Abstract
The study is a continuation of the research cycle concerning the oxidation of propylene in dielectric-barrier discharge plasma to propylene oxide and the corresponding hydroxyl and carbonyl compounds. Comparison of experimental data on the propylene conversion in air and carbon dioxide made it possible to reveal the features of the process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Previously, we have shown that the oxidation of propylene with oxygen or air in dielectric-barrier discharge (DBD) plasma in the presence of water leads to the formation of propylene oxide and hydroxyl and carbonyl compounds [1]. Based on experimental data and theoretical calculations, it was established that the presence of nitrogen molecules in the reactant mixture does not significantly affect the composition and the mechanism of formation of reaction products. The main contribution to the formation of propylene oxide is made by processes involving atomic oxygen. Molecular oxygen also takes part in the oxidation of propylene, leading to the formation of hydroxyl and carbonyl compounds.
It is known that the dissociation of oxygen [2] and carbon dioxide [3] molecules in DBD leads to the formation of oxygen atoms, but molecular oxygen is absent in the reaction mixture in the case of carbon dioxide. Comparison of experimental and calculated data on the oxidation of propylene in air and carbon dioxide will clarify the possible mechanism of the formation of oxygen-containing products with the general formula C3HnO: propylene oxide, propanal, acetone, allyl alcohol, acrolein, isopropanol, and propanol.
EXPERIMENTAL
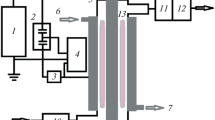
The study was carried out using the experimental setup described earlier [4]. A flow of propylene and air or carbon dioxide is mixed with water at room temperature, and then the gas–liquid mixture is sent to the reactor, where it is exposed to DBD.
The plasma reactor is made of duralumin and has a planar structure with one dielectric barrier (glass fiber, 1 mm thick). The gap in the discharge zone is 1 mm, and the area of the discharge zone is 48 cm2. In all experiments, the amplitude of high-voltage pulses did not exceed 9 kV, and their repetition frequency was 400 Hz. The active discharge power was 1.9 or 2.5 W for mixtures of propylene with air or carbon dioxide, respectively. The volumetric flow rate of the gas mixture was 60 cm3/min, and that of water was 0.3 cm3/min. The gaseous and liquid products were analyzed using a gas chromatograph equipped with a thermal conductivity detector and a flame ionization detector.
The concentration of the main chemically active particles formed at the stage of discharge initiation in the reaction mixture was calculated using the Kintecus software suite [5] according to a previous procedure [4]. The necessary data on the energy losses of DBD electrons in propylene–air and propylene–oxygen mixtures, as well as the values of the rate constants of electron–molecule reactions and the electron drift velocity, were obtained using the Bolsig+ software [6], and the cross sections for electron scattering by molecules were taken from the database [7]. The rate constants of electron–molecule reactions were calculated taking into account published data [3, 8].
RESULTS AND DISCUSSION
Table 1 shows the products of propylene conversion in carbon dioxide in comparison with air [1]. It can be seen that the products markedly differ in composition and amount. Methanol, acrolein, acetic and propionic acids are not formed. The proportion of gaseous hydrocarbons (methane, ethane, ethylene, acetylene) is smaller by a factor of ~2, while the amount of products represented mainly by unsaturated C4–C6 hydrocarbons (line “Other,” Table 1) is greater by a factor of ~2.
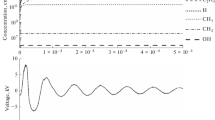
The energy consumption for the conversion of propylene is comparable in both cases, but the conversion with CO2 is higher because of the higher active discharge power. Figure 1 shows charge–voltage characteristics (CVCs) of the discharge for propylene mixtures with air and CO2. The calculation of the electrical characteristics of the DBD according to the CVC data was carried out graphically [4]. The discharge operating voltage (Udis) was determined by the expression:
where Cb and Cg are the capacitances of the dielectric barrier and the discharge gap, respectively.
Charge–voltage characteristic of DBD in a mixture of propylene with carbon dioxide or air in the presence of water. Umin is the minimum external voltage at which microdischarges are observed in the discharge gap; lines AB and BC correspond to the capacitance of the dielectric barrier and the discharge gap, respectively; and q is the value of the transferred charge per pulse.
