Abstract
The radiolysis of a radiochemical extraction system based on N,N,N',N'-tetra-n-octyl diglycolamide (TODGA) dissolved (0.15–0.2 M) in a mixture of Isopar-M with n-decanol or n-nonanol has been studied. The alcohol content was 6 or 20 vol %. A beam of 8-MeV electrons was used for irradiation. It has been found that the predominant radiolytic transformation of TODGA is fragmentation with the major formation of N,N-dioctylacetamide and 2-hydroxy-N,N-dioctylacetamide. Products of the dissociative addition of alkoxy radicals to the carbonyl groups of TODGA were detected. The total yield of TODGA degradation in the extraction system was no higher than 0.5 μmol/J. Degradation was insensitive to the type of alcohol, but it depended on the alcohol content of the solution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Radiation chemistry plays a key role in the selection of durable and safe extraction systems for the recovery of radioisotopes from spent nuclear fuel. Radiolytic processes can lead to different rates of decline in the performance characteristics of an extractant depending on the composition of the extraction system. Therefore, the elucidation of the mechanism of radiolysis of extraction media plays a fundamentally important role in the optimization of their composition [1]. Diglycolamides [2–4] are among the most interesting current extractants with high extraction capacity and ease of utilization. Among them, N,N,N',N'-tetra-n-octyl diglycolamide (TODGA) is one of the most promising extractants for the extraction of An(III) and Ln(III) from highly concentrated solutions of nitric acid [5–7]. The radiation resistance of an extraction system is closely related to the issues of extraction efficiency, regenerability, and fire and explosion safety in the development of diluents for TODGA. Practical considerations indicate the feasibility of using hydrocarbon–alcohol diluents. In particular, the hydrocarbon fraction can be a mixture of C13–C14 isoparaffins (Isopar-M), and the alcohol fraction can be represented by n-nonanol or n‑decanol [8, 9]. Information on the radiation resistance of TODGA in these mixed diluents is scarce and contradictory, although the main fragmentation channels of the TODGA molecule are well known [10, 11]. In this paper, we consider the transformations of TODGA in a mixture of a heavy alcohol with Isopar-M at a high absorbed dose of accelerated electrons.
EXPERIMENTAL
The extraction mixtures of N,N,N',N'-tetra-n-octyl diglycolamide (TODGA), Isopar-M (a mixture of isoparaffins with a boiling range of 208–257°С), and an aliphatic alcohol (n-decanol or n-nonanol) were obtained from the Khlopin Radium Institute (St. Petersburg). In addition to Isopar-M, mixture S1 contained 0.15 M TODGA and 6 vol % n-decanol, mixture S2 contained 0.15 M TODGA and 6 vol % n‑nonanol, mixture S3 contained 0.2 M TODGA and 20 vol % n-decanol, and mixture S4 contained 0.2 M TODGA and 20 vol % n-nonanol.
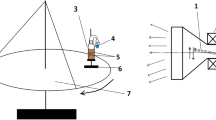
The mixtures were irradiated in a cylindrical glass reactor with a hydraulic seal at 17 ± 2°C using a scanned beam of accelerated electrons from an UELV-10-10-S-70 linear accelerator (energy, 8 MeV; pulse duration, 6 μs; pulse repetition rate, 300 Hz; average beam current, 700 μA; vertical scanning frequency, 1 Hz; and scanning width, 245 mm). Intermittent irradiation was used: an interval of irradiation to a dose of 10 kGy (dose rate, 0.22 kGy/s) was alternated with an interval of sample cooling for 10 min. Dosimetry was carried out using film dosimeters of a SO PD(F)R-5/50(GSO 7865-2000) copolymer with a phenazine dye. The total absorbed dose in each sample was 500 kGy.
