Abstract
This work is a continuation of research on the oxidation of propylene in dielectric-barrier discharge. On the basis of calculated and experimental data, it has been concluded that reactions involving atomic oxygen play the leading role in the formation of the main products—propylene oxide and hydroxyl and carbonyl compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Propylene oxide is a popular petrochemical feedstock. The main processes for its production are hydroperoxide methods; simultaneously with them, catalytic processes develop [1–3] and new alternative approaches to its synthesis appear.
Previously, we have shown the fundamental possibility of propylene oxidation with oxygen in dielectric- barrier discharge (DBD) plasma in the presence of n-octane to propylene oxide and hydroxyl and carbonyl compounds [4]. The maximum concentration of propylene oxide in liquid products reaches 45 wt %. The paper considers a possible reaction mechanism, in which the main role is assigned to processes that involve atomic oxygen:
where e are DBD electrons.
The choice of air as the propylene oxidation medium leads to a decrease in the propylene oxide content in the products to ~23 wt %. It has been assumed that nitrogen molecules contained in the initial mixture can affect the reaction mechanism.
In this study we attempt to test this assumption. The main idea is to select the initial ratio of the components of propylene–oxygen and propylene–air mixtures, in which the energy losses of DBD electrons for the excitation of oxygen molecules will be comparable, all other things being equal. If the composition and concentration of the products are close, the effect of nitrogen on the mechanism of propylene oxidation with air can be ignored.
EXPERIMENTAL
The study was carried out using, as an example, the oxidation of a mixture of propylene with oxygen or air in the presence of water, instead of n-octane used in [4]. The replacement of n-octane with water is caused by both the lack of data on the cross sections for electron scattering by n-octane molecules, which are necessary in calculating the energy losses of DBD electrons in collisions with molecules of the reactant mixture, and its undesirable oxidation along with propylene in DBD.
The results of preliminary experiments on the oxidation of propylene with oxygen in the presence of water in DBD [8] showed that replacing n-octane with water does not lead to a significant change in the product composition. There is a decrease in the concentration of propylene oxide in the products from 45 to ~30 wt %. Acetic and propionic acids were identified; otherwise, the composition of the oxidation products was almost identical to that obtained in the presence of n-octane.
The experiments were carried out on a laboratory setup sketched in [9]. The flow of propylene and air/oxygen is mixed with water, and then the gas–liquid mixture at room temperature is sent to the plasma reactor for its DBD treatment.
The plasma reactor has a dismountable design of a planar type with one dielectric barrier. The gap in the discharge zone is 1 mm, and the area of the discharge zone is 48 cm2. In all experiments, the amplitude of high-voltage pulses did not exceed 15 kV, and their repetition rate was 400 Hz. The active discharge power was 1.7 W. The volumetric flow rate of the reaction mixture was 60 cm3/min, and the water flow rate was 0.3 cm3/min. The analysis of gaseous and liquid products was carried out using an HP 6890 gas chromatograph equipped with a thermal conductivity detector and a flame ionization detector.
The energy loss of DBD electrons for the propylene–air and propylene–oxygen mixtures was calculated using the program Bolsig+ [10]; the cross sections for electron scattering by molecules were taken from the database [11].
RESULTS AND DISCUSSION
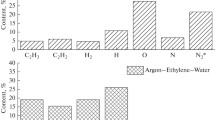
Figure 1 shows calculated values for the total energy losses of DBD electrons in propylene–air and propylene–oxygen mixtures with water vapor, depending on the propylene content in the feed mixture. The concentration of water molecules in the gas mixture was ~2%.
The figure shows that in an excess of propylene, the main energy losses of electrons occur in collisions with propylene molecules in both cases, with a significant loss of electron energy in the case of oxidation with air being due to excitation of nitrogen molecules. In all the cases, water molecules account for less than 1% of the electron energy losses. It can be seen that comparable values of the energy losses of DBD electrons for the excitation of oxygen molecules can be achieved in propylene mixtures with an initial oxygen or air concentration of 50 or 90%, respectively.
Table 1 shows the product composition for the oxidation of these mixtures in the presence of water. It can be seen from the table that products formed are similar in composition and amount of components, thereby indicating a similar mechanism of propylene oxidation by oxygen and air and confirming the above assumptions about the reaction mechanism. Oxygen atoms play the main role in the formation of the products, and nitrogen molecules do not have a noticeable effect on the mechanism of the reaction process.
