Abstract
Endogenous monoamine 5-hydroxytryptamine (5-HT, serotonin) is a phylogenetically ancient neurotransmitter present in vertebrates. The functions of 5-HT in central nervous system are intensively studied; however, the presynaptic effects of 5-HT in frog spinal motoneurons are practically unexplored. We have previously shown that 5-HT decreases the frequency of glycinergic miniature inhibitory postsynaptic potentials (mIPSPs), but does not affect the frequency of GABAergic mIPSPs and increases the frequency of glutamatergic postsynaptic potentials. In the present study, using pharmacological methods and intracellular recordings in motoneurons from an adult frog’s isolated spinal cord, we aimed to identify the 5-HT receptor subtype responsible for inhibiting the release of glycine. Аn agonist of 5-HT1A and 5-HT7 receptors, 8-OH-DPAT, and a selective agonist of 5-HT2 receptors, α-Ме-5-НТ, did not show any significant effect on inhibitory transmission, indicating that 5-HT1A, 5-HT2, and 5-HT7 receptors are not involved in the modulation of glycine release in the adult frog spinal cord. An agonist of 5-HT1B/D receptors sumatriptan decreased the frequency (but not the amplitude) of glycinergic mIPSPs similar to 5-HT. An antagonist of 5-HT1,2 receptors, methysergide, abolished the effect of sumatriptan. Together our results suggest that 5-HT inhibits the release of glycine by activation of 5-HT1B/D receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endogenous monoamine 5-hydroxytryptamine (5-HT, serotonin) is a phylogenetically ancient neurotransmitter present in vertebrates (Jacobs and Azmitia 1992). Widely distributed in the central nervous system, 5-HT contributes to several essential physiological functions, such as sleep and wake cycle, affective state, appetite regulation, learning and memory, pain modulation, sexual arousal, fatigue, and motor control (Jacobs and Azmitia 1992; Ciranna 2006; Perrier et al. 2013). In the spinal cord and brainstem, 5-HT can both activate and modulate locomotor networks (Miles and Sillar 2011).
In vertebrates, most serotonergic neurons are located in the reticular formation, in particular in the raphe nuclei. The serotonergic neurons project to the dorsal horn and the motor nuclei of the spinal cord (Tan and Miletic 1990a; Ridet et al. 1994; Woolston et al. 1994; Alvarez et al. 1998; Perrier et al. 2013). Serotonergic neurons have also been detected in the spinal cord of rats, both in autonomic regions and in lamina VII and X (Newton et al. 1986; Zhang 2015). Therefore, in mammalians and probably in other vertebrates, 5-HT may also be produced in the spinal cord.
Few studies have investigated the effect of 5-HT on neurons in the spinal cord of amphibians. For example, bath-applied 5-HT changes the excitability of dorsal root afferent fibers (Holz et al. 1985; Holohean et al. 1990b; Ovsepian and Vesselkin 2004), interneurons (Tan and Miletic 1990b), and motoneurons (Cardona and Rudomin 1983; Holohean et al. 1990a) leading to the decrease of evoked polysynaptic spinal reflex (Holohean et al. 1992). Ovsepian and Vesselkin (2006) investigated the effect of exogenously applied 5-HT on monosynaptic segmental and descending evoked responses in frog lumbar motoneurons. They showed depression of the segmental excitatory postsynaptic potentials (EPSPs) by 5-HT and no effect on the descending monosynaptic EPSPs conditioned by ventrolateral column stimulation (Ovsepian and Vesselkin 2006). The presynaptic action of 5-HT on the spinal motoneurons of the adult frog is practically unexplored. In our previous study (Kalinina et al. 2016), we showed that 5-HT reduces the frequency of glycinergic miniature inhibitory postsynaptic potentials (mIPSPs) without exerting a noticeable effect on the frequency of GABAergic mIPSPs. However, it is not known what subtypes of 5-HT receptors are involved in the regulation of glycine release from presynaptic terminals. There are 14 genetically, pharmacologically, and functionally different 5-HT receptors belonging to 7 families, termed 5-HT1 through 5-HT7 (Fink and Göthert 2007; Hannon and Hoyer 2008). Except for 5-HT3 receptors, which are the ligand-controlled ion channels, all other 5-HT receptors are metabotropic. Spinal neurons of mammals express 5-HT1A,B, 5-HT2A,B,C, 5-HT5A, and 5-HT7 receptors (Helton et al. 1994; Ridet et al. 1994; Rekling et al. 2000; Xu et al. 2007; Perrier et al. 2013).
