Abstract—
We have synthesized hybrid membranes based on N-phosphorylated polybenzimidazole, containing different percentages of silica (2–20 wt %). The materials have been characterized by scanning electron microscopy, transmission electron microscopy, thermogravimetric analysis, IR spectroscopy, and impedance spectroscopy. The membranes have been shown to contain silica nanoparticles with a bimodal size distribution: 3–5 and 20–60 nm. The hybrid membranes have high proton conductivity (9.7 mS/cm at 130°C), which has a maximum when the dopant content is 2–10 wt %. The phosphonic groups grafted onto the polymer ensure additional hydration of the membranes at increased humidity. The addition of silica helps to reduce the gas permeability of the membranes by a factor of ~1.5.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Fuel cells (FCs) are thought to be promising power supplies for use in stationary and mobile devices owing to their high theoretical power and chemical-to-electrical energy conversion efficiency. In particular, polymer electrolyte membrane fuel cells (PEMFCs) are of both fundamental and practical interest as one of the most convenient types of electrochemical device for electric power generation [1]. Such an FC contains a proton-conducting membrane sandwiched between two porous electrodes. Such membranes should meet a number of requirements, including high proton conductivity in combination with negligible electronic conductivity, low fuel permeability, high mechanical and thermal stability, and the ability to operate at elevated temperatures in order to raise the tolerance of catalysts to CO impurities [2, 3].
Researchers have examined many polymer systems for application in FCs and searched for membranes possessing high transport characteristics. The most often used commercially available membranes based on perfluorinated polyelectrolytes (Nafion, Flemion, and Dow membranes) are expensive, their proton-conducting properties depend on humidity, and their maximum service temperature is no higher than 100°C [4]. The last two problems arise from the fact that proton transport in such membranes follows the Grotthuss mechanism in pores and channels containing a considerable amount of water [2].
A viable alternative is systems in which proton transport takes place not in water but in acids. In particular, phosphoric acid-containing materials are capable of conducting even at a very low humidity, through the system of hydrogen bonds formed by acid molecules [2]. Phosphoric acid-doped polybenzimidazoles (PBIs) are successfully used in PEMFCs. Owing to the basic nature of the polymer, high phosphoric acid doping levels can be reached, which ensures a rather high electrical conductivity at service temperatures of up to 190°C [5, 6]. The main drawback to such materials is that the water forming during FC operation can leach the acid out of the membrane.
This problem can be resolved by modifying the PBI polymer with inorganic particles capable of stabilizing the acid in the polymer matrix [7–10] and by grafting polymer chains with acid groups [11, 12]. In the latter case, the polymer is most frequently functionalized with sulfo groups (–SO3H), which have high acidity. However, as mentioned above the electrical conductivity of sulfonic acid polymers is highly dependent on relative humidity [12]. Grafting ethylphosphonic groups on a polymer matrix can make the material conductive at a low relative humidity and less humidity-dependent. In the literature, this issue has been addressed in a comparatively small number of reports. Sukumar et al. [1] obtained phosphorylated PBI-based membranes possessing high proton conductivity, which are regarded as potentially attractive polyelectrolytes for FCs.
The polymer matrix used in this work is N-phosphonoethylated cardo polybenzimidazole, a polymer based on 3,3',4,4'-tetraaminodiphenyl oxide and 3,3'-bis(p-carboxyphenyl)phthalide (PhEPBI) (Fig. 1). Its conductive properties were the subject of a previous study, where it was compared to unphosphorylated PBI [13]. In this paper, we report the preparation and properties of PhEPBI-based hybrid membranes containing hydrous silica particles.
EXPERIMENTAL
Preparation of PhEPBI composite membranes. PhEPBI was provided by Dr. I.I. Ponomarev (Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences). The synthesis procedure was described in detail elsewhere [14]. The degree of phosphorylation was 92%. The phosphonic groups of the PhEPBI were in ethyl form.