Table 2 shows the calculated distribution of the total energy losses of DBD electrons for the propylene–oxygen and propylene–air mixtures with water vapor over the internal degrees of freedom of the feed mixture molecules.
From the data in Fig. 1 and Table 2 it is seen that for the propylene–air mixture, ~60% of all energy losses of DBD electrons are due to the excitation of nitrogen molecules. It is known that energy consumption for ozone production from air in DBD is lower than in pure oxygen and this difference is attributed to the formation of additional atomic oxygen according to the reactions:
Comparison of the data given in Table 1 for the energy consumption for oxidation shows that the contribution of reactions (3) and (4) to the formation of an additional amount of atomic oxygen can be neglected in this case. It is likely that the presence of propylene in the feed mixture facilitates the dissipation of energy of excited nitrogen molecules:
but, judging by the composition of the gaseous products (Table 1), this does not lead to the appearance of additional pathways of propylene fragmentation along with electron–molecule reactions [13]
In general, the value obtained for energy consumption is slightly lower than in the oxidation of propylene at an oxygen concentration of 91% in the presence of n-octane [4] in a coaxial reactor with two dielectric barriers, 17.8 kWh/kg. Perhaps, the difference is due to the choice of the planar reactor design with one dielectric barrier. In addition, a positive point is that replacing n-octane with water precludes contamination of propylene oxidation products with n-octane conversion products and increases the overall selectivity of the process.
CONCLUSIONS
On the basis of simple theoretical calculations and experimental data, an indirect confirmation of the conclusions about the possible mechanism of the propylene oxidation reaction in DBD has been obtained:
The main contribution to the formation of the products is made by processes involving atomic oxygen, which is formed by the interaction of DBD electrons with oxygen molecules;
In the case of oxidation with air, the presence of nitrogen in the feed mixture does not significantly affect the composition of the products and their formation mechanism.
REFERENCES
Terzan, J., Hus, M., Likozar, B., and Djinovic, P., ACS Catal., 2020, vol. 10, p. 13415.
Van-Huy Nguyen, Ba-Son Nguyen, Hieu-Thao Vo, Chinh Chien Nguyen, Sa-Rang Bae, Soo Young Kim, and Quyet Van Le, Catalysts, 2020, vol. 10, p. 87.
Nijhuis, TA., Makkee, M., Moulijn, J.A., and Weckhuysen, B.M., Ind. Eng. Chem. Res., 2006, vol. 45, no. 10, p. 3447.
Kudryashov, S.V., Ochered’ko, A.N., Ryabov, A.Yu., and Shchyogoleva, G.S., Plasma Chem. Plasma Process., 2011, vol. 31, p. 649.
Samoilovich, V.G., Gibalov, V.I., and Kozlov, K.V., Fizicheskaya khimiya bar’ernogo razryada (Physical Chemistry of Dielectric Barrier Discharge), Moscow: Izd. MGU, 1989.
Tsang, W., J. Phys. Chem. Ref. Data, 1991, vol. 20, p. 221.
Cvetanovic, R.J., J. Phys. Chem. Ref. Data, 1987, vol. 16, p. 261.
Mukusheva, G., Dankovtsev, G.Yu., Ryabov, A.Yu., Ochered’ko, A.N., and Kudryashov, S.V., AIP Conf. Proc., 2020, vol. 2310, p. 020214.
Kudryashov, S., Ryabov, A., and Shchyogoleva, G., J. Phys. D: Appl. Phys., 2016, vol. 49, p. 025205.
Hagelaar, G.J.M. and Pitchford, L.C., Plasma Sources Sci. Technol., 2005, vol. 14, no. 4, p. 722.
Viehland Database. http://www.lxcat.net.
Herron, J.T., J. Phys. Chem. Ref. Data, 1999, vol. 28, p. 1453.
Janev, R.K. and Reiter D., Phys. Plasmas, 2004, vol. 11, p. 780.
Funding
The work was carried out under the program of fundamental scientific research of the state academies of sciences for 2021–2025.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by S. Zatonsky
Rights and permissions
About this article
Cite this article
Ryabov, A.Y., Kudryashov, S.V., Ochered’ko, A.N. et al. On the Possible Mechanism of Propylene Oxidation in Dielectric-Barrier Discharge. High Energy Chem 55, 336–339 (2021). https://doi.org/10.1134/S0018143921040111
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0018143921040111