We could not find immunohistochemical data on the presence and distribution of subtypes of 5-HT receptors in the spinal cord of amphibians. Therefore, the purpose of this study is to identify the possible subtypes of 5-HT receptors participating in the modulation of spontaneous inhibitory synaptic activity in the lumbar motoneurons of the adult frog Rana ridibunda.
Methods
Animals and preparation of spinal cord sections
The experiments were carried out on the frog Rana ridibunda (weight 120–170 g, n = 27 animals). Experiments were performed in accordance with the protocols approved by the Animal Care Committee of the Sechenov Institute of Evolutionary Physiology and Biochemistry of the Russian Academy of Sciences to minimize animal use and suffering. Frogs were deeply anesthetized with diethyl ether, killed by decapitation, and spinal cords were isolated. After removing the meninges, segments IX and X were separated along with their roots in frontal section 2–3 mm thick. The thickness of the frontal section was chosen to preserve the motoneurons intact, as frog motoneurons have prolonged dendrites that extend to a distance of 2 mm in the rostrocaudal direction (Dityatev et al. 2001). The preparation procedure, chamber layout, and experimental design have been previously described in detail (Kurchavyi et al. 2005).
Electrophysiology
The perfusion solution contained (in mM): 100 NaCl, 2 KCl, 0.5 MgCl, 5.5 glucose, 1.5 CaCl2, 9 NaHCO3, and 2 Tris (pH 7.4–7.6), and was aerated with a gas mixture (98% O2 and 2% CO2). The flow rate was 6 ml/min, and the bath volume was 0.5 ml. Experiments were performed at a temperature of 16–18 °C. Postsynaptic potentials in the motoneurons were recorded intracellularly using glass microelectrodes with a resistance of 10–20 MΩ, filled with 3 M KCl. Motoneurons were identified by the antidromic action potentials evoked by electrical stimulation of the ventral root (Fig. 1). Potentials were registered using a microelectrode differential amplifier with a bandwidth up to 5 kHz, digitized at a frequency of 10–20 kHz using an ADC NI USB-6211 (National Instruments, USA), and recorded using a WinWCP computer program (Strathclyde Electrophysiology Software, UK).
To record the miniature postsynaptic potentials (mPSPs), tetrodotoxin (TTX, 1 μM), a sodium channel blocker, was added to the perfusion solution. The TTX block was confirmed by the disappearance of the evoked EPSP in motoneurons after stimulation of the dorsal root. To pharmacologically isolate the inhibitory fraction of mPSPs (mIPSP), antagonists of the AMPA and NMDA receptors (6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 20 μM; D-AP5, 40 μM, respectively) were added in a perfusion solution. Then, GABAergic and glycinergic mIPSPs were isolated by the addition of either strychnine (2 μM) or gabasin (20 μM), respectively.
Spontaneous/miniature PSPs were recorded for 100–150 s. Peak events were first detected automatically using a generated template response with Clampfit 10.2 (Axon Instruments, USA). The average RMS noise for the mIPSPs was ∼ 0.1 mV (± 0.05 mV). Therefore, the minimum amplitude of the detected events was more than 0.075 mV. After automatic analysis, events were re-checked by visual inspection of the traces and were accepted for analysis if they had a monophasic rising phase and decayed to baseline exponentially. Between 100 and 3000 synaptic events in each motoneuron were included in the analysis. Amplitudes of miniature responses were determined from baseline to peak. The rise time was estimated as the time necessary to rise between 10 and 90% of the peak response and the decay time as the time needed to decay between 90 and 10% of the peak response. The frequency of events was also analyzed.