The samples were analyzed using a Thermo Scientific Trace 1310 gaschromatograph with an ISQ mass spectrometric detector (electron ionization, 70 eV) and a Trace 1310 gas chromatograph with a flame-ionization detector. The split injection mode (1 : 20 and 1 : 5) was used in helium (flow rate, 1.2 mL/min). Acetone was used as a diluent for the samples. Thermo Scientific TG-5MS columns 15 and 30 m long with a polydiphenylsiloxane/polydimethylsiloxane ratio of 5 : 95 (15 m × 0.25 mm and 30 m × 0.25 mm, respectively) were used. The products were identified based on the mass spectra and retention indices using the NIST-2017 database. According to the results of repeated experiments, the relative measurement error did not exceed 10%.
RESULTS AND DISCUSSION
Among the components of samples S1 and S2, TODGA has the lowest radiation resistance. At 0.5 MGy, the decrease in the TODGA content was almost 3/4 of its initial concentration. The radiation-chemical yields of TODGA degradation (0.41–0.43 µmol/J) were approximately four times higher than the yield of alcohol degradation (0.11–0.12 µmol/J), and the replacement of n-decanol (sample S1) with n-nonanol (S2) had little effect on these yields. An increase in the alcohol content to 20 vol % (samples S3 and S4) led to a slight increase in the degradation of TODGA (to 0.48 μmol/J), while the yield of degradation of the alcohol increased by a factor of almost 5 to 0.52 μmol/J.
In all irradiated samples S1–S4, only products lighter than TODGA were detected. Heavier products were not observed. This fact indicates that the cleavage of skeletal bonds is the main radiolytic transformation of TODGA molecules in an alkane/alcohol mixture. Figure 1 shows the key products of TODGA conversion. Compounds P1–P5 and P7 are products of the rupture of internal bonds in the TODGA molecule. The other compounds belong to the products of the combination of TODGA fragments with alkyl and alkoxy radicals. In this case, the carbonyl groups of TODGA were the predominant sites of attachment.
The radiolytic formation of alkyl radicals was due to several competing processes: the decay of excited alkane molecules and ions (Isopar-M), ion–molecule reactions with the participation of primary alkane radical cations, pair neutralization of primary ions, the detachment of H atoms in reactions of alkanes with small radicals (primarily, H, OH, and CH3), and fragmentation of aliphatic alcohol molecules [12, 13]. The ratio between the yields of C–C and C–H bond cleavage is about 0.5 for both alkanes and aliphatic C5–C16 alcohols [14, 15]. In isoalkanes, the yield of C–C bond cleavage is higher than that in linear homologues. Isoalkanes were the dominant components in the test extraction system. They were directly exposed to radiation, that is, directly absorbed radiation energy. Alcohol was contained in a smaller amount, but its electron fraction was also sufficient for direct energy absorption. Accordingly, radiolysis provided a wide range of alkyl radicals, the length of which was shorter than or equal to the length of the parent Isopar-M molecules and an alkyl substituent in the alcohol molecule. Obviously, products P9 and P10 were formed with the participation of alkyl radicals. In particular, light C4–C9 hydrocarbons, which are lighter than the Isopar-M components, were observed among the radiolysis products of the extraction system. With the exception of C7 products, the longer the hydrocarbon skeleton, the higher the yield of alkanes and alkenes (Fig. 2).
The precursors of alkoxy radicals were alcohol molecules. The O–H bond cleavage in alcohol is easier than the C–H bond cleavage. Therefore, in spite of the predominance of C–H bonds, the yield of O–H bond cleavage the formation of alkoxy radicals can reach 0.2 µmol/J [13, 15]. Alkoxy radicals easily detach H from surrounding molecules, or they are attached at double bonds [16]. It is likely that the formation of esters P6, P8, P10, and P11 was due to the following reactions of the dissociative addition of alkoxy radicals:

The breaking of C–H bonds leads to the formation of much less reactive hydroxyalkyl radicals, mainly, α-hydroxyalkyl radicals [13]. It is likely that they disappear in recombination reactions with alkyl radicals.
