Abstract—
We have synthesized silica-based sorbents with an MSM-48 pore structure, modified with hydrazide and amide groups based on Versatic tret-carboxylic acids, and investigated their textural and structural properties. The kinetics of Cu(II), Ni(II), and Co(II) sorption on the sorbents from aqueous solutions have been studied, and the effects of the silica modification process and modifier concentration on the sorption capacity of the silicas for the three metals present together have been examined. The synthesized sorbents have been shown to be capable of separating the nonferrous metals studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Mesoporous silicas are of obvious interest to materials researchers. The reason for this is that, owing to their considerable specific surface area (>1000 m2/g) and ordered mesopore structure, these materials offer significant advantages over other silicas in catalytic and sorption processes. Mesoporous silica materials can find application in wastewater and indoor air purification and as nanostructured “host–guest” compounds and sorbents capable of group extraction of metal ions and their isolation from solutions of complex composition.
In recent years, many mesoporous silicas (SBA-15, SBA-16, MCM-41, MCM-48, and others) with various (hexagonal, cubic, and other) pore geometries have been studied and it has been well documented that chemical modification of their surface helps to significantly improve their sorption characteristics [1–3].
The use of hydrazides and amides prepared using Versatic tert-carboxylic acids for surface modification of amorphous silica having good texture characteristics will allow for preconcentration of trace components from large volumes of solutions on a relatively small mass of a sorbent without using organic solvents.
The purpose of this work was to study the effect of the surface modification of MCM-48 mesoporous silica with hydrazide and amide functional groups on their texture, structural properties, particle shape and size, and sorption properties for nickel(II), cobalt(II), and copper(II) compounds.
EXPERIMENTAL
The silica basis of sorbents with an MCM-48 pore structure was prepared by template synthesis under hydrothermal conditions at a holding temperature of 120°C over a period of two days [4]. The silicon source used was tetraethyl orthosilicate (TEOS), and the structure-forming agent was cetyltrimethylammonium bromide (CTAB). In our syntheses, the starting reagents were used in the following ratio: TEOS : CTAB : NaOH : H2O = 1 : 0.44 : 0.4 : 100.
The surface of the mesoporous silicas was modified by two procedures: by impregnation (I) of silica having the MCM-48 structure (samples I-DMH and I-DBA) and during the hydrothermal synthesis (HS) of the silica basis (samples HS-DMH and HS-DBA), where DMH and DBA stand for N',N'-dimethylhydrazides and N',N'-dibutylamides, respectively, based on Versatic CH3R1R2CC(O)ОH tert-carboxylic acids. Here R1 and R2 are alkyl radicals, and the total number of carbon atoms is ten.
The I-DMH and I-DBA sorbents were prepared by refluxing a mixture of the silica basis (MCM-48) and a modifying agent—dimethylhydrazide or dibutylamide—in various molar ratios in a nonpolar solvent (hexane) for 4 h. The HS-DMH and HS-DBA sorbents were synthesized as follows: A template (CTAB) was dissolved in an aqueous NaOH solution with stirring on a magnetic stirrer, and then the silica source (TEOS) was added. After the formation of SiO2 sol, a modifying agent—dimethylhydrazide or dibutylamide—was added. The mixture was held under hydrothermal conditions at a temperature of 120°C for 48 h. The template was then removed by extraction with ethanol, following which the samples were dried at a temperature of 80°C.
The structure of the modified silica sorbents was studied by IR spectroscopy at room temperature on an IFS-66/S Fourier transform IR spectrometer (Bruker, Germany) in the range 150–4000 cm–1 (number of scans, 100; resolving power, 2 cm–1).
Textural characteristics of the sorbents (specific surface area, pore volume, pore diameter, and pore size distribution) were determined by low-temperature nitrogen sorption measurements (at –196°C) using an ASAP 2020 gas adsorption analyzer (Micromeritics, the United States), after vacuum degassing of the material at a temperature of 90°C for 3 h.
The sorption properties of the silicas were studied as follows: To 100 mL of a pH-adjusted solution containing only copper(II) ions or Cu(II), Ni(II), and Co(II) ions together was added 0.2 g of modified silica. After 20 min, the residual metal ion concentration in the aqueous phase was determined on a SOLAAR iCE 3500 flame atomization atomic absorption spectrometer (Thermo Fisher Scientific, the United States). Titers of standard copper(II), nickel(II), and cobalt(II) sulfate solutions were also determined on the SOLAAR iCE 3500 atomic absorption spectrometer. The solution pH was adjusted by hydrochloric acid or ammonia of appropriate concentration.