PhEPBI-based composite membranes were produced by casting a polymer solution (4 g/100 mL) in N-methylpyrrolidone (N-MP, Aldrich) onto a glass substrate. The polymer solution contained an appropriate amount of a precursor (tetramethoxysilane (TMOS), Aldrich) for silica synthesis and was homogenized by sonication for 5 min. After casting, the solutions were dried at 50°C for 48 h and then in vacuum at 160°C for 3 h in order to remove the residual solvent. The amount of the precursor was calculated so as to obtain a final content of the oxide from 2 to 20 wt %. In all cases, we obtained visually homogeneous, robust films 60–70 μm in thickness. Hereafter, the membranes will be denoted as PhEPBI/SiO2-ω, where ω is the weight fraction of the oxide in the membrane.
The methoxy groups of TMOS and phosphonic groups of PhEPBI were hydrolyzed by holding the membranes in 18% HCl for five days, followed by washing in deionized water until a qualitative test for chloride ions was negative. After that, the membranes were dried at 120°C. For doping with phosphoric acid, the membranes were held in a 50% H3PO4 solution (KhimMed, extrapure grade) for seven days. As a result, the weight of the membranes increased by a factor of 2.1–2.5 and their elasticity was observed to increase, while their strength remained sufficient for subsequent measurements.
Characterization techniques. The phosphoric acid doping level in the membranes, x (the number of H3PO4 molecules per repeating unit of the polymer), was determined from the weight of the absorbed acid as described by Özdemir et al. [15].
Water absorption was measured by holding the undoped membranes in water at 25 and 80°C for 24 h. The samples were weighed before and after the experiment and the amount of the absorbed water was determined as

where m0 and m are the sample weights before and after the experiment.
The morphology of the membranes was examined by scanning electron microscopy (SEM) on a Carl Zeiss NVision 40 scanning electron microscope equipped with an Oxford Instruments X-Max X-ray detector (accelerating voltage, 0.5 or 1 kV). The microstructure of the samples was examined on a JEOL JEM 2100 transmission electron microscope.
Phase compositions were determined by X-ray diffraction on a Rigaku D/MAX 2200 diffractometer (CuKα radiation).
The samples were characterized by thermogravimetric analysis (TGA) using a Netzsch TG 209 F1 thermobalance. Samples weighing 15–20 mg were placed in platinum crucibles and heated in argon at a rate of 10°C/min in the temperature range from 25 to 130°C. To determine water content, the samples were additionally thermostated at 130°C for 2 h. The oxide content of the membranes was determined by prolonged annealing at 800°C, until all of the polymer burnt out, followed by weighing of the annealing product.
Fourier transform IR spectra were measured in the wavelength range 520–4000 cm–1 on a Nicolet iS5 IR spectrometer equipped with a Specac Quest attenuated total reflection (ATR) accessory.
Ionic conductivity was measured with a Z500-PRO impedance meter (Elins, Russia) at frequencies from 10 to 2 × 106 Hz in potentiostatic mode using a sinusoidal voltage of 100 mV peak and graphite electrodes. The conductivity was evaluated by extrapolating semicircles representing bulk conductivity to the real axis.
In addition, the ionic conductivity was measured as a function of the relative humidity (RH) of air at a constant temperature of 90°C in a Binder MKF 115 dynamic climate chamber. Prior to measurements, the membranes were held for 1 h at each RH value. The ionic conductivity was measured as a function of temperature in a tube furnace under an air atmosphere in the temperature range 20–130°C at 10 to 15°C intervals. In all of the membranes, the contribution of dc electronic conductivity to total conductivity was within 0.01%.
The gas permeability of the membranes was evaluated using gas chromatography as described elsewhere [13].