The radiolysis of alcohols is also characterized by the breaking of C–O bonds. The OH radical formed in this case can [13, 15, 17], on the one hand, generate water (due to H abstraction) and, on the other hand, attach to unsaturated compounds, in particular, to the carbonyl groups of TODGA or to C=C bonds in the TODGA tautomer. The addition of OH is similar to reaction (1); however, it is likely that the resulting OH adduct is unstable in this case, and it decomposes with the elimination of CO2 (an important component in the gaseous products of radiolysis) and P3:

In turn, the dissociative addition of H more often leads to a weakening of the N–C bond in the octylamine group with the formation of P7.
The observed yield of TODGA degradation is significantly higher than that expected from the electron fraction. In particular, the yield of radiolytic degradation of alcohol in solutions S1 and S2 was lower by a factor of almost 4 than the yield of decomposition of TODGA, although their electron fractions were comparable. This effect can be due to the fact that TODGA molecules are more effective radical and ion acceptors in comparison with alkane and alcohol molecules. In addition, the ionization and excitation potentials of TODGA are lower than those of alkanes and alcohol [18]. Consequently, excess energy and charge transfer from diluent molecules to TODGA likely occurred in the irradiated extraction system. Accordingly, a significant portion of the TODGA degradation products resulted from the decay of excited molecules and ions. Figure 3 shows the observed yields of the main products in S1 and S2. It can be seen that the yields only slightly depended on the type of alcohol. An equally weak effect of replacing decanol with nonanol was observed in the case of solutions S3 and S4. Figure 4 shows the yields of products in S4 as an example.
From Figs. 3 and 4, it follows that the highest yields were observed for compounds P1 and P2, which are the products of C–O bond cleavage. The predominant cleavage of ether bonds is typical for the radiolysis of both ethers and esters [15, 19]. This cleavage can occur on the decomposition of the primary TODGA radical cations (RC+)

and on the homolytic decay of excited TODGA molecules (M*)

Next, the alkoxyl-type radical Rox is converted into P2 through the rapid H-atom elimination from the neighboring Isopar-M or alcohol molecules. In turn, Р1 is formed as a result of neutralization of the fragment cation K or by disproportionation of the alkyl-type radical Ralk with alkyl radicals resulting from an alkane or alcohol. The disproportionation of two Ralk with each other does not occur, probably, because of the structure peculiarities of these radicals and their relatively low concentrations. In mass spectrometry, when the ionization of TODGA is performed with low-energy electrons, the cleavage of C–C and C–N bonds adjacent to the carbonyl group plays the main role. It is likely that these cleavages with the formation of Р3, Р4, Р5, and Р7 can also occur upon irradiation with high-energy electrons; however, the ether bond cleavage at the center of the TODGA molecule is dominant in this case. In particular, the relatively low probability of the α-C–C bond cleavage can be due to keto–enol tautomerism, which is characteristic of the excited molecules of carbonyl compounds [20]:

As shown above, the α-C–C and C–N bond cleavage mainly occurs in the processes caused by the indirect action of radiation with the participation of alkoxy radicals formed from an alcohol. An increase in the alcohol concentration led to an increase in the degree of decomposition of TODGA and to an increase in the yields of products of the recombination of alkoxy and alkyl radicals (P6, P8–P11). Products P5 and P7 resulted from the cleavage of C–N bonds upon the dissociative addition of alkoxy radicals to the carbonyl group of the TODGA molecule. At the same time, some of the adducts underwent C–C bond cleavage in the α-position relative to the carbonyl group with the formation of P8. The α-С–С bond cleavage also resulted in P9.