The effects of 5-НТ (30 μM, Sigma–Aldrich) and several subtype-selective agonists and an antagonist on the properties of mIPSPs were tested: (1) (±)-8-Hydroxy-2-(dipropylamino)tetralin hydrobromide ((±)-8-OH-DPAT, 10 μM, Sigma-Aldrich), a 5-HT1A and 5-HT7 receptor agonist; (2) 3-[2-(dimethylamino)ethyl]-N-methyl-1H-indole-5-methanesulfonamide succinate (sumatriptan succinate, 10 μM, Sigma-Aldrich), a 5-HT1B and 5-HT1D receptor agonist; (3) α-methyl-5-hydroxytryptamine maleate (α-Ме-5-НТ, 10 μM, Tocris Bioscience), a 5-HT2 receptor agonist with high affinity for subtypes 5-HT2B, 5-HT2A, and 5-HT2C; and (4) [8β(S)]-9,10-didehydro-N-[1-(hydroxymethyl)propyl]-1,6-dimethylergoline-8-carboxamide maleate (methysergide maleate, 10 μM, Tocris Bioscience), a mixed 5-HT1/5-HT2 receptor antagonist. The substance concentrations were chosen based on published data (Holohean and Hackman 2004; Perrier et al. 2013).
Statistical analysis
Statistical analysis and plotting were conducted using SigmaPlot 12.5 software (SYSTAT Software; San Jose, CA, USA). The Student’s t test was used to evaluate statistically significant differences between the values. Differences were considered as statistically significant at p ≤ 0.05. The results were expressed as a mean ± standard error of the mean.
Results
The effects of 5-HT and 5-HT1,2 agonists on mIPSP properties in frog motoneurons
Miniature IPSPs were recorded in frog spinal motoneurons in the presence of sodium channel blocker TTX (1 μM) and AMPA and NMDA glutamate receptor blockers CNQX (20 μM) and D-AP5 (40 μM), respectively (Fig. 2). Miniature IPSPs were registered in 46 motoneurons with a stable resting membrane potential (RMP) ranging from − 65 to − 75 mV. In our experimental conditions, the recorded mIPSPs were depolarizing at the RMP. This is due to the increase in [Cl−]i because of Clˉ diffusion from the microelectrode, which increases the reversal potential of Clˉ above RMP.
The effects of sumatriptan (Sum) on mIPSP properties in frog motoneurons. a Representative examples of mIPSPs in control conditions (black line) and after application of sumatriptan (10 μM, gray line). Inter-event interval (b) and amplitude (c) distributions of mIPSPs in cumulative forms for a representative motoneuron
A short application of 5-HT (30 μM, 5 min) decreased the frequency of mIPSPs by 22%, from 5.9 ± 0.4 to 4.6 ± 0.7 Hz (n = 5; paired t test, p < 0.05, Fig. 3a). Application of sumatriptan, a 5-HT1B/D receptor agonist, reduced the frequency of mIPSPs by 44%, from 6.1 ± 0.5 to 3.4 ± 0.4 Hz (n = 8; paired t test, p < 0.05, Figs. 2a, b, 3a). Application of (±)-8-OH-DPAT (10 μM), a selective agonist of 5-HT1A and 5-HT7 receptors, and α-Ме-5-НТ (10 μM), a selective agonist of 5-HT2 receptors, did not significantly change the mIPSP frequency (less than 10%, p > 0.05, Fig. 3a). None of the drugs changed the average amplitude (Figs. 2c, 3b) or kinetic parameters of mIPSPs.
