Abstract
Ultra-high-temperature Ta4HfC5 ceramics with a submicron structure has been manufactured from a 4Ta + Hf + 5С powder mixture by electro-thermal explosion under pressure. The effect of the duration of mechanical activation of starting 4Ta + Hf powder mixtures on the structure of the resulting precursor has been studied. It has been demonstrated that mechanical activation in hexane for 40–60 min leads to amorphization of metals and nucleation of crystallites of the carbide phase with a cubic structure. This makes it possible to synthesize a single-phase solid solution of tantalum and hafnium carbides with a submicron structure in the course of an electro-thermal explosion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Tantalum and hafnium carbides, which have record high melting points and high strength, are of interest for use in the aerospace, nuclear, and metalworking industries etc. A characteristic feature of these carbides is the formation of continuous solid solutions with physical and mechanical properties superior to those of monocarbides [1]. This class of ultra-high temperature ceramics includes solid solutions based on the TaC–HfC system [2, 3]. It has been found that the maximum melting point of the Ta4HfC5 solid solution is ~3990°C [3–5]. The high melting point, electrochemical activity, and stability of the TaC–HfC solid solution make it possible to use it in aerospace engineering, power engineering, and catalysis [6, 7].
Ceramic materials based on carbide solid solutions are fabricated mainly by hot isostatic pressing (HIP) [3, 8, 10], spark plasma sintering (SPS) [1, 2, 10], a combined method of self-propagating high-temperature synthesis (SHS) and SPS [10, 11], and the sol–gel method [12]. All these methods are energy-intensive and require sophisticated equipment. Previously, an efficient one-stage method for manufacturing ultra-high-temperature ceramics has been developed [13], which combines exothermic synthesis in the electro-thermal explosion (ETE) mode and consolidation of the hot product under quasi-isostatic compression conditions. In ETE, the reaction mixture is heated due to the passage of current. When the ignition temperature is reached, the exothermic interaction of the reactants in the volume of the mixture is initiated. Simultaneously with the ignition of the mixture, pressure is applied to the sample, which is responsible for the consolidation of the hot product. The method is highly efficient, since it makes it possible to eliminate the intermediate stages of synthesizing monocarbides, their grinding and fractionation, followed by hot pressing or spark plasma sintering.
A feature of this process is the roughly inertialess heating of the synthesized product to a temperature that ensures the complete conversion of the initial reagents into the final product of equilibrium composition and its efficient consolidation. Previously, this method has been used for the synthesis of double carbides Ta4ZrC5 and Hf4ZrC5 [13].
This study is aimed at manufacturing consolidated ultra-high-temperature Ta4HfC5 ceramics with a submicron structure using ETE under pressure; the synthesis proceeds from commercial metal powders previously subjected to mechanical activation (MA) in a planetary mill.
Reaction mixtures were prepared from commercial powders of tantalum (TaPM-5B grade, dispersity <6 μm), hafnium (GFM-1 grade, dispersity 60–80 μm), and carbon black (P804-T grade, dispersity <0.2 μm). The ratio between the components was calculated according to the reaction:
The powders were mixed in two stages. At the first stage, Ta and Hf powders were mixed in hexane for 5, 20, 40, and 60 min in an AGO-2 planetary ball mill (Russia) at a rotation speed of 2220 rpm. The mill drums were made of steel 40Kh13, and the balls were made of steel ShKh-15. Each 150-mL drum was loaded with 30 g of a 4Ta + Hf mixture, 35 mL of hexane, and 240 g of 8 mm balls. At the second stage, carbon black was added to the resulting mixture in accordance with reaction (1), and the mixture was further stirred for 4 min. Next, the mixture was dried in air in a SNOL drying cabinet at 60°C for 2 h.
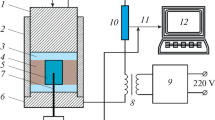
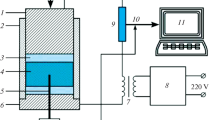
The synthesis of carbide ceramics was carried out in an ETE setup described in [9]. The prepared charge (30 g) was placed in a ceramic mold with a diameter of 21 mm. A pressure of 100 MPa was applied to it and it was heated by passing an electric current to carry out the exothermic interaction of the reagents in the ETE mode. After completion of the reaction, the synthesized product was kept under pressure for 3 s.