A high absorbed dose makes possible multiple interactions of alkoxy and alkyl radicals with TODGA molecules and with TODGA fragmentation products. In particular, as a result of repeated reactions of alkoxy radicals with the TODGA molecule, a small amount of P11 was formed (Fig. 4). Alkoxy and alkyl radicals were also involved in the formation of esters P10. In particular, the addition of an alcoholic alkoxy radical to the carbonyl group in P4 can lead to the rupture of the adjacent C–N bond and the subsequent formation of P10 with the participation of an alkyl radical:


The radiolysis of the test extraction solutions also led to the formation of gaseous products, including hydrogen, fragmented hydrocarbons (Fig. 2), СО2 (reaction (2)), and ammonia. The formation of ammonia played a relatively minor role; it occurred likely due to the successive radiolytic dealkylation of P5. The higher the alcohol content, the higher the gas evolution in the irradiated solutions [9]. The amount of products released into a gas phase ranged from 6 to 10 mg/MGy [21].
CONCLUSIONS
The radiolysis of the solutions of TODGA in a mixture of Isopar-M and an aliphatic alcohol is an illustrative example of the physical and chemical protection of some solution components by others. In this case, TODGA acts as a victim, and the yield of degradation of this compound is significantly higher than that expected from the electron fractions of the components. A significant portion of TODGA is destroyed due to the direct absorption of radiation energy and as a result of energy and charge transfer from alkane and alcohol molecules (physical protection). Another portion of TODGA is destroyed due to the addition of H, OH, and alkoxy radicals to the carbonyl group (chemical protection). The presence of double bonds (carbonyl C=O or tautomeric C=C) makes the TODGA molecule an effective radical scavenger. The formation of radical adducts often entails the rupture of a C–N or α-C–C bond with the elimination of a low-molecular-weight fragment (for example, CO2). At the same time, the radiolytic degradation of TODGA weakens at high absorbed doses due to the partial conversion of the diluent (alkanes and an alcohol) into alkenes and aldehydes, which are also effective radical scavengers. Accordingly, as secondary unsaturated compounds accumulate, they compete with TODGA molecules in the capture of radicals.
The alcoholic fraction of the diluent plays the greatest role in the degradation of TODGA in the irradiated solutions. High alcohol content contributes to an increase in the fraction of reactive alkoxy radicals among other radicals and, thereby, enhances the degradation of TODGA. Therefore, from the point of view of extending the service life of TODGA, 6 vol % alcohol is preferable to 20 vol %. At the same time, the replacement of n-decanol with n-nonanol does almost not change the above effect.
REFERENCES
Mincher, B.J., Reprocessing and Recycling of Spent Nuclear Fuel, Taylor, R., Ed., Amsterdam: Elsevier–Woodhead Publishing, 2015, p. 191. https://doi.org/10.1016/B978-1-78242-212-9.00008-3
Sugo, Y., Izumi, Y., Yoshida, Y., Nishijima, S., Sasaki, Y., Kimura, T., Sekine, T., and Kudo, H., Radiat. Phys. Chem., 2007, vol. 76, p. 794. https://doi.org/10.1016/j.radphyschem.2006.05.008
Leoncini, A., Ansari, S.A., Mohapatra, P.K., Boda, A., Ali, S.M., Sengupta, A., Huskens, J., and Verboom, W., Dalton Trans., 2017, vol. 46, p. 1431. https://doi.org/10.1039/C6DT04034A