The effects of 5-HT and 5-HT1,2 agonists on mIPSP frequency (a) and amplitude (b) in frog motoneurons. Statistically significant differences from the control value are marked with the asterisks (*p < 0.05 according to a paired t test). Sum sumatriptan (10 μM), a 5-HT1B/D receptor agonist, α-Me α-Ме-5-НТ (10 μM), a selective agonist of 5-HT1A receptors, 8-OH-DPAT (±)-8-OH-DPAT (10 μM), a selective agonist of 5-HT1A and 5-HT7 receptors
The effects of 5-HT and 5-HT1,2 agonists on GABAergic and glycinergic mIPSPs
Application of 5-HT or 5-HT receptor agonists (sumatriptan, (±)-8-OH-DPAT, α-Ме-5-НТ) in the presence of strychnine (2 μM) does not change the frequency of the GABAergic mIPSPs (Fig. 4a). In contrast, after application of 5-HT, the frequency of glycinergic mIPSPs recorded in the presence of GABAA receptor blocker gabazine (20 μM) decreased by 28% (from 3.9 ± 1.1 to 2.8 ± 0.4 Hz, n = 5; paired t test, p < 0.05, Fig. 4a). Sumatriptan also reduced the frequency of glycinergic mIPSPs by 55% (from 4.2 ± 0.5 to 1.9 ± 0.2 Hz, n = 5; paired t test, p < 0.05, Fig. 4a). The presence of 8-OH-DPAT (10 μM) and α-Me-5-HT (10 μM) did not affect the frequency of the glycinergic mIPSPs (Fig. 4a). No significant changes in the amplitude and the kinetic parameters of glycinergic or GABAergic mIPSPs were observed after application of either agonist (Fig. 4b, c).
The effects of 5-HT1,2 receptor antagonist methysergide maleate on properties of the glycinergic mlPSPs
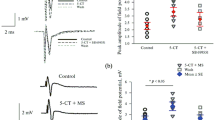
Application of 5-HT1/5-HT2 receptor antagonist methysergide maleate (10 μM) reduced the effect of 5-HT1B and 5-HT1D receptor agonist sumatriptan on glycinergic mIPSPs (Fig. 5). After application of sumatriptan, the frequency of glycinergic mIPSPs increased from 2.0 ± 0.3 to 3.5 ± 0.4 Hz (n = 6) and reached 78% of the control value (4.2 ± 0.2 Hz).
The effects of 5-HT1,2 receptor antagonist methysergide maleate on frequency (b) and amplitude (c) of the glycinergic mlPSPs. a A representative example of mIPSPs recorded in a frog motoneuron in a solution containing TTX (1 μM), CNQX (20 μM), d-AP5 (40 μM) and gabazine (20 μM) (upper trace), after application of sumatriptan (Sum, 10 μM, middle trace), after application of methysergide (Mserg, 10 μM, lower trace). Statistically significant difference from the control value is marked with the asterisk (*p < 0.05 according to a paired t test)
Discussion
In the present study, using pharmacological methods and intracellular recordings in motoneurons from an adult frog’s isolated spinal cord, we aimed to identify the 5-HT receptor subtype responsible for inhibiting the release of glycine. Аn agonist of 5-HT1A and 5-HT7 receptors, 8-OH-DPAT, and a selective agonist of 5-HT2 receptors, α-Ме-5-НТ, did not show any significant effect on inhibitory transmission, indicating the 5-HT1A, 5-HT2, and 5-HT7 receptors are not involved in the modulation of glycine release in the adult frog’s spinal cord. An agonist of 5-HT1B/D receptors sumatriptan decreased the frequency (but not the amplitude) of glycinergic mIPSPs similar to 5-HT. An antagonist of 5-HT1,2 receptors, methysergide, abolished the effect of sumatriptan. Based on the results of electrophysiological experiments in which selective agonists and antagonists of various 5-HT receptor subtypes have been used, we assume that 5-HT inhibits the release of glycine by activation of 5-HT1B/D receptors.
The expression of 5-HT1B/D receptors in the spinal cord
There are few studies of serotonergic innervation in the frog spinal cord, and knowledge about the expression of different types of 5-HT receptors is scarce. In general, the organization of bulbospinal serotonergic pathways in frog is similar to that of mammals. The spinal cord neurons receive serotonergic innervation from the bulbar raphe nuclear region (Tan and Miletic 1990a; Woolston et al. 1994). Also, 5-HT immunopositive descending fibers have been seen terminating in the dorsal and ventral horns, as well as in the intermediate gray matter (Tan and Miletic 1990a). The expression of 5-HT receptors in the mammalian CNS has been studied with different techniques, including immunohistochemistry, pharmacology, in situ hybridization, and expression of fluorescent proteins under the promoter of the gene of one particular receptor (Perrier et al. 2013). The 5-HT1B/D receptors expressed in the CNS are concentrated in the basal ganglia, striatum, and frontal cortex. In the spinal cord, only low concentrations of 5-HT1B,D receptors have been found (Palacios 2016). Using mice expressing GFP driven by the 5-ht1d promotor, Enjin et al. (2012) showed that 5-HT1D receptors are specifically expressed by γ-motoneurons (innervating muscle spindles) and proprioceptive sensory neurons, but not in α-motoneurons (innervating skeletal muscle fibers). The 5-HT1B/D receptors serve as terminal autoreceptors controlling the release of not only 5-HT itself, but also of other neurotransmitters, such as acetylcholine, glutamate, dopamine, noradrenaline, and GABA (Pauwels 1997).
The functions of 5-HT receptors in the spinal cord
We found that 5-HT and sumatriptan inhibited the frequency of glycinergic mIPSPs but not of GABAergic mIPSPs. In our previous study (Kalinina et al. 2016), we showed that 5-HT increased the frequency of glutamatergic mEPSPs. These findings suggest that different subtypes of serotonergic receptors are unevenly distributed between glutamatergic, glycinergic, and GABAergic terminals in frog spinal cord. The decrease in frequency of miniature glycinergic mIPSPs by 5-HT1B/D receptors revealed in the present study is consistent with Jacobs and Fornal’s hypothesis that the primary functions of the 5-HT system in the brain are to facilitate motor output and concurrently inhibit sensory information processing (Jacobs and Fornal 1993; Jacobs et al. 2002). Our results are in line with data obtained in a study of Xenopus tadpoles (McDearmid et al. 1997), where the authors showed that glycinergic inhibitory potentials occurring mid-cycle in motoneurons during swimming activity were reduced by 5-HT. However, another study showed that in spinal motoneurons from immature (12- to 20-day-old) rats, 5-HT inhibits dorsal-root evoked excitatory and inhibitory postsynaptic responses via presynaptic 5-HT1 receptors (Wu et al. 1991). The effects of 5-HT on inhibitory synaptic transmission in the substantia gelatinosa of the rat spinal cord are significantly different. The frequencies of GABAergic and glycinergic mIPSCs were both enhanced after application of 5-HT; however, the amplitude of mIPSCs was not affected. Using pharmacological methods, Xie et al. (2012) found that 5-HT modulated the inhibitory transmission in substantia gelatinosa by the activation of 5-HT2A and 5-HT2C receptor subtypes located predominantly at inhibitory interneuron terminals, and of 5-HT3 receptors located at inhibitory interneuron terminals and soma-dendrites, consequently enhanced both the frequency and amplitude of IPSCs. The 5-HT1B receptor-mediated inhibition of glycinergic eIPSCs has also been shown in rat hypoglossal motoneurons (Berger and Huynh 2002). Also, in a study of neonatal rat, 5-HT (10 µM) presynaptically inhibited glycinergic synaptic transmission by 85.5%, and a selective 5-HT1B receptor activation by N-(3-trifluoromethylphenyl)piperazine (TFMPP, 10 µM) reduced the frequency of glycinergic mIPSCs without changing their mean amplitude in hypoglossal motoneurons (Umemiya and Berger 1995).
The mechanisms of 5-HT modulation of glycine release
Both the pre- and post-synaptic mechanisms of serotonergic modulation of neuronal activity have been described in the spinal cord of different species of vertebrates (Sillar and Simmers 1994; Skydsgaard and Hounsgaard 1996; El Manira et al. 1997; McDearmid et al. 1997). Multiple postsynaptic mechanisms have been suggested to underlie the effects of 5-HT on motoneurons in the spinal cord: the modulation of calcium-activated potassium conductance (Brownstone et al. 1992; Grunnet et al. 2004; Perrier and Delgado-Lezama 2005), leak potassium conductance (Ziskind-Conhaim et al. 1993; Perrier et al. 2003), inward rectifying potassium conductance (Kjaerulff and Kiehn 2001), hyperpolarization-activated inward current (Larkman and Kelly 1997; Kjaerulff and Kiehn 2001), low-voltage-activated calcium conductance (Berger and Takahashi 1990; Perrier and Hounsgaard 2003; Perrier and Delgado-Lezama 2005), and persistent sodium current (Harvey et al. 2006). Presynaptic mechanisms include the changes in the membrane properties of sensory axons (Carstens et al. 1987; Lopez-Garcia and King 1996) and a decrease in neurotransmitter release at synaptic terminals (Lopez-Garcia and King 1996).
In the present study, we did not aim to investigate the exact mechanisms of 5-HT modulation of glycine release. The fact that 5-HT and sumatriptan affected only the frequency but not the amplitude or other properties of mIPSPs suggests the presynaptic location of 5-HT1B, 1D receptors. Among 5-HT receptor subtypes, 5-HT1B and 5-HT1D receptors are coupled to Gi/o protein-coupled receptors, suggesting inhibitory effects (Hoyer et al. 2002). The 5-HT1B receptor-mediated inhibition of EPSCs at the calyx of Held in immature rats could be fully explained by the reduction of presynaptic voltage-dependent Ca2+ currents, suggesting that exocytotic mechanisms downstream of Ca2+ influx are not involved (Mizutani et al. 2006). However, in lamprey spinal cord synapses, 5-HT acts via G-protein βγ subunits, directly inhibiting exocytotic machinery downstream of Ca2+ influx (Blackmer et al. 2001). The exact mechanisms of 5-HT modulation of glycine release in frog spinal cord need to be further investigated.
Abbreviations
- 5-HT-serotonin:
-
5-hydroxytryptamine;
- 8-OH-DPAT:
-
(±)-8-Hydroxy-2-(dipropylamino)tetralin hydrobromide;
- Sum:
-
3-[2-(Dimethylamino)ethyl]-N-methyl-1H-indole-5-methanesulfonamide succinate (sumatriptan succinate)
- α-Ме-5-НТ:
-
α-Methyl-5-hydroxytryptamine maleate;
- Methysergide maleate:
-
[8β(S)]-9,10-Didehydro-N-[1-(hydroxymethyl)propyl]-1,6-dimethylergoline-8-carboxamide maleate;
- CNQX:
-
6-Cyano-7-nitroquinoxaline-2,3-dione;
- D-AP5:
-
D-(−)-2-Amino-5-phosphonopentanoic acid;
- mIPSPs:
-
Miniature inhibitory postsynaptic potentials;
- RMP:
-
Resting membrane potential;
- TTX:
-
Tetrodotoxin
References
Alvarez FJ, Pearson JC, Harrington D, Dewey D, Torbeck L, Fyffe RE (1998) Distribution of 5-hydroxytryptamine-immunoreactive boutons on alpha-motoneurons in the lumbar spinal cord of adult cats. J Comp Neurol 393:69–83
Berger AJ, Huynh P (2002) Activation of 5HT1B receptors inhibits glycinergic synaptic inputs to mammalian motoneurons during postnatal development. Brain Res 956:380–384
Berger AJ, Takahashi T (1990) Serotonin enhances a low-voltage-activated calcium current in rat spinal motoneurons. J Neurosci 10:1922–1928
Blackmer T, Larsen EC, Takahashi M, Martin TF, Alford S, Hamm HE (2001) G protein betagamma subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science 292:293–297. https://doi.org/10.1126/science.1058803
Brownstone RM, Jordan LM, Kriