Abstract
When studying a preparation of the isolated spinal cord segment of an adult frog, damaged and intact lumbar motoneurons were found to differ significantly in the membrane potential, input resistance and the action potential properties (amplitude, duration, fast and medium phases of the afterhyperpolarization, and the frequency of spikes). Serotonin (5-HT) reduced the amplitude of afterpolarization and increased the frequency of the spikes of the intact neurons, while in the damaged motoneurons, 5-HT increased the amplitude of afterpolarization and had no effect on the frequency of discharges.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The role of serotonin in the motor function recovery after the spinal cord injury is currently confirmed by increasingly much evidence [1, 2]. In healthy brain, serotonergic fibers descending from the nuclei raphe make direct contacts with motoneurons [3, 4]. In brain injuries, serotonin delivery to the spinal cord motor nuclei is disrupted, and dendrites of both motorneurons and premotor interneurons are damaged [5, 6]. At the same time, the bulk of synapses, including those modulated by serotonin, is located on these neurons. Dendrite damage may or may not lead to motoneuron death, but the functional properties of motoneurons may be affected. These changes are important to know and take into account in developing proper treatment of the spinal cord injuries.
We aimed at comparing the electrophysiological properties of the damaged and intact motoneurons and at studying the modulating effect of serotonin on them.
Studying the electrophysiological properties of the spinal cord motoneurons remains a challenging task because of low viability of the spinal-cord thin sections of adult mammals [5, 6]. In this study, we used an isolated preparation of the spinal cord lumbar segment of an adult frog (Rana ridibunda). The preparation technique has been earlier described in detail [7, 8]. Frog spinal cord preparations have some advantages over those obtained from mammals. First, the frog spinal neurons are less prone to cell death at hypoxia. Second, the damaged and intact motoneurons can be readily and distinctly obtained in the same preparation owing to specific morphology of these cells. The lumbar motoneuron is the largest cell of the frog spinal cord. It has an extremely branched dendrite tree with dendrites extending in the rostro-caudal direction over a distance of up to 2 mm [9]. In our experiments, we used frontal 2- to 3-mm-thick slices (Fig. 1). At this thickness, mainly intact neurons remain in the middle of a slice.
Scheme of the experiment. The isolated spinal-cord lumbar segment of the Rana ridibunda frog (a frontal 2- to 3-mm-thick section); IM, intact motoneuron located at a depth of 300–1200 μm from the rostral surface of the slice; DM, damaged motoneuron with partially cut-off dendrites that is located at a depth of down to 300 μm.
A solution of the following composition was used for perfusion (mM): NaCl, 100; KCl, 2; MgCl2, 0.5; glucose, 5.5; CaCl2, 1.5; NaHCO3, 9; Tris-HCl buffer, 2; рН 7.4–7.6, aerated with a gas mixture (98% О2 and 2% СО2). It had a temperature of 16–18°С. Serotonin (10 μM) and tetrodotoxin (TTX, 1 μM), which blocks sodium channels, were added into the perfusion solution.
Intracellular potentials were measured with sharp glass microelectrodes having a tip diameter of 1–1.5 μm and resistance of 10–20 MΩ; they were filled with a KCl solution (3 М). The potentials were recorded using a differential microelectrode amplifier, digitized at a frequency of 10–20 kHz using ADC NIUSB-6211 (National Instruments, United States) and the WinWCP computer software (Strathclyde Electrophysiology Software, United Kingdom). Motoneurons were identified by the antidromic action potential (AP) arising in response to the ventral root stimulation.
In statistical analysis and plotting, SigmaPlot 11.0 and MS Excel software were used. Significant differences between the motoneuron groups were assessed using unpaired Student’s t test; the 5-HT effect was measured using the paired t test.
To determine the area of damaged motoneurons within a 2-mm-thick brain slice, the antidromic field potential caused by the ventral root stimulation was measured during the mcroelectrode vertical movement down from the rostral surface and along the motoneuron column with a step of 100 μm (Fig. 2). Both the amplitude and the area of the antidromic field potential were found to be significantly less in the rostral and caudal parts (0–300 and 1700–2000 μm, respectively) than in the middle of a slice (400–1600 μm), because the motoneuron dendrites were cut-off in the rostral/caudal layers. The average areas under the curves of the antidromic field potentials were 0.26 ± 0.10 (hereinafter, M ± m, n = 5) and 0.5 ± 0.2 mV ms in the surface and depth of a slice, respectively (p < 0.05).
Examples of the antidromic field potential recorded in locations of motoneurons with partially damaged (top) and intact (bottom) structure. Figures indicate the depth of microelectrode immersion from the rostral surface of a 2-mm-thick slice. Individual ranges are shown in gray; averaging of 30 ranges, in black.
Based on this observation, we divided all of the motoneurons studied into two groups: intact motoneurons (IM), whose soma was located at a depth of 300–1200 μm (n = 13), and damaged motoneurons (DM) located at a depth of down to 300 μm from the slice surface (n = 7). The resting potential of IM was lower than that of DM (–67.2 ± 1.6 mV and –55.0 ± 1.4 mV, respectively; p < 0.01). The IM input resistance was 7.3 ± 0.7 MΩ, while that of DM was 2.5 times higher (17.1 ± 2.5 MΩ, p < 0.01), because many dendrites were cut off during manipulations, and, hence, the membrane surface area of DM was smaller. The highest antidromic spike amplitude measured from the resting potential to peak, was 60 ± 3 mV for DM and 25% higher for IM (75.1 ± 1.9 mV, p < 0.01). The fast and medium afterhyperpolarization phases (fAHP and mAHP) were higher in IM (–12.9 ± 1.2 mV and –3.0 ± 0.2 mV,), than in DM (–8.4 ± 0.8 mV and –0.8 ± 0.1 mV, respectively, p < 0.05). The half-widths of AP values (0.8 ± 0.3 ms in IM and 1.6 ± 0.1 ms in DM) also differed significantly (p < 0.05).
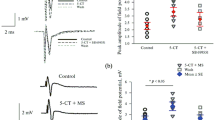
Eight IM and six DM were examined to study the effect of 5-НТ (10 μM). 5-НТ added into the perfusion solution caused a slight depolarization for 3–5 min (by 3.4 ± 0.5 mV in IM and 2.0 ± 0.3 mV in DM), which retained under TTX blocking of spike activity. The antidromic AP amplitude and half-width remained almost unchanged under the effect of 5-НТ in both IM and DM. In contrast, a significant and differently directed 5-НТ effect on afterhyperpollarization was observed in both IM and DM. In IM, 5-НТ reduced the fAHP amplitude by 33% from –12.9 ± 1.2 mV to –8.6 ± 1.5 mV (p < 0.05), while the mAHP amplitude was reduced by 43% from –3.0 ± 0.2 mV to –1.7 ± 0.5 mV (p < 0.05, Fig. 3a). In DM, 5-HT addition increased fАНР amplitude one and a half times from –8.4 ± 0.8 mV to –12.3 ± 1.1 mV (p < 0.01), while mAHP amplitude increased by 62% from –0.8 ± 0.1 mV to –1.3 ± 0.2 mV (p < 0.05, Fig. 3b). The nearer were DM to the rostral surface of a slice, the larger was fAHP increase caused by 5-НТ, i.e., this parameter depended on the degree of cell dendrite damage.
The effect of 5-HT on fAHP and mAHP of the antidromic AP within (a) intact and (b) damaged motoneurons. Control (black color) and 5-HT applications at doses of 5 and 10 μM (light gray and dark gray color, respectively). (b) Diagrams that illustrate a significant decrease of both АНР phases after 5-HT application within IM (n = 8) and their increase within DM (n = 6), M ± m. * p < 0.05, ** p < 0.01.
Since changes in AHP have an effect on the spike frequency [10], we have determined how the number of antidromic AP caused by a single stimulation of the ventral root varies after 5-НТ addition to the perfusion solution. 5-HT increased the number of spikes recorded for 1 s from 2.1 ± 0.5 to 8.2 ± 0.4 in IM, while in DM the number of spikes remained unchanged (Fig. 4).
