Abstract
Ultra-high-temperature TiC–ZrC composites with submicron structure were synthesized by electro-thermal explosion (ETE) under pressure. The precursors for synthesis were prepared from a mixture containing Ti, Zr, and carbon black powders by high energy ball milling in hexane. The influence of mechanical activation (MA) on the metal crystalline structure was studied. It was shown that the phase composition of the precursor depends on MA duration. The partial amorphization of metal particles occurred after 40 min of MA; while after 90 min, the amorphization was completed. In the last case, carbide phase crystallites with a cubic structure were formed. It was shown that the composite synthesized from precursor activated for 40 min contains Zr0.50Ti0.50C single-phase solid solution with a grain size of 3–5 μm, while the composite synthesized from precursor activated for 90 min consists of Zr0.14Ti0.86C and Zr0.74Ti0.26C phases with a grain size of about 0.2 μm. The Vickers hardness of composites with a residual porosity of no more than 10% was found to be in the range from 11.3 to 18.53 GPa.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 INTRODUCTION
TiC and ZrC with high melting point (3343 and 3693 K, respectively) have low density, high hardness (above 24 GPa), thermal stability, and good corrosion resistance in extreme conditions [1, 2]. Ultra-high-temperature ceramics seem promising for use in metalworking as cutting tools, in thermal protection systems for aerospace applications, and in nuclear power. These carbides with fcc crystal structure dissolve indefinitely in each other, forming TixZr1–xC solid solutions [3]. Recently, much attention has been paid to the preparation and properties of TiC–ZrC solid solution. It was noted in [2] that these solid solutions have higher hardness than their corresponding monocarbides. Single-phase Ti0.9Zr0.1C solid solution was synthesized by spark plasma sintering (SPS) at 2100°C [4, 5]. Studies [6, 7] showed that prolonged annealing of TiC–ZrC solid solution in the range of 1150–1800°C for 100 and 500 h leads to a spinodal decomposition into Ti and Zr-rich nanolayers. The hardness of the resulting lamellar composite was higher than that of TiC and ZrC. As a result of annealing at 1300°C, Ti0.97Zr0.03C and Zr0.94Ti0.06C phases were formed. It was shown in [8] that the (Ti,Zr)C composite synthesized by forced SHS compaction decomposes during annealing at 950°C for 60 min that is accompanied by an increase in the microhardness by 2–4 GPa.
We developed an effective method for producing ultra-high-temperature composites that combines electro-thermal explosion (ETE) and consolidation of hot product under quasi-static compression. In the basis of ETE is heating of reaction mixture of metal and non-metal powders capable of exothermic interaction, as differentiated from SPS where pre-synthesized and chemically inert refractory compounds are used. ETE method offers freedom of intermediate stages for the synthesis of monocarbide powders or their refinement and fractionation.
The present work was aimed at preparing high-temperature TiC–ZrC composites with a submicron structure by ETE under pressure with special emphasis on the study of the effect of mechanical activation duration on the reaction mixture characteristics, phase composition, and microstructure.
2 EXPERIMENTAL
In the experiments, commercial powders of titanium (PTM grade, d < 40 μm), zirconium (PTsrK-1, d < 5 μm), and carbon black (P804-T, d < 0.2 μm) were used as starting reagents. The exothermic synthesis can be represented by the following scheme:
The molar ratio of initial reagents was selected from the equality of TiC and ZrC in Ti0.54Zr0.46C composite. Green mixtures were mechanically activated in hexane (35 mL) for 10 (precursor 1), 40 (precursor 2), and 90 min (precursor 3) using an AGO-2 planetary mill at 2220 rpm.
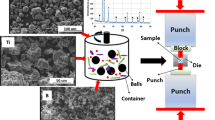
30 g of Ti + Zr mixture with steel balls (240 g, 8 mm in diameter) were placed into a 150-mL drum. The prepared precursor was dried in air at room temperature for 24 h. Then it was placed in a reaction mold with an internal diameter of 21 mm and heated by electric current under a pressure of 100 MPa to ignition temperature. When a reaction was completed, the synthesized product was kept under pressure for 3 s. The schematic showing experimental set up for synthesis of high-temperature composite by ETE under pressure is presented in Fig. 1.