The static sorption capacity for metals (EM, mol/g), the degree of extraction (E, %), distribution coefficient (D, L/g), and separation factor β were evaluated with an accuracy within 5% using the following formulas:
where C0 (mol/L) is the initial metal concentration in solution, Ceq (mol/L) is the residual equilibrium concentration of the metal in solution, V (L) is the volume of the solution, and m (g) is the weight of the sorbent.
The thermodynamic characteristics of adsorption equilibria, namely, the limiting adsorption of the sorbent (Γ∞, mol/g) and the adsorption equilibrium constant (K) were evaluated by linearizing the Langmuir isotherm [5]:
where Γ (mol/g) is the amount of adsorption and C (mol/L) is the equilibrium concentration of the substance in solution.
The surface morphology of the sorbents was examined by scanning electron microscopy (SEM) on a FEI Quanta 650FEA. The surface of the samples was scanned at a magnification of 1500×.
RESULTS AND DISCUSSION
Structural characterization of the silica basis by X‑ray diffraction at small angles, 2θ = 1.4°–10°, showed that the X-ray diffraction pattern of the silica contained small-angle reflections—211, 220, 420, 332, and others—confirming a cubic pore structure (MCM-48) in the material. X-ray diffraction data indicated that the modification of the MCM-48 silica with hydrazide and amide groups led to disordering of the pore structure.
The present IR spectroscopy results lead us to assume that the silica basis modification process most likely involves interaction of the silica matrix with the modifying agents, resulting in the formation of, at least, hydrogen bonds and associates. This is evidenced by the observed decrease in the intensity of the band between 3900 and 2900 cm–1 and the emergence of bands at 3252, 1523, and 1469–1488 cm–1, corresponding to bending and stretching vibrations of the NH, CN, and C=N groups.
Examination of textural characteristics of the MCM-48 silica basis showed that the sorption isotherms of the sample had a shape characteristic of ordered mesoporous structures (type IV in the IUPAC classification): isotherms with a well-defined capillary condensation of nitrogen. The adsorption and desorption curves almost coincided, pointing to a high degree of structural order in the sorbent.
Investigation of the textural and structural properties of the sorbents by low-temperature nitrogen sorption measurements showed that the samples consisted of a mesoporous material with a rather large specific surface area (up to 1420 m2/g). Surface modification reduced the specific surface area of the sorbents and increased their particle size.
The SEM data in Fig. 1 demonstrates that the silica with an MCM-48 pore structure consists of spherical particles ranging in size from 100 to 500 nm (Fig. 1a). The particles of the silica sorbent modified with dimethylhydrazide (I-DMH) have the shape of polyhedra and a slightly larger particle size (Fig. 1b). The silica sample modified with dibutylamide (I‑DBA) contains agglomerates of particles differing in shape and size (Fig. 1c).
From thermogravimetry (TG) data, we evaluated the activation energy characterizing the bonding between the carrier and functional groups of the sorbent (Table 1). Increasing the grain size of the sorbent and, accordingly, reducing its specific surface area only slightly reduces the sorbability of metals and the rate of the sorption process. Raising the temperature activates the desorption process, in agreement with data in the literature [5].
Given that the modified samples contain high concentrations of functional groups (13–22 mmol/g) [6] capable of forming six-membered chelate cycles with complexing ions, it is reasonable to conclude that the materials under consideration are potentially attractive for use as efficient sorbents for the preconcentration, separation, and extraction of nonferrous ions from complex mixtures.
Since the synthesized samples are planned for use as sorbents for wastewater purification, we assessed their sorption capacity for nickel(II), cobalt(II), and copper(II) ions.
The amount of a metal extracted depends on the acidity of the medium and the time the sorbent is in contact with the solution, so first of all we examined the influence of these factors on the amount of adsorption.
The pore structure of a material can have a significant effect on adsorption kinetics. Adsorption on porous materials involves mass transport in pores, a process that typically follows a diffusion mechanism. This step often determines the time needed for adsorption equilibrium to be reached. The adsorption mechanism in adsorbents whose pores are comparable in size to molecules being adsorbed differs significantly from that on large-pore adsorbents. Adsorption in micro- or mesopores is not accompanied by the formation of adsorption layers but proceeds through the filling of the volume of adsorption spaces [7].
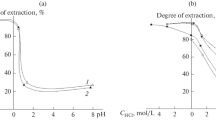
Figure 2 shows kinetic curves for cobalt ion extraction by the modified sorbents. In an alkaline medium, adsorption was almost instantaneous, which allowed us to assume a chemical nature of the adsorption process. The time-dependent adsorption data obtained at pH 1.1 and 1.5 yielded straight lines in plots of F against τ1/2, with correlation coefficients of 0.996 and 0.998, respectively, suggesting that the rate of the adsorption process was limited by internal diffusion [8]. As the solution pH was raised from 1.5 to 5.5, the rate constant increased from 0.098 to 0.104 mmol/(g min1/2).
According to the present results, copper can be extracted by the MCM-48 sorbent only in an alkaline medium. The highest degree of limiting adsorption is reached in a few minutes. Cobalt and nickel adsorption reaches its limits in acid and neutral media in 60 min and in an alkaline medium in 20 min.
The use of atomic absorption spectroscopy for monitoring metal ion concentrations in equilibrium solutions during the sorption process allowed us to study simultaneous sorption of transition metal ions and take into account their combined effect on the sorption process.
Since the synthesized sorbents are planned for commercial use as well, we evaluated the metal sorption capacity of the I-DMH silica sorbents with SiO2 : DMH ratios of 0.05, 0.1, 0.2, and 0.4. The results are presented in Table 2. It is seen that the highest sorption capacity for the metals is offered by the sorbents with DMH : SiO2 ratios from 0.1 to 0.2. A comparative estimation of the sorption capacity of the I-DMH sorbents (Table 2) with unmodified SiO2 indicates that the surface modification of MCM-48 with the N',N'-dimethylhydrazide of Versatic acids enables cobalt separation from nickel and copper in the pH range 0.8–4 when these metals are present together.
The procedure used to modify the surface of mesoporous silicas also has a significant effect on the degree of metal extraction. Using the I-DMH, I-DBA, HS-DMH, and HS-DBA samples and unmodified silica (MCM-48) as examples, we examined the effect of solution pH on the sorption capacity of the different sorbents (Tables 3, 4; Fig. 3) for copper(II), nickel(II), and cobalt(II) present together. The I-DMH sorbents were found to have the highest sorption capacity.
The data thus obtained suggest that the three nonferrous metals can be extracted from solutions with high efficiency. The three materials—MCM-48, I-DMH, and I-DBA—extract nickel(II), cobalt(II), and copper(II) ions from solutions in going from weakly acidic to strongly alkaline solutions. Qualitatively, the solution pH has similar effects on the adsorption of metal ions on HS-DMH and HS-DBA. Maximum extraction is observed from neutral and weakly alkaline solutions.
Impregnation increases the sorption capacity of the modified material to a greater extent than does hydrothermal synthesis. It follows from the data in Fig. 3 that, when the three metals are present together, the highest degree of extraction is observed for cobalt, which can be isolated from a solution containing all three ions. Besides, the pH range where it caextracted (1.5–6) is broader than that in the case of the sorbents modified during hydrothermal synthesis.
With the aim of utilizing the sorbents in high-temperature sorption, we examined the effect of temperature on the sorption of metals on the synthesized modified silicas. The effect of pH on the temperature dependence of the degree of extraction was studied by bringing a solution under study into contact with the sorbent for 20 min, as prompted by previously obtained data on the kinetics of the sorption process.
In the general case, the sorption capacity decreases with increasing temperature at low and intermediate pH values, which points to physisorption. Figure 4 plots ln EM against 1/T for MCM-48 and I-DMH. It is seen that, at high pH values, adsorption on the unmodified sorbent is due to physical interaction. The situation changes when the formation of nonferrous ion complexes with DMH groups is possible. The slope of the plot of ln EM against 1/T (Fig. 4) changes; that is, the amount of adsorption increases with increasing temperature. This is possible if a chemical reaction occurs on the surface [5].
CONCLUSIONS
We have studied the effect of the method used to modify the surface of mesoporous silica with hydrazide and amide groups on its structure and adsorption properties. The results demonstrate that the presence of a modifier reduces the specific surface area of the sorbents. Impregnation has been shown to cause a larger increase in their sorption capacity in comparison with the hydrothermal synthesis of silicas modified with hydrazide groups. At pH 1.5 and 5.5, the rate of the adsorption process is limited by the internal diffusion step. At pH 8, copper and cobalt ions chemisorb on the surface of modified MCM-48.
The present detailed study has made it possible to optimize conditions for the interaction of metal (copper(II), nickel(II), and cobalt(II)) ions with the functional groups of the synthesized chelate-forming sorbents. The present results on the adsorption of nonferrous metal cations demonstrate that both coextraction and separation of such cations are possible, depending on solution pH.
The MCM-48, I-DMH, and I-DBA samples sorb nickel(II), cobalt(II), and copper(II) ions from solutions in going from weakly acidic to strongly alkaline solutions.
The highest degree of extraction (80–90%) of the three nonferrous metals is reached in a neutral and an alkaline medium by the sorbent modified with amide groups via hydrothermal synthesis. The highest degree of extraction has been obtained for cobalt (EM = 4–5.3 mmol/g). In the pH range 1.5–6.0, cobalt can be isolated from solutions containing the three ions.
REFERENCES
Balakain, V.M., Dranitsina, N.V., Kholmanskaya, Yu.B., et al., New nitrogen- and phosphorus-containing ampholytes based on a polyacrylate matrix and copper, zinc, and iron sorption on them from sulfuric acid solutions, Zh. Prikl. Khim. (S.-Peterburg), 1981, vol. 54, no. 4, pp. 781–785.
Nikolaev, A.V., Fokin, A.V., Kolomiets, A.F., et al., Sorption of copper and nonferrous metals on sulfur-, nitrogen-, and sulfur/nitrogen-containing sorbents, Izv. Sib. Otd. Akad. Nauk SSSR,Ser. Khim. Nauk, 1977, vol. 4, no. 9, pp. 34–40.
Oskotskaya, E.P., Basargin, H.H., Ignatov, D.E., et al., Copper, cobalt, and nickel group preconcentration with a polymer chelate sorbent, Zav. Lab. Diagn. Mater., 1999, vol. 65, no. 3, pp. 10–14.
Kondrashova, N., Saenko, E., Lebedeva, I., Valtsifer, V., and Strelnikov, V., Effect of organic-silane additives on textural–structural properties of mesoporous silicate materials, Microporous Mesoporous Mater., 2012, vol. 153, pp. 275–281. https://doi.org/10.1016/j.micromeso.2011.12.017
Frolov, Yu.G., Kurs kolloidnoi khimii: poverkhnostnye yavleniya i dispersnye sistemy (A Course in Colloid Chemistry: Surface Phenomena and Disperse Systems), Moscow: Khimiya, 1988.
Batueva, T.D., Kondrashova, N.B., Kuz’micheva, N.D., et al., Physicochemical properties of mesoporous silicas modified with hydrazide and amide functional groups, Russ. J. Appl. Chem., 2017, vol. 90, no. 11, pp. 1746–1752. https://doi.org/10.1134/S1070427217110039
Tager, A.A., Fizikokhimiya polimerov (Physical Chemistry of Polymers), Moscow: Nauchnyi Mir, 2007.
Alosmanov, R.M., Kinetics of sorption of lead and zinc ions on a phosphorus-containing cation, Vestn. Mosk. Gos. Univ., Ser. 2:Khim., 2011, vol. 52, no. 2, pp. 145–148.
ACKNOWLEDGMENTS
In this study, we used equipment at the Materials and Matter Characterization Shared Research Facilities Center, Perm Federal Research Center, Ural Branch, Russian Academy of Sciences.
Funding
This work was supported by the Russian Federation Ministry of Science and Higher Education (state research target no. AAAA-A18-118032790022-7).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Batueva, T.D., Kondrashova, N.B. & Shcherban’, M.G. Modified MCM-48 Mesoporous Materials and Their Sorption Capacity for Nonferrous Ions. Inorg Mater 56, 360–365 (2020). https://doi.org/10.1134/S0020168520040020
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168520040020