The microstructure of the samples was studied using a Zeiss Ultra plus ultrahigh resolution scanning electron microscope. X-ray diffraction (XRD) analysis of powder samples taken during the first stage of mixing, as well as synthesized ceramics, was carried out on a DRON-3 diffractometer (Russia) using CuKα radiation. Thin sections of the synthesized samples were used for XRD. The unit cell parameters of the carbide phase were calculated from 5 reflections, the profile of which was approximated by the pseudo-Voigt function.
Microhardness was measured on a PMT-3 hardness tester (Russia) by the Vickers method at a load of 100 g according to State standard GOST 2999-75. The ceramic density was determined by hydrostatic weighing according to State standard GOST 25281-82 on an analytical balance with an accuracy of 1 × 10–4 g.
The content of bound carbon was determined by oxidative melting in a ceramic crucible in an induction furnace from the amount of released carbon dioxide by the infrared adsorption method using a LECO CS600 instrument. Analysis accuracy was 0.01 wt %.
Figure 1 shows X-ray diffraction patterns of 4Ta + Hf mixtures after the first stage of preliminary mechanical activation (MA) in comparison with the mixture prepared by manual mixing for 10 min. The diffraction pattern of the mixture without activation treatment shows reflections of Ta and Hf, as well as reflections of hafnium hydrides that are present in the Hf powder.
In the diffraction patterns of the mixtures after 5 and 20 min of MA, there are no hafnium reflections, while the intensity of the Ta reflections significantly decreases, and their width increases (Fig. 1). Thus, Ta and HfH2 phases are present in the mixture in the crystalline form, while Hf passes into the amorphous state.
The broadening of the Ta and HfH2 reflections indicates the imperfection of their crystal structure and a decrease in the coherent scattering length. Obviously, the change in the structural state and phase composition of the metal mixture is a result of the deformation effect during high-energy treatment.
MA for more than 40 min leads to complete amorphization of the crystal structure of the initial metals. The Ta, Hf, and HfH2 reflections disappear, and the features of the diffraction pattern indicate the absence of long-range order in the Ta, Hf, and HfH2 structure.
At the same time, the X-ray diffraction pattern of the 4Ta + Hf mixture after 60 min of MA shows broadened reflections with intensities close to the background ones at 2θ angles of approximately 34.5°, 40°, and 58°. The angular position of these reflections corresponds to the positions of the 111, 200, and 220 TaC reflections, which indicates the formation of crystalline nuclei of the carbide phase. It should be noted that, at the first stage, the MA of the 4Ta + Hf mixture was carried out without the addition of carbon black.
The formation of the carbide phase at this stage can be explained by the interaction of tantalum and hafnium with carbon, which is released during the decomposition of hexane during MA [14, 15]. A significant error in determining the angular position of the precursor reflections makes it impossible to reliably determine the unit cell parameter of the carbide and estimate the Ta/Hf ratio using the Vegard rule.
The formation of carbide upon MA of the mixture in hexane is confirmed by chemical analysis data. It has been shown that, after MA for 60 min, the content of bound carbon Сb reaches 1.6 wt %, which corresponds to the 25% conversion of metals to the carbide phase. Thus, we can conclude that 60 min of MA of the 4Ta + Hf mixture in hexane results in the formation of a precursor containing an amorphous metal phase and crystallites of the carbide phase.
Flashover was carried out by passing an electric current through the sample. The process includes the stages of pre-explosion heating and thermal explosion. The pre-explosion heating stage ends when the ignition temperature is reached. At the stage of thermal explosion, an exothermic interaction of reactants occurs with a sharp increase in temperature. The duration of the first stage is a few seconds, and the second stage lasts a few tens of milliseconds. The adiabatic combustion temperature of the 4Ta + Hf + С mixture, calculated by the THERMO program [16], is about 3000°С.
Table 1 presents the consolidation parameters and physical and mechanical characteristics of carbide ceramics synthesized in this work and known in the literature [1–3, 10, 17, 19]. The relative density of the synthesized carbide ceramics is 90%, which is inferior to the Ta4HfC5 ceramics manufactured by hot isostatic pressing and spark sintering.
Due to the high residual porosity, the microhardness of the synthesized ceramics is ~14.5 GPa. The high porosity of the synthesized ceramics is caused by the insufficient time of exposure of the sample to pressure, which under the experimental conditions was about 3 s.
It should be noted that the short consolidation time, which is a few seconds at ETE in contrast to the HIP and SPS methods, is a significant advantage. To reduce the residual porosity of the samples, it is necessary to reduce the heat removal, which should lead to an increase in the time the material being in the plastic state and should increase the time of consolidation.