The microstructure of the synthesized product was studied using a Carl Zeiss Ultra Plus scanning electron microscope equipped with an INCA 350 energy dispersive spectrometer.
X-ray diffraction (XRD) analysis was performed using a DRON-3 diffractometer with CuKα radiation. The scanning range 2θ was set from 20° to 80° with a step size of 0.02° and an exposure of 4 s. Quantitative XRD analysis was carried out using the JANA2006 program [9]. The weighted discrepancy factor for all samples was in the range: Rwp = 2–3%. The unit cell parameters were determined by the internal reference method with using Si (NIST SRM 640b) as a marker. The accuracy of determining the composition of carbide phases according to the cell parameters was 0.01–0.02 mol.
The Vickers hardness of composites was measured using a PMT-3 tester under a load of 100 g. The density was determined by hydrostatic weighing using analytical scales with an accuracy of 1 × 10–4 g.
The content of bound carbon was determined by oxidative melting in a ceramic crucible in an induction furnace; the amount of carbon dioxide released by infrared adsorption was determined using a CS600 device. The accuracy of the analysis was 0.01 wt %.
3 RESULTS AND DISCUSSION
3.1 MA of Reaction Mixtures
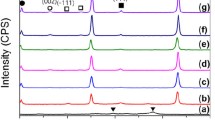
The coarse particle size of initial Ti and Zr powders prevents the synthesis of a single-phase solid solution of titanium and zirconium carbides. To reduce the particle size, the mixture of Ti + Zr powders was subjected to high-energy ball milling in hexane. Figure 2 shows diffractograms of activated Ti + Zr mixtures. The XRD pattern of mixture activated for 10 min is seen to contain reflexes of Ti, Zr, and ZrH2. The presence of ZrH2 in the alloy is caused by its content in the starting zirconium powder. After 40-min MA, complete amorphization of metals occurs. This is pointed out by the broadening of Ti and Zr diffraction peaks, associated with defects of crystal structure and a decrease in coherent scattering regions. MA for 90 min leads to an appearance of low-intensity diffraction peaks of (Ti,Zr)C solid solution. The formation of the carbide phase can be explained by the interaction of Ti and Zr with C, which is released during decomposition of hexane in the MA process [10, 11].
The formation of titanium and zirconium carbides during MA is confirmed by the results of chemical analysis of the powder mixture (Fig. 3). It was found that the content of bound carbon in the Ti + Zr mixture after 90-min MA increases to 4.26 wt %. Thus, it can be concluded that mechanical activation of the Ti + Zr mixture in hexane for 90 min results in amorphous metals and crystallites of the carbide phase.
3.2 ETE Parameters
Figure 4 shows the typical ETE thermogram of precursor 2 at 100 MPa. It is seen that the ETE process includes pre-heating, thermal explosion, and post-processing. Pre-explosion heating is completed by reaching the ignition temperature Tign = 520 K, at which the thermal equilibrium is disrupted. At the second stage, exothermic reactions result in an abrupt increase in the maximal ETE temperature up to Tm = 2350 K. After completion of the exothermic reaction, the final product is cooled from maximum ETE temperature to room temperature. At this stage of the post-process, the microstructure of the composite is completely formed.
ETE parameters are given in Table 1. It is seen that an increase in the MA time results in an increase in the ignition temperature and a decrease in the maximum ETE temperature. This is due to the formation of an inert product (carbide phase). The content of bound carbon in mixtures activated for 40 and 90 min is 3.2 and 4.26 wt %, respectively. The formation of carbides reduces a chemical energy reserve in the system and, as a consequence, the maximal ETE temperature.
3.3 Phase Composition and Microstructure of Composites
Figure 5 shows the XRD patterns of synthesized composites. It is seen that the composite synthesized from precursor 1 contains three carbide phases: Zr0.14Ti0.86C, Zr0.52Ti0.48C, and Zr0.74Ti0.26C. The single-phase solid solution Zr0.52Ti0.48C is formed in the composite derived from precursor 2. In this case, amorphous metals provide high rates of diffusion of carbon atoms and self-diffusion of Ti and Zr atoms. The composite obtained from precursor 3 is seen to contain Zr0.16Ti0.84C and Zr0.73Ti0.27C phases. It was due to an incomplete mutual dissolution of Ti and Zr carbides.
Table 2 shows the content of carbide phases in prepared composites. Differences in phase compositions can be explained by the influence of MA duration and ETE temperature. In case of precursor 2, the high diffusion rate of carbon atoms provided high ETE temperature (3000 K). However, short activation time (10 min) did not allow for a high rate of self-diffusion of Ti and Zr atoms due to an insufficient concentration of crystal lattice defects because there is no amorphization of metals. Low ETE temperature (1600 K) for precursor 3 prevented the complete solubility of Ti and Zr carbides. This is confirmed by low content of Zr0.48Ti0.52C phase and high content of Zr0.16Ti0.84C and Zr0.73Ti0.27C phases. Note that the formation of Zr0.16Ti0.84C and Zr0.73Ti0.27C phases is not associated with the spinodal decomposition of the solid solution Zr0.50Ti0.50C.
It was demonstrated in [4] that the decomposition of (Ti,Zr)C solid solution occurs via the spinodal mechanism after 500 h of annealing at 1300°C. In this work, the synthesized composite was subjected to no additional heat treatment. The time of exothermic synthesis of (Ti,Zr)C solid solution during ETE (5 ms) is much shorter than the annealing time, at which the decomposition takes place [12].
The data obtained are confirmed by the results of microstructural analysis (Fig. 6). It can be seen that the composite synthesized from precursor 2 consists of a single-phase solid solution Ti0.5Zr0.5C with a grain size of 3–5 μm (Fig. 6a). While the fine structured composite synthesized from precursor 3 contains two ZrC and TiC based solid solutions (Fig. 6b) with a grain size of about 0.2 μm.
3.4 Mechanical Characteristics of Composites
Table 3 presents the mechanical properties of synthesized composites. It is seen that the composite synthesized from precursor 3 has the highest hardness. This is explained by the formation of a submicron microstructure (Fig. 6b). For comparison, Table 4 collects data for TiC–ZrC composites prepared by different methods [8, 12–17].
4 CONCLUSIONS
Ultra-high-temperature composites based on TiC–ZrC with a submicron structure can be successfully synthesized in one stage by ETE under pressure from mixtures mechanically activated in hexane. The phase composition of composites is shown to be controlled by the duration of mechanical activation and ETE temperature. The fine structured composite with an average grain size smaller than 0.2 µm is synthesized from precursor activated for 60 min. It is worth noting that the fine structured composite is obtained directly during exothermic synthesis by ETE under pressure, without the need for a long-term annealing stage. Thus, the ETE under pressure is an effective technique to synthesize ultra-high-temperature TiC–ZrC composites with submicron structure.
REFERENCES
Shabalin, I.L., Ultra-High Temperature Materials II. Refractory Carbides I (Ta, Hf, Nb and Zr Carbides), Dordrecht: Springer, 2019. https://doi.org/10.1007/978-94-024-1302-1
Hollek, H., Material selection for hard coatings, J. Vac. Sci. Tech. A, 1986, vol. 4, no. 6, pp. 2661–2669. https://doi.org/10.1116/1.573700
Cedillos-Barraza, O., Grasso, S., Al Nasiri, N., Jayaseelan, D., Reece, M., and Lee, W., Sintering behaviour, solid solution formation and characterization of TaC, HfC, and TaC–HfC fabricated by spark plasma sintering, J. Eur. Ceram. Soc., 2016, vol, 36, pp. 1539–1548. https://doi.org/10.1016/j.jeurceramsoc.2016.02.009
Li, Y., Katsui, H., and Goto, T., Phase decomposition of TiC−ZrC solid solution prepared by spark plasma sintering, Ceram. Int., 2015, vol. 41, no. 10, pp. 14258–14262. https://doi.org/10.1016/j.ceramint.2015.07.055
Li, Y., Katsui, H., and Goto, T., Effect of heat treatment on the decomposition of TiC–ZrC solid solutions by spark plasma sintering, J. Eur. Ceram. Soc., 2016, vol. 36, no. 15, pp. 3795–3800. https://doi.org/10.1016/j.jeurceramsoc.2016.01.039
Borgh, I., Hedström, P., Blomqvist, A., Ågren, J., and Odqvist, J., Synthesis and phase separation of (Ti,Zr)C, Acta Mater., 2014, vol. 66, pp. 209–218. http://dx.doi.org/doi:10.1016/j.actamat.2013.11.074
Ma, T., Hedström, P., Ström, V., Masood, A., Borgh, I., Blomqvist, A., and Odqvist, J., Self-organizing nanostructured lamellar (Ti,Zr)C - A superhard mixed carbide, Int. J. Refr. Met. Hard Mater., 2015, vol. 51, pp. 25–28. https://doi.org/10.1016/j.ijrmhm.2015.02.010
Levashov, E., Kurbatkina, V., Zaitsev, A., Rupasov, S., Patsera, E., Chernyshev, A., Zubavichus, Ya., and Veligzhanin, A., Structure and properties of precipitation-hardening ceramic Ti–Zr–C and Ti–Ta–C materials, Phys. Met. Metallogr., 2010, vol. 109, pp. 102–112. https://doi.org/10.1134/S0031918X10010102
Petříček, V., Dušek, M., and Palatinus, L., Crystallographic computing system JANA2006: General features, Z. Kristallogr. Cryst. Mater., 2014, vol. 229, no. 5, pp. 345–352. https://doi.org/10.1515/zkri-2014-1737
Eremina, M.A., Lomaeva, S.F., Burnyshev, I.N., Kalyuzhnyi, D.G., and Konygin, G.N., Titanium carbohydride synthesis by mechanical activation in liquid hydrocarbon, Russ. J. Inorg. Chem., 2018, vol. 63, no. 10, pp. 1274–1282. https://doi.org/10.1134/S0036023618100066
Kozawa, T., Fukuyama, K., Kushimoto, K., Ishihara, S., Kano, J., Kondo, A., and Naito, M., Effect of ball collision direction on a wet mechanochemical reaction, Sci. Rep., 2021, vol. 11, no. 1, p. 210. https://doi.org/10.1038/s41598-020-80342-w
Yung, L., Kollo, L., Hussainova, I., and Žikin, A., Reactive sintering of ZrC–TiC composites, Key Eng. Mater., 2012, vol. 527, pp. 20–25. https://doi.org/10.4028/www.scientific.net/KEM.527.20
Li, Y., Katsui, H., and Goto, T., Spark plasma sintering of TiC–ZrC composites, Ceram. Int., 2015, vol. 41, pp. 7103–7108. https://doi.org/10.1016/j.ceramint.2015.02.019
Shaocun, L., Wentao, H., Jianyong, X., Fusheng, W., Bo, X., Dongli, Y., Julong, H., Yongjun, T., and Zhongyuan, L., Mechanical properties of nanocrystalline TiC–ZrC solid solutions fabricated by spark plasma sintering, Ceram. Int., 2014, vol. 40, no. 7, pp. 10517–10522. https://doi.org/10.1016/j.ceramint.2014.03.024
Niu, B., Zhang, F., Ji, W., Zhang, J., Wang, H., and Weimin, W., Effect of solid solution formation on densification of spark plasma sintered ZrC ceramics with TiC as sintering aid, Adv. Appl. Ceram., 2015, vol. 115, pp. 55–59. https://doi.org/10.1179/1743676115Y.0000000037
Mroz, C., Processing and properties of microcomposite TiZrC and TiZrB2 materials, Proc. 17th Annu. Conf. on Composites and Advanced Ceramic Materials, 1993, vol. 4, no. 9, pp. 725–735. https://doi.org/10.1002/9780470314234.ch5
Umalas, M., Hussainova, I., Reedo, V., Young, D.L., Cura, E., Hannula, S.P., Lohmus, R., and Lohmus, A., Combined sol–gel and carbothermal synthesis of ZrC–TiC powders for composites, Mater. Chem. Phys., 2015, vol. 153, pp. 301–306. https://doi.org/10.1016/J.MATCHEMPHYS.2015.01.017
ACKNOWLEDGMENTS
This work was carried out by using modern scientific instruments available for multiple access at ISMAN.
Funding
The work was financially supported by the state research program for ISMAN.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Shcherbakov, V.A., Semenchuk, I.E., Gryadunov, A.N. et al. Synthesis of TiС–ZrC Composite with Submicron Structure by Electro-Thermal Explosion under Pressure. Int. J Self-Propag. High-Temp. Synth. 31, 261–267 (2022). https://doi.org/10.3103/S1061386222050077
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1061386222050077