability of small silver nanoclusters (Liu et al. 2011) showed that with an Ag89 and Ag153 cluster size, minima of energy corresponding to the Dh structure were observed, while Ag90 and Ag152 clusters already had a stable fcc structure. It is this fact that can explain the competition we observe between various crystalline modifications.
Silver clusters without the magic structural numbers
Further, the structure of silver nanoparticles with a size different from the structural magic numbers was studied to explore the abovementioned patterns. For this purpose, there was taken a cluster ensemble of Ag120 (D = 1.53 nm), Ag141 (D = 1.59 nm), and Ag177 (D = 1.8 nm). The size of Ag120 and Ag177 clusters was chosen so that it was possible to decrease the influence of different kinds of magic numbers, which allows to determine the “free” evolution of silver clusters under thermal effects. Therefore, the first example to consider is an ensemble of Ag120 clusters (Fig. 5).
The silver clusters of this size demonstrated the so-called classic behavior which consists in the fact that the result of thermally determined diffusion is that the surface area of small clusters starts to optimize. Thus, the initial crystal structure (FCC) turns out to be very unstable and quickly converts to a structure with lower value of the surface energy (Ih, Dh) during the thermal energy input. The increasing cluster size makes this transition less possible, resulting in the shift of the polytypic transition point to the melting temperature.
It is clearly shown in Fig. 5. So at temperature up to about 200 K, Ag120 cluster is of initial FCC structure. Further, diffusive rearrangement of atoms takes place to form nearly perfect icosahedral structure which is stable up to the melting point. Such behavior of Ag120 clusters is predictable and not very interesting. However, the structural evolution of Ag141 clusters is much more complex.
The analysis of the modification of the internal structure of Ag141 nanoclusters when heated highlighted three possible scenarios of their evolution. So, in about 10% of the experiments, the approximately linear increase in the particle potential energy was observed at the cluster temperature up to 400 K, with the FCC structure being preserved. At T = 401 K, there was a sharp decrease of the potential energy of Ag141 cluster to form a clearly defined icosahedral modification. This five-part structure was completely destroyed only when the cluster turned into a liquid state at Tm = 658 K.
In the second case, the heat of Ag141 nanocluster causes twice a sharp decrease of the particle potential energy, with its structure modified. Thus, the case described allows to conclude about a fairly rare, double structural transition in the cluster (Gafner et al. 2018). A similar situation was recorded in 50% of the conducted computer experiments. The comparison of emerging structures in this silver cluster of 141 atoms showed that the initial ideal FCC modification of the particle remained unchanged up to about 300 K. The increasing of the temperature to 350 K led to partial displacement of silver atoms in the cluster and formation of several HCP planes.
At T = 351 K, the potential energy decreases sharply, which indicates the formation of energetically more stable structure of the cluster. However, some atomic planes of the cluster are slightly twisted, which points to the possible seed of icosahedron (Ih) or decahedron (Dh) structure. The thermal energy in this temperature range is likely to be still not enough to form a complete five-part combination of atoms in the nanoparticle. Upon further heating of Ag141, there was only a small change observed in the cluster structure, but at temperature T = 491 K, there comes a spontaneous transition to the icosahedral phase, which continued with the further temperature increase.
The third type of the thermodynamic curve obtained at the heating of Ag141 nanoparticles was recorded in 40% of the experiments. At the initial stage, the thermal evolution is similar to scenario number 2. That is, the original FCC structure at large retains to the temperature of about 300 K. In the interval of 300–350 K, there was also some distortion of this structure with the inclusion of HCP. However, this structure appears to be unstable; at the same temperature, T = 351 K, the potential energy of the cluster dramatically decreases so that the cluster creates a more energetically favorable form as well. However, unlike the second type of the thermal evolution, the restructuring of the cluster gives a more stable structure with a decahedral seed. Thus, due to the deeper fall of the potential energy than it was with the second scenario, this structure being intermediate between FCC and icosahedron turns to be quite stable, shifting the second structural transition Dh → Ih almost to the melting point of the cluster.
In our view, the main reason for such a complex behavior of Ag141 cluster is the functionality of the structural magic number of the distorted octahedron that occurs at N = 140 atoms. Normally, this structure is not considered a major one in clusters, as it has higher energy, if compared with decahedron or icosahedron, but the distortion of octahedron along an atomic plane allows to reduce the energy gap between the structures. The example of Ag141 cluster clearly illustrates that it is the distortion of the primary FCC structure, but not its transition into icosahedron that allows to gain energy at the first stage of the cluster reconfiguration. Only further heat supply that weakens the atomic interaction makes the transition to icosahedron, the energetically most favorable structure, possible.
The next stage of the simulation was aimed at studying the structure formation of Ag clusters consisting of 177 atoms. Obtained in the computer simulation, curves of the thermal dependence of the potential energy prove that the structural transition with a sharp decrease in potential energy is not typical of the silver cluster of this size. When the temperature increases from 20 to 400 K, the simulated system keeps the original ideal FCC phase, while the further heating of the cluster slightly deforms the crystal lattice. The melting of the considered nanoparticle (Ag177) was at temperature Tm = 651 K.
This behavior of Ag177 nanoclusters upon heating was observed in 60% of the experiments; the other 40% of the experiments showed the potential energy fluctuations near the melting point, which still caused some reconfiguration of the cluster structure. Analyzing modifications of the internal structure of such Ag177 nanoclusters upon heating, we found a structural transition from FCC phase to the decahedral modification in the solid-liquid phase transition. The figure shows typical structures of Ag177 nanoclusters at different temperatures for 40% of the simulation cases.
The figure shows that heating of the simulated system to 600 K in the initial ideal FCC configuration of the cluster (Fig. 6a) develops an atomic plane with the local HCP structure (Fig. 6b). Such a combined structure (FCC + HCP) is preserved at the further increase of the cluster temperature (Fig. 6c, d). But there occurs a spontaneous transition to the decahedral phase at T = 638 K (Fig. 6e). The Dh structure is deformed closely to the phase transition (Fig. 6f); the structural modification in the silver nanocluster with N = 177 is completely destroyed at Tm = 651 K, when it transforms to the liquid state (Fig. 6g).
It is necessary to point out that the size of N = 177 atoms is far enough from various structural and electronic magic numbers. In our opinion, this very circumstance determines the type of the thermal evolution of Ag177 cluster. In most cases, its original FCC structure in one way or another remained unchanged almost up to the melting temperature, which is similar to the clusters of Ag79 or Ag201. But the FCC structure of these clusters was stable until its destruction when melted; the key factor was the structural stabilization due to the FCC structural magic number. If the cluster size does not match this number (N = 177 atoms), the structural stabilization will not be complete. The weaker atomic binding observed near the melting point provokes another type of the atomic structure with five-part symmetry, which is experimentally confirmed for silver nanoparticles of this size (Baletto and Ferrando 2005). It is also possible to conclude that Ag177 cluster size appears to be that very limit for five-part symmetry structures to show themselves. It becomes very difficult for them due to the significant growth of the elastic stress in the energy cluster suppressing possible gain in the surface energy.
Thus, the computer simulation with the MD method studied the heating processes for FCC silver clusters of up to 2.0 nm. The comparison of the obtained cluster structures showed that, in general, magic structural numbers of different types are sure to participate in their reconfiguration. Moreover, the configuration transition may be associated with the difference in the energy of the cluster elastic deformation and its surface energy which depend on the number of atoms composing the nanoparticle.
Conclusion
The computer simulation with the MD method studied the heating processes for FCC silver nanoclusters to 201 atoms. The analysis of the results showed that silver nanoparticles of more than 200 atoms do not undergo spontaneous reconfiguration of the cluster structure; it means that FCC structure of such clusters is thermally stable up to the melting point. The situation for silver nanoparticles of smaller size is much more complex, as there were numerous cases of thermally induced changes in the cluster structure, often under different scenarios. Thus, the use of small silver clusters (N < 200 atoms) in plasmonic applications requires a detailed study of the thermal stability of the cluster structure, apparently, with due consideration of various kinds of magic structural numbers.
References
Aikens CM (2011) Electronic structure of ligand-passivated gold and silver nanoclusters. J Phys Chem Lett 2:99–104
Akbarzadeh H, Yaghoubi H (2014) Molecular dynamics simulations of silver nanocluster supported on carbon nanotube. J Colloid Interface Sci 418:178–184
Alkis S, Krause JL, Fry JN, Cheng H-P (2009) Dynamics of Ag clusters on complex surfaces: molecular dynamics simulations. Phys Rev B 79:121402(R)
Attia YA, Buceta D, Blanco-Varela C, Mohamed MB, Barone G, López-Quintela MA (2014) Structure-directing and high-efficiency photocatalytic hydrogen production by Ag clusters. J Am Chem Soc 136:1182–1185
Baletto F, Ferrando R (2005) Structural properties of nanoclusters: energetic, thermodynamic, and kinetic effects. Rev Mod Phys 77:371
Baletto F, Ferrando R, Fortunelli A, Montalenti F, Mottet C (2002) Crossover among structural motifs in transition and noble-metal clusters. J Chem Phys 116:3856–3863
Becerril D, Noguez C (2015) Adsorption of a methylthio radical on silver nanoparticles: size dependence. J Phys Chem C 119:10824–10835
Chiu Y-P, Wei C-M, Chang C-S (2008) Density functional study of surface-supported planar magic Ag nanoclusters. Phys Rev B 78:115402
Cleri F, Rosato V (1993) Tight binding potentials for transition metals and alloys. Phys Rev B 48:22–33
Copp SM, Schultz D, Swasey SM, Faris A, Gwinn EG (2016) Cluster plasmonics: dielectric and shape effects on DNA-stabilized silver clusters. Nanoletters 16:3594–3599
Cuenya BR (2010) Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Films 518:3127–3150
Demtröder W (2000) Molekülphysik: Theoretische Grundlagen und experimentelle Methoden. Oldenburg: Heidelberg 349 p
Dhoubhadel MS, Rout B, Lakshantha WJ, Das SK, D’Souza F, Glass GA, McDaniel FD (2014) Investigation of structural and optical properties of Ag nanoclusters formed in Si(100) after multiple implantations of low energies Ag ions and post-thermal annealing at a temperature below the Ag-Si eutectic point. AIP Conf Proc 1607:16
Engemann DC, Roese S, Hövel H (2016) Preformed 2 nm Ag clusters deposited into ionic liquids: stabilization by cation-cluster interaction. J Phys Chem C 120:6239–6245
Gafner SL, Redel LV, Golovenko ZV, Gafner YY, Samsonov VM, Kharechkin SS (2009) Structural transitions in small nickel clusters. JETP Lett 89:364–369
Gafner Y, Gafner S, Redel L, Zamulin I (2018) Dual structural transition in small nanoparticles of Cu-Au alloy. J Nanopart Res 20:51
Garzón IL, Michaelian K, Beltrán MR, Posada-Amarillas A, Ordejón P, Artacho E, Sánchez-Portal D, Soler JM (1998) Lowest energy structures of gold nanoclusters. Phys Rev Lett 81:1600–1603
Garzón IL, Michaelian K, Beltan MR, Posada-Amarillas A, Ordejon P, Artacho E, Sanchez-Portal D, Soler JM (1999) Structure and thermal stability of gold nanoclusters: the Au38 case. Eur Phys J D 9:211–215
Guo C, Irudayaraj J (2011) Fluorescent Ag clusters via a protein-directed approach as a Hg(II) ion sensor. Anal Chem 83:2883–2889
Horta-Fraijo P, Cortez-Valadez M, Hurtado RB, Vargas-Ortiz R, Perez-Rodriguez AA, Flores-Acosta M (2018) Ultra-small Ag clusters in zeolite A4: antibacterial and thermochromic applications. Phys E 97:111–119
Hua D, Hongtao Y (2015) A mini review on controlling the size of Ag nanoclusters by changing the stabilizer to Ag ratio and by changing DNA sequence. Adv Nat Sci 8:1–9
Kuznetsov AS, Cuong NT, Tikhomirov VK, Jivanescu M, Stesmans A, Chibotaru LF, Velázquez JJ, Rodŕiguez VD, Kirilenko D, Van Tendeloo G, Moshchalkov VV (2012) Effect of heat-treatment on luminescence and structure of Ag nanoclusters doped oxyfluoride glasses and implication for fiber drawing. Opt Mater 34:616–621
Liu D, Wen Z, Jiang Q (2011) Surface energy and site dependent cohesive energy of Ag clusters. Curr Nanosci 7:463–470
Lu Y, Liu GL, Lee LP (2005) High-density silver nanoparticle film with temperature-controllable interparticle spacing for a tunable surface enhanced Raman scattering substrate. Nanoletters 5:5–9
Manninen NK, Figueiredo NM, Carvalho S, Cavaleiro A (2014) Production and characterization of Ag nanoclusters produced by plasma gas condensation. Plasma Process Polym 11:629–638
Mirguet C, Fredrickx P, Sciau P, Colombian P (2008) Origin of the self-organisation of Cu°/Ag°nanoparticles in ancient lustre pottery. A TEM study. Phase Transit 81:253–266
Novikov SM, Popok VN, Evlyukhin AB, Hanif M, Morgen P, Fiutowski J, Beermann J, Rubahn H-G, Bozhevolnyi SI (2017) Monocrystalline highly stable silver clusters for plasmonic applications. Langmuir 33:6062–6070
Numazawa S, Ranjan M, Heinig K-H, Facsko S, Smith R (2011) Ordered Ag nanocluster structures by vapor deposition on pre-patterned SiO2. J Phys Condens Matter 23:R. 222203
Padmos JD, Boudreau RTM, Weaver DF, Zhang P (2015) Structure of tiopronin-protected silver nanoclusters in a one-dimensional assembly. J Phys Chem C 119:24627–24635
Panáček A, Kvítek L, Prucek R, Kolář M, Večeřová R, Pizúrová N, Sharma VK, Nevěčná T, Zbořil R (2006) Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B 110:16248–16253
Pang T (2006) An introduction to computational physics. University Press, Cambridge 385 p
Roese S, Engemann D, Hoffmann S, Latussek K, Sternemann C, Hövel H (2016) PDMS embedded Ag clusters: coalescence and cluster-matrix interaction. J Phys Conf Ser 712:012068
Rycenga M, Cobley CM, Zeng J, Li W, Moran Ch H, Zhang Q, Qin D, Xia Y (2011) Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem Rev 111:3669–3712
Starace AK, Neal CM, Cao B, Jarrold MF, Aguado A, Lopez JM (2008) Correlation between the latent heats and cohesive energies of metal clusters. J Chem Phys 129:144702
Tamaru H, Kuwata H, Miyazaki HT, Miyano K (2002) Resonant light scattering from individual Ag nanoparticles and particle pairs. Appl Phys Lett 80:1826–1828
Velázquez JJ, Tikhomirov VK, Chibotaru LF, Cuong NT, Kuznetsov AS, Rodríguez VD, Nguyen MT, Moshchalkov VV (2012) Energy level diagram and kinetics of luminescence of Ag nanoclusters dispersed in a glass host. Opt Express 20:13582–13591
Wilcoxon JP, Abrams BL (2006) Synthesis, structure and properties of metal nanoclusters. Chem Soc Rev 35:1162–1194
Wiley BJ, Chen Y, McLellan JM, Xiong Y, Li Z-Y, Ginger D, Xia Y (2007) Synthesis and optical properties of silver nanobars and nanorice. Nanoletters 7:1032–1036
Yang SH, Pettiette CL, Conceicao J, Cheshnovsky O, Smalley RE (1987) Ups of buckminsterfullerene and other large clusters of carbon. Chem Phys Lett 139:233–274
Acknowledgments
The work was supported by grants from the Russian Foundation for Basic Research, project numbers 18-42-190001and 19-48-190002.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gafner, Y., Gafner, S. & Bashkova, D. On measuring the structure stability for small silver clusters to use them in plasmonics. J Nanopart Res 21, 243 (2019). https://doi.org/10.1007/s11051-019-4691-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-019-4691-2