The obtained Udis values were respectively ~3300 and 2500 V for experiments with air and CO2. It can be seen that in the case of CO2, microdischarges appear in the reactor at a lower external voltage, whereas the charge transferred in the pulse is higher and corresponds to values of 1.8 and 2.4 × 10−6 C (Fig. 1).
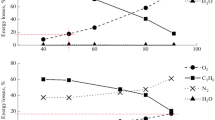
Figure 2 shows the amount of oxygen-containing products with the general formula C3HnO depending on the initial concentration of air or CO2 in the feed mixture. It can be seen that for both mixtures there is a general trend in the product formation with a decrease in the concentration of propylene in the feed mixture, namely, the amount of propylene oxide and propanal increases and that of acetone and isopropanol decreases. The common features indicate a similar mechanism for the formation of substances with different compositions of the feed mixture.
Figure 3 shows the calculated composition of the main chemically active species formed at the stage of discharge initiation of the reaction for propylene–air–water and propylene–CO2–water mixtures in a DBD per high-voltage pulse.
The upper limits of the yield of atoms and radicals as a result of electronically excited dissociation of molecules were evaluated on the basis of threshold dissociation energies, which have values of ~3.6, ~7.0, ~4.5, ~8.9, and ~ 7.0 eV for propylene, CO2, O2, N2, and H2O, respectively.
The DBD treatment of the feed mixture results in the predominant formation of oxygen atoms and hydrocarbon radicals as propylene degradation products. It can be seen that due to the lower dissociation energy of molecular oxygen, more oxygen atoms are generated in the propylene–air mixture than in the presence of CO2. In the case of treatment of the propylene–air mixture, atomic nitrogen appears.
The formation of oxygen and nitrogen atoms occurs according to the following reactions [2, 3, 10, 11]:
As shown earlier [1], the presence of nitrogen molecules in the feed mixture has no significant effect on the formation of oxygen-containing products during the oxidation of propylene. Most likely, nitrogen atoms interact with molecular oxygen, subsequently leading to the formation of various nitrogen oxides [2, 12].
According to the published data [8, 9], electronically excited propylene molecules dissociate to give gaseous hydrocarbons and various radicals according to the reactions:
It should be noted that the concentration of hydrocarbon radicals and hydrogen is comparable for both mixtures (Fig. 3). Thus, it can be assumed that the mechanism of propylene oxidation with air or CO2 includes two routes of the appearance of principal radicals—the formation of atomic oxygen and the degradation of the olefin. A significant difference is the involvement of molecular oxygen in the formation of products during the oxidation of propylene with air.
It is known that oxygen atoms interact with the olefin double bond to form an adduct, which rearranges into the final products by ring closure (an oxide is formed) or by migration of a hydrogen atom or an alkyl group from the oxygen-linked carbon atom to the other carbon atom of the original double bond (carbonyl compounds are formed). The main products of propylene reaction with the O atom are propylene oxide, propanal, and acetone with the general formula C3H6O:
It can be seen from Table 1 that the concentration of propylene oxide in the products in both cases has similar values, 15.6 and 16.9%, respectively, for experiments with air and CO2. For propanal and acetone, the values differ and can be explained by additional reactions of their formation in the mechanism of propylene oxidation.
When propylene is oxidized with air, the interaction of the propyl radical with an oxygen molecule leads to the appearance of acetone and alcohol (isopropanol or propanol) as part of the reaction products. The formation of the propyl radical is possibly a result of the hydrogenation of propylene molecules with hydrogen atoms:
In the case of propylene conversion in a CO2 atmosphere, the formation of acetone or propanal is possible as a result of the interaction of an oxygen atom with a propyl radical:
Consequently, in experiments with air, propyl radicals predominantly interact with oxygen molecules according to reactions (11) and (12), increasing the amount of acetone in the products. In a carbon dioxide medium, propyl radicals can interact with oxygen atoms according to reaction (13), increasing the proportion of propanal in the mixture, or react with other hydrocarbon radicals, increasing the amount of unsaturated С4–С6 hydrocarbons in the products (line “Others,” Table 1):
The data in Table 1 show that the formation of acrolein and acetic and propionic acids occurs only in the presence of molecular oxygen in the feed mixture.
From the calculation data for the composition of chemically active species generated at the step of discharge initiation of the reaction (Fig. 2), it follows that the concentration of propenyl radicals in experiments with air and carbon dioxide is comparable; therefore, their interaction with molecular oxygen yields the corresponding peroxide radicals, similarly to reactions (11) and (12), the further transformation of which leads to the appearance of unsaturated alcohol and aldehyde (allylic alcohol and acrolein):
A detailed insight into the mechanism of formation of propylene oxidation products is possible by studying the kinetic model of propylene oxidation, which is a separate and time-consuming task.
CONCLUSIONS
Comparison of experimental data on the conversion of propylene in carbon dioxide and air [1] made it possible to reveal the following features of the process:
(1) the discharge in experiments involving CO2 is excited at a lower external voltage, and the charge transferred per pulse is greater in comparison with that in the case of air;
(2) energy consumption for propylene conversion has a close value, but the conversion per pass is higher;
(3) the overall selectivity of the process in CO2 is higher: a smaller number of products are formed;
(4) there are common trends in the formation of oxygen-containing products with the general formula С3НnО. The yield of propylene oxide and propanal depends on the amount of oxygen atoms in the reaction mixture, and the formation of C2–C6 hydrocarbons, acetone, and alcohols is associated with the activation of the propylene molecule in DBD;
(5) the formation of methanol, acrolein, and acetic and propionic acids requires the presence of molecular oxygen in the feed mixture.
REFERENCES
Ryabov, A.Yu., Kudryashov, S.V., and Ochered’ko, A.N., High Energy Chem., 2021, vol. 22, no. 3, p. 238.
Samoilovich, V.G., Gibalov, V.I., and Kozlov, K.V., Fizicheskaya khimiya bar’ernogo razryada (Physical Chemistry of Dielectric Barrier Discharge), Moscow: Izd. MGU, 1989.
Itikawa, Y., J. Phys. Chem. Ref. Data, 2002, vol. 31, no. 3, p. 749.
Kudryashov, S., Ryabov, A., and Shchyogoleva, G.S., J. Phys. D: Appl. Phys., 2016, vol. 49, p. 025205.
Ianni, J.C., Kintecus V5.5, 2015. http://www.kintecus.com.
Hagelaar, G.J.M. and Pitchford, L.C., Plasma Sources Sci. Technol., 2005, vol. 14, no. 4, p. 722.
Viehland database. http://www.lxcat.net.
Janev, R.K. and Reiter, D., Phys. Plasmas, 2004, vol. 11, p. 780.
Tsang, W., J. Phys. Chem. Ref. Data, 1991, vol. 20, p. 221.
Ponduri, S., Becker, M.M., Welzel, S., et al., J. Appl. Phys., 2016, vol. 119, p. 093301.
Cosby, P.C., J. Chem. Phys., 1993, vol. 98, p. 9544.
Herron, J.T., J. Phys. Chem. Ref. Data, 1999, vol. 28, p. 1453.
Kurylo, M.J., Chem. Phys. Lett., 1972, vol. 14.
Tsang, W., J. Phys. Chem. Ref. Data, 1988, vol. 17, p. 1.
Atkinson, R., Baulch, D.L., Cox, R.A., et al., J. Phys. Chem. Ref. Data, 1997, vol. 26, p. 521.
Wallington, T.J., Dagaut, P., and Kurylo, M.J., Chem. Rev., 1998, vol. 92, p. 667.
Funding
The work was carried out under the 2021–2023 program of fundamental scientific research of the state academies of sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by S. Zatonsky
Rights and permissions
About this article
Cite this article
Ryabov, A.Y., Kudryashov, S.V. & Ochered’ko, A.N. Features of Propylene Oxidation by Carbon Dioxide in Dielectric Barrier Discharge. High Energy Chem 56, 217–222 (2022). https://doi.org/10.1134/S0018143922030092
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0018143922030092