X-ray diffraction analysis of samples of consolidated ceramics after ETE showed that the preliminary MA of a metal mixture in hexane ensured the formation of a single-phase material. All diffraction patterns of ceramics (Fig. 2) manufactured from mechanically activated mixtures contain reflections of the cubic carbide phase (space group Fm–3m).
The diffraction reflections of the carbide are significantly broadened, which can be associated with both the small coherent scattering length and the distortion of the Ta1 – хHfхC crystal structure caused by the difference in the atomic radii of Hf (0.167 nm) and Ta (0.149 nm).
With an increase in the MA duration from 5 to 60 min, the 110 reflection of α-Fe is noted on the diffraction patterns, the appearance of which is associated with the grinding of iron from the drum walls and grinding balls.
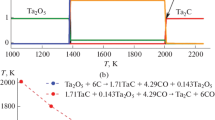
The X-ray diffraction pattern of carbide ceramics fabricated from a manually prepared mixture differs radically from the diffraction patterns of ceramics synthesized from preliminarily mechanically activated mixtures (Fig. 3). In the first case, the synthesized ceramics is not single-phase. It contains phases with parameters close to those of the unit cell of TaC and HfC monocarbides (Table 2). In addition, the presence of diffuse reflections in the angular ranges between the narrow reflections of TaC and HfC indicates the partial formation of a carbide solid solution Ta1 – хHfхC.
The unit cell parameters of a single-phase carbide ceramic depending on the MA time and literature data are presented in Table 2.
The obtained cell parameters are close to the known parameters of the Ta0.8Hf0.2C solid solution [2, 3, 10, 18–20]. The change in the unit cell metric in the series of TaC–HfC solid solutions is linear, i.e., obeys Vegard’s rule (Fig. 3).
On the basis of the data obtained, it can be concluded that ETE of the preliminarily mechanically activated metal powder mixtures affords a single-phase solid solution of tantalum and hafnium carbides with the composition corresponding to Ta4HfC5.
The results of XRD are consistent with the data of microstructural analysis. Figure 4 shows the microstructure of the carbide ceramics synthesized by ETE from a reaction mixture subjected to MA for 64 min. The size of carbide particles is less than 500 nm, and they have a faceting characteristic of a cubic crystal structure.
CONCLUSIONS
Thus, for the first time, a consolidated ultra-high-temperature Ta4HfC5 ceramics based on a single-phase solid solution with a submicron structure has been synthesized from a 4Ta + Hf + 5C powder mixture by electrothermal explosion under pressure. For the synthesis of single-phase carbide ceramics, a mixture of Ta and Hf metals has been used after preliminary MA in a planetary mill. It has been shown that the MA time of the 4Ta + Hf powder mixture significantly affects the structure of the resulting precursor. MA leads to a change in the structural state and phase composition of the metal mixture as a result of the deformation effect. During MA in hexane for 40–60 min, amorphization of metals and the nucleation of crystallites of the carbide phase with a cubic structure occur. The formation of carbide is associated with the interaction of Ta and Hf with carbon formed upon the decomposition of hexane. The ETE of the reaction mixture containing the precursor and the required amount of carbon black produces a single-phase solid solution of tantalum and hafnium carbides. The unit cell parameter of the carbide as a function of the MA time of the metals is 4.4796(9)–4.4951(8) Å and corresponds to the cell parameter of the Ta0.8Hf0.2C carbide. The synthesized ceramics is characterized by a submicron structure; the Vickers hardness is 14.5 GPa at a porosity of 10%.
REFERENCES
Cedillos-Barraza, O., Grasso, S., Al Nasiri, N., Jayaseelan, D.D., Reece, M.J., and Lee, W.E., J. Eur. Ceram. Soc., 2016, vol. 36, no. 7, pp. 1539–1548. https://doi.org/10.1016/j.jeurceramsoc.2016.02.009
Zhang, C., Gupta, A., Seal, S., and Boesl, B., Arvind Agarwal. A., J. Am. Ceram. Soc., 2017, vol. 100, no. 5, pp. 1853–1862. https://doi.org/10.1111/jace.14778
Gaballa, O., Cook, B.A., and Russell, A.M., Int. J. Refract. Met. Hard Mater., 2013, vol. 41, pp. 293–299. https://doi.org/10.1016/j.ijrmhm.2013.04.018
Ghaffari, S.A., Faghihi-Sani, M.A., Golestani-Fard, F., and Nojabayy, M., Int. J. Refract. Met. Hard Mater., 2013, vol. 41, pp. Ð. 180–184. https://doi.org/10.1016/j.ijrmhm.2013.03.009
Gusev, A.I., Russ. J. Phys. Chem., 1985, vol. 59, no. 3, pp. 579-584.