Thus, the results of our study suggest that motoneuron properties and the effect of neuromodulators on them, such as 5-HT, in particular, may be drastically changed after injuries. In IM, 5-НТ reduced mAHP, which has been demonstrated on various animal species: guinea pig, mice, turtles [10, 11, 13]. mAHP phase depends on activity of the apamin-sensitive Са2+-dependent K+ channels [10, 14]. A decrease in mAHP reduces the spike accommodation, and the motoneuron is discharged longer and with a higher frequency [12]. Thus, the settings of motoneuron output depend on mAHP modulation [13]. Of interest is that 5-НТ had the opposite effect on DM. The fAHP and mAHP amplitudes increased, while the frequency of discharges did not. Perhaps, weakening of DM activity prevents their death.
The specific molecular mechanisms involved in this DM response remain unclear. No evidence is available on the effect of 5-НТ on DM; therefore, further experiments are required.
Differences in AP parameters of the intact and partially damaged motoneurons, which were shown on thin slices, should be taken into account because of possible damage of the big branched cells.
ACKNOWLEDGMENTS
This study was a part of the State Contract with the Russian Agency of Scientific Organizations (FASO) under the Programs of Basic Scientific Research of the State Academies of Sciences-63 (PBSR SAS-63), topic “Neurophysiologic Mechanisms of the Function Regulation and Evolution”, no. АААА-А18-118012290372–0.
The study was in part supported by the Russian Foundation for Basic Research, project no. 18-04-00247.
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
REFERENCES
Schmidt, B.J. and Jordan, L.M., Brain Res. Bull., 2000, vol. 53, no. 5, pp. 689–710.
Murray, K.C., Stephens, M.J., Ballou, E.W., et al., J. Neurophysiol., 2011, vol. 105, pp. 731–748.
Alvarez, F.J., Pearson, J.C., Harrington, D., et al., J. Comp. Neurol., 1998, vol. 393, pp. 69–83.
Xia, Y., Chen, D., Xia, H., et al., Neurosci. Let., 2017, vol. 649, pp. 70–77.
Davies, M.L., Kirov, S.A., and Andrew, R.D., J. Neurosci. Methods, 2007, vol. 166, pp. 203–216.
Carp, J.S., Tennissen, A.M., Mongeluzi, D.L., et al., J. Neurophysiol., 2008, vol. 100, pp. 474–481.
Kalinina, N.I., Kurchavyi, G.G., Zaitsev, A.V., and Vesselkin, N.P., J. Evol. Biochem. Physiol., 2016, vol. 52, no. 5, pp. 359–368.
Kalinina, N.I., Zaitsev, A.V., and Vesselkin, N.P., J. Comp. Physiol. A, 2018, vol. 204, no. 3, pp. 329–337.
Dityatev, A.E., Chmykhova, N.M., Dityateva, G.V., et al., J. Comp. Neurol., 2001, vol. 430, pp. 433–447.
Diaz-Rios, M., Dombeck, D.A., Webb, W.W., and Harris-Warrick, R.M., J. Neurophysiol., 2007, vol. 98, pp. 2157–2167.
Hsiao, C.F., Trueblood, P.R., Levine, M.S., and Chandler, S.H., J. Neurophysiol., 1997, vol. 77, pp. 2910–2924.
Miles, G.B. and Sillar, K.T., Physiology, 2011, vol. 26, pp. 393–441.
Perrier, J-F., Rasmussen, H.B., Christensen, R.K., and Petersen, A.V., Curr. Pharm. Des., 2013, vol. 19, no. 24, pp. 4371–4384.
Sah, P., TIN, 1996, vol. 19, no. 4, pp. 150–154.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by A. Nikolaeva
Rights and permissions
About this article
Cite this article
Kalinina, N.I., Zaitsev, A.V. & Vesselkin, N.P. Serotonin Modulates Differently the Functional Properties of Damaged and Intact Motoneurons in the Frog Spinal Cord. Dokl Biol Sci 484, 5–9 (2019). https://doi.org/10.1134/S0012496619010046
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0012496619010046