RESULTS AND DISCUSSION
Morphology and composition of the hybrid membranes. The micrograph of a section of a membrane in Fig. 2a demonstrates that the polymer contains isolated pores. Their formation is probably due to the presence of inorganic particles and interaction of the polymer matrix with the silica particles, intensified by the presence of phosphonic groups grafted onto its surface. A similar picture was observed in hybrid PBI membranes containing mesoporous silica [16]. According to electron probe X-ray microanalysis data, the dopant is uniformly distributed across the membranes (Fig. 2b). Transmission electron microscopy (TEM) data confirm the formation of silica particles in the polymer matrix. The micrographs show particles 3–5 nm in size (Fig. 2c), evenly distributed over the membrane. There are also larger particles, 20–60 nm in size, more rarely encountered, which were possibly formed in the surface layer of the membranes [17]. Energy dispersive X-ray microanalysis confirms the presence of silicon in the composition of the PhEPBI/SiO2 hybrid membranes.
The dopant content estimated via annealing of the hybrid membranes exceeded the calculated one (Table 1). X-ray diffraction characterization of the annealing product showed that it consisted of amorphous silica and crystalline SiP2O5 (PDF-2 database, card no. 390189). The latter resulted from reaction between the dopant and the phosphonic groups of the membrane. Its formation led to a considerable weight gain of the residue.
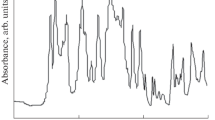
IR spectroscopy. The IR spectrum of PhEPBI (Fig. 3) contains bands around 3500 cm–1 attributable to stretching vibrations of N–H groups, slightly involved in the formation of hydrogen bonds. The broader band peaking at 3300 cm–1 is due to stretching vibrations of –OH and –NH groups, involved in the formation of stronger H bonds [18]. The stretching bands of the C=N groups of the imidazole ring are located around 1620 cm–1, those of the benzene ring are located near 1450 and 1600 cm–1, and those of the phosphonic groups lie in the range 1150–1280 cm–1 [19, 20]. The participation of silica in the formation of H bonds leads to broadening and an increase in the intensity of the –OH and –NH stretching bands in the spectrum of the hybrid membrane (Fig. 3, spectrum 2). In the other ranges, the difference between the spectra of the hybrid membrane and the unmodified PhEPBI polymer (Fig. 3, spectrum 3) is the spectrum of silica: the strong absorption bands between 1000 and 1080 cm–1 arise from Si–O–Si bonds and the band at 780 cm–1 is due to Si–O vibrations.
Water content and phosphoric acid doping level. The addition of silica leads to a slight decrease in the water content of the samples and the phosphoric acid doping level of the hybrid membranes (x, the number of H3PO4 molecules per PBI unit) (Table 2), which is probably associated with the decrease in free volume in the polymer on the addition of SiO2. At the same time, having good sorption properties, the surface of silica can participate in water and phosphoric acid sorption. Because of this, these parameters change little. Moreover, the interaction of the silica surface with the NH and phosphonic groups of the polymer leads to an additional “cross-linking” of the polymer due to the formation of hydrogen bonds, which can show up as the narrowing of pore spaces and influence acid absorption and the water content of the membranes.
Water absorption in the phosphoric acid-free membranes decreases with increasing oxide content and heat-treatment temperature: the weight fraction of the water absorbed by the membrane at 70°C is 32.6% in the case of PhEPBI and decreases to 29.0 and 23.0% on the addition of 2 and 10% SiO2, respectively.
Proton conductivity of the hybrid membranes. With increasing relative humidity, the conductivity of the membranes rises (Fig. 4). This is due to the increase in proton mobility in the “intrapore” solution on account of the decrease in its concentration on dilution with sorbed water and to the increase in pore and channel size in the membrane [21]. At temperatures below 100°C, we observe a tendency for the proton conductivity of the PhEPBI/SiO2 hybrid membranes to decrease with increasing dopant concentration, which is due to the decrease in water content.
At the same time, at temperatures above 100°C the addition of silica leads to an increase in electrical conductivity relative to the undoped PhEPBI membrane throughout the composition range studied (Fig. 5a). The maximum increase in conductivity is observed at 2–10% silica. Above 80°C, the conductive properties of the undoped PhEPBI membrane begin to degrade (Fig. 5b) as a result of dehydration. At the same time, this effect in the hybrid membranes is much weaker. It seems likely that this is due to the increase in the size of the phosphoric acid-containing pores and channels as a result of doping. Moreover, the oxide surface can also be involved in transport processes.
The ability of the membranes to retain phosphoric acid was assessed by holding them in flowing air with increased humidity (2 × 104 Pa) at 150°C. Such holding simulates conditions similar to those under which FCs operate. After that, the membrane was withdrawn from the reactor and patted dry while hot to remove the liquid phase from the surface. The exposure of the membranes to a humidified atmosphere increased their electrical conductivity (Fig. 6), which was due to the absorption of an additional amount of water, accompanied by an increase in the elasticity of the polymer. These results differ fundamentally from those obtained for PBI without phosphonic groups. Treatment of the H3PO4-doped PBI membrane led to a decrease in its electrical conductivity by two orders of magnitude, whereas the electrical conductivity of the PBI composite membrane containing silica and zirconia particles remained constant to within the measurement error [7]. Above 100°C, the as-prepared PhEPBI/SiO2 membrane and the PhEPBI/SiO2 membrane treated in a humidified atmosphere are similar in electrical conductivity, which is due to dehydration processes in the samples tested.
Gas permeability. It is worth noting that the incorporation of silica into the polymer leads to a decrease in its hydrogen permeability. In this process, the permeability decreases with increasing oxide content (Table 3). In ion exchange membranes, the reason for this is that dopant particles force out the electrically neutral solution, which determines the co-ion and gas permeability, from the central part of the pores [2]. This situation is rather favorable for the use of membranes in FCs, because this reduces fuel crossover and power loss.
CONCLUSIONS
We have prepared and characterized hybrid membranes based on N-phosphorylated polybenzimidazole and silica. The high proton conductivity of such membranes makes them potentially attractive for use as polymer electrolytes in FCs at temperatures below 140°C without additional humidification. Moreover, the addition of silica helps to reduce the hydrogen permeability of the membranes.
REFERENCES
Sukumar, P.R., Wu, W., Markova, D., Unsal, O., Klapper, M., and Mullen, K., Functionalized poly(benzimidazole)s as membrane materials for fuel cells, Chem. Phys., 2007, vol. 208, pp. 2258–2267.
Stenina, I.A. and Yaroslavtsev, A.B., Low- and intermediate-temperature proton-conducting electrolytes, Inorg. Mater., 2017, vol. 53, no. 3, pp. 253–262.
Hickner, M., Ghassemi, H., Kim, Y.S., Einsla, B.R., and McGrath, J.E., Alternative polymer systems for proton exchange membranes (PEMs), Chem. Rev., 2004, vol. 104, pp. 4587–4612.
Moore, R.B. and Mauritz, K.A., State of understanding of Nafion, Chem. Rev., 2004, vol. 104, pp. 4535–4586.
Melchior, J.-P., Majer, G., and Kreuer, K.-D., Why do proton conducting polybenzimidazole phosphoric acid membranes perform well in high-temperature PEM fuel cells?, Phys. Chem. Chem. Phys., 2017, vol. 19, pp. 601–612.
Wainright, J.S., Wang, J.T., Weng, D., Savinell, R.F., and Litt, M.H., Acid-doped polybenzimidazoles: a new polymer electrolyte, J. Electrochem. Soc., 1995, vol. 142, pp. L121–L123.
Lysova, A.A., Ponomarev, I.I., and Yaroslavtsev, A.B., Composite materials based on polybenzimidazole and inorganic oxides, Solid State Ionics, 2011, vol. 188, pp. 132–134.
Lysova, A.A., Ponomarev, I.I., and Yaroslavtsev, A.B., Composites based on cardo polybenzimidazole and hydrated silicon dioxide for phosphoric acid fuel cells, Russ. J. Inorg. Chem., 2012, vol. 57, no. 1, pp. 1–5.
Lysova, A.A., Ponomarev, I.I., and Yaroslavtsev, A.B., Hybrid membrane materials based on poly(benzimidazole) and hydrous zirconia, Membr. Membr. Tekhnol., 2012, vol. 2, no. 2, pp. 85–91.
Quartarone, E., Angioni, S., and Mustarelli, P., Polymer and composite membranes for proton-conducting, high-temperature fuel cells: a critical review, Materials, 2017, vol. 10, paper 687. https://doi.org/10.3390/ma10070687
Hou, H., Di Vona, M.L., and Knauth, P., Building bridges: crosslinking of sulfonated aromatic polymers—a review, J. Membr. Sci., 2012, vols. 423–424, pp. 113–127.
Bock, T., Mohwald, H., and Mulhaupt, R., Arylphosphonic acid-functionalized polyelectrolytes as fuel cell membrane material, Macromol. Chem. Phys., 2007, vol. 208, pp. 1324–1340.
Lysova, A.A., Ponomarev, Iv.I., Volkova, Yu.A., Ponomarev, I.I., and Yaroslavtsev, A.B., Effect of phosphorylation on the conductive properties of polybenzimidazole, Membr. Membr. Tekhnol., 2018, vol. 8, no. 5, pp. 353–359.
Ponomarev, Iv.I., Ponomarev, I.I., Petrovskii, P.V., Volkova, Yu.A., Razorenov, D.Yu., Goryunova, I.B., Starikova, Z.A., Fomenkov, A.I., and Khokhlov, A.R., Synthesis of N-phosphonoethylated cardo poly(benzimidazole) and testing of proton-conducting membranes made of it, Dokl. Chem., 2010, vol. 432, no. 2, pp. 168–174.
Özdemir, Y., Üregen, N., and Devrim, Y., Polybenzimidazole based nanocomposite membranes with enhanced proton conductivity for high temperature PEM fuel cells, Int. J. Hydrogen Energy, 2017, vol. 42, pp. 2648–2657.
Zhang, J., Aili, D., Bradley, J., Kuang, H., Pan, C., De Marco, R., Li, Q., and Jiang, S.P., In situ formed phosphoric acid/phosphosilicate nanoclusters in the exceptional enhancement of durability of polybenzimidazole membrane fuel cells at elevated high temperatures, J. Electrochem. Soc., 2017, vol. 164, pp. F1615–F1625.
Novikova, S.A., Yaroslavtsev, A.B., and Yurkov, G.Yu., Synthesis and transport properties of membrane materials with incorporated metal nanoparticles, Mendeleev Commun., 2010, vol. 20, no. 2, pp. 89–91.
Karelin, A.I., Pisareva, A.V., Pisarev, R.V., and Dobrovolsky, Yu.A., IR study of a polymer proton-conducting electrolyte based on Poly(vinyl alcohol) and phenol-2,4-disulfonic acid, Polym. Sci., Ser. B, 2018, vol. 60, no. 1, pp. 69–83.
Jiang, F., Pua, H., Meyer, W.H., Guana, Y., and Wan, D., A new anhydrous proton conductor based on polybenzimidazole and tridecyl phosphate, Electrochim. Acta, 2008, vol. 53, pp. 4495–4499.
Bouchet, R. and Siebert, E., Proton conduction in acid doped polybenzimidazole, Solid State Ionics, 1999, vol. 118, pp. 287–299.
Yaroslavtsev, A.B., Karavanova, Yu.A., and Safronova, E.Yu., Ionic conductivity of hybrid membranes, Petroleum Chem., 2011, vol. 51, no. 7, pp. 473–479.
ACKNOWLEDGMENTS
This work was supported by the Russian Science Foundation, project no. 17-73-10447.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lysova, A.A., Yaroslavtsev, A.B. New Proton-Conducting Membranes Based on Phosphorylated Polybenzimidazole and Silica. Inorg Mater 55, 470–476 (2019). https://doi.org/10.1134/S0020168519050121
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168519050121