Valencia, D.P., Yate, L., Aperador, W., Yanguang, LiY., and Coy, E., J. Phys. Chem. C, 2018, vol. 122, no. 44, pp. 25433–25440. https://doi.org/10.1021/acs.jpcc.8b08123
Coy, E., Babacic, V., Yate, L., Zaleski, K., Kim, Y., Reparaz, J.S., Dorling, B., Graczykowski, B., Iatsunsky, I., and Siuzdak, K., Chem. Eng. J., 2021, vol. 415, p. 128987. https://doi.org/10.1016/j.cej.2021.128987
Guo, S., Adv. Appl. Ceram., 2021, vol. 120, no. 2, pp. 117–126. https://doi.org/10.1080/17436753.2021.1892363
Ghaffari, S.A., Faghihi-Sani, A., Golestani-Fard, F., and Mandal, H., J. Eur. Ceram. Soc., 2013, vol. 33, no. 8, pp. 1479–1484.https://doi.org/10.1016/j.jeurceramsoc.2013.01.017
Kurbatkina, V.V., Patsera, E.I., Levashov, E.A., and Timofeev, A.N., Ceram. Int., 2018, vol. 44, no. 4, pp. 4320–4329. https://doi.org/10.1016/j.ceramint.2017.12.024
Patsera, E., I., Kurbatkina, V.V., Levashov, E.A., and Timofeev, A.N., Izv. Vyssh. Uchebn. Zaved. Poroshk. Matall. Funkts. Pokryyiya, 2017, no. 2, pp. 55–63. https://doi.org/10.17073/1997-308X-2017-2-55-63
Simonenko, E.P., Simonenko, N.P., Petrichko, M.I., Sevast’yanov, V.G., and Kuznetsov, N.T., Russ. J. Inorg. Chem., 2019, vol. 64, no. 11, pp. 1127–1135. https://doi.org/10.1134/S0036023619110196
Shcherbakov, V.A., Gryadunov, A.N., Vadchenko, S.G., and Alymov, M.I., Dokl. Chem., 2019, vol. 488, no. 2, pp. 242–245. https://doi.org/10.1134/S0012500819090027
Lubnin, A.N., Dorofeev, G.A., Nikonova, R.M., Mukhgalin, V.V., Lad’yanov, V.I., Phys. Solid State, 2017, vol. 59, no. 11, pp. 2206–2217. https://doi.org/10.21883/FTT.2017.11.45063.015
Eremina, M.A., Lomaeva, S.F., Burnyshev, I.N., Kalyuzhnyi, D. G., and Konygin, G.N., Russ. J. Inorg. Chem., 2018, vol. 63, no. 10, pp. 1257–1265. https://doi.org/10.1134/S0044457X18100069
Shiryaev, A.A., Int. J. Self-Propag. High-Temp. Synth., 1995, vol. 4, no. 4, pp. 351–362.
Ha, D., Kim, J., Han, J., and Kang, S., Ceram. Int., 2018, vol. 44, no. 16, pp. 19247–19253. https://doi.org/10.1016/j.ceramint.2018.07.149
Andrievskii, R.A., Strel’nikova, N.S., Poltoratskii, N.I., Kharkhardin, E.D., and Smirnov, V.S., Soviet Powder Metall. Met. Ceram., 1967, vol. 6, no. 1, pp. 65–67.
Simonenko, E.P., Ignatov, N.A., Simonenko, N.P., Ezhov, Yu.S., Sevastyanov, V.G., and Kuznetsov, N.T., Russ. J. Inorg. Chem., 2011, vol. 56, no. 11, pp. 1681–1687. https://doi.org/10.1134/S0036023611110258
Jiang, J., Wang, S., and Li, W., J. Am. Ceram. Soc., 2016, vol. 99, no. 10, pp. 3198–3201. https://doi.org/10.1111/jace.14436
ACKNOWLEDGMENTS
The work was performed using the equipment of the ISMAN Shared Facility Center.
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 19-08-01085 A).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Additional information
Translated by G. Kirakosyan
Rights and permissions
About this article
Cite this article
Shcherbakov, V.A., Gryadunov, A.N., Semenchuk, I.E. et al. Synthesis of Ta4HfC5 Ceramics with a Submicron Structure by Electro-Thermal Explosion under Pressure. Dokl Chem 501, 259–263 (2021). https://doi.org/10.1134/S0012500821120041
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0012500821120041





 ) experimental and (
) experimental and (