Sasaki, Y., Sugo, Y., Suzuki, S., and Tachimori S. Solvent, Extr. Ion Exch., 2001, vol. 19, no. 2001, p. 91. https://doi.org/10.1081/SEI-100001376
Ansari, S.A., Pathak, P.N., Manchanda, V.K., Husain, M., Prasad, A.K., and Parmar, V.S., Solvent Extr. Ion Exch., 2005, vol. 23, no. 2005, p. 463. https://doi.org/10.1081/SEI-200066296
Iqbal, M., Huskens, J., Verboom, W., Sypula, M., and Modolo, G., Supramol. Chem., 2010, vol. 22, p. 827. https://doi.org/10.1080/10610278.2010.506553
Whittaker, D., Geist, A., Modolo, G., Taylor, R., Sarsfield, M., and Wilden, A., Solvent Extr. Ion Exch., 2018, vol. 36, no. h. 2018, p. 223. https://doi.org/10.1080/07366299.2018.1464269
Skvortsov, I.V., Belova, E.V., and Yudintsev, S.V., Nucl. Eng. Technol., 2020, vol. 52, p. 2034. https://doi.org/10.1016/j.net.2020.02.024
Nikitina, Yu.V., Yudin, N.V., Belova, E.V., and Ponomarev, A.V., J. Radioanal. Nucl. Chem., 2020, vol. 326, p. 1185. https://doi.org/10.1007/s10967-020-07375-3
Zarzana, C.A., Groenewold, G.S., Mincher, B.J., Mezyk, S.P., Wilden, A., Schmidt, H., Modolo, G., Wishart, J.F., and Cook, A.R., Solvent Extr. Ion Exch., 2015, vol. 33, no. 2015, p. 431. https://doi.org/10.1080/07366299.2015.1012885
Zsabka, P., van Hecke, K., Wilden, A., Modolo, G., Hupert, M., Jespers, V., Voorspoels, S., Verwerft, M., Binnemans, K.,and Cardinaels, T., Solvent Extr. Ion Exch., 2020, vol. 38, no. 2020, p. 212. https://doi.org/10.1080/07366299.2019.1710918
Metreveli, A.K. and Ponomarev, A.V., High Energy Chem., 2016, vol. 50, p. 97. https://doi.org/10.1134/S0018143916020053
Ponomarev, A.V., Vlasov, S.I., and Kholodkova, E.M., High Energy Chem., 2019, vol. 53, p. 314. https://doi.org/10.1134/S0018143919040106
Cserep, G., Gyorgy, I., Roder, M., and Wojnarovits, L., Radiation Chemistry of Hydrocarbons, Budapest: Akademiai Kiado, 1981.
Woods, R.J. and Pikaev, A.K., Applied Radiation Chemistry: Radiation Processing, New York: Wiley–Interscience, 1994.
Rauk, A., Boyd, R.J., Boyd, S.L., Henry, D.J., and Radom, L., Can. J. Chem., 2003, vol. 81, p. 431. https://doi.org/10.1139/v02-206
Ponomarev, A.V., Vlasov, S.I., Kholodkova, E.M., Chulkov, V.N., and Bludenko, A.V., Radiat. Phys. Chem., 2019, vol. 165, p. 108405. https://doi.org/10.1016/j.radphyschem.2019.108405
Sugo, Y., Sasaki, Y., and Tachimori, S., Radiochim. Acta, 2002, vol. 90, p. 161. https://doi.org/10.1524/ract.2002.90.3_2002.161
Ponomarev, A.V. and Kholodkova, E.M., Mendeleev Commun., 2018, vol. 28, p. 375. https://doi.org/10.1016/j.mencom.2018.07.011
Grajales-González, E., Monge-Palacios, M., and Sarathy, S.M., J. Phys. Chem. A, 2018, vol. 122, p. 3547. https://doi.org/10.1021/acs.jpca.8b00836
Emel’yanov, A.S., Belova, E.V., Ponomarev, A.V., and Myasoedov, B.F., Radiochemistry, 2020, vol. 62, p. 587. https://doi.org/10.1134/S1066362220050045
ACKNOWLEDGMENTS
We are grateful to the Shared-Use Center for Instrumental Research Methods at the Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences for the equipment provided.
Funding
This work was funded by the Russian Academy of Sciences, project no. AAAA-A18-118011190130-0.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Makhlyarchuk
Rights and permissions
About this article
Cite this article
Serenko, Y.V., Ponomarev, A.V. & Belova, E.V. Direct and Indirect Effects of an Electron Beam on N,N,N',N'-Tetra-n-Octyl Diglycolamide in Hydrocarbon–Alcohol Solutions. High Energy Chem 55, 482–487 (2021). https://doi.org/10.1134/S0018143921060114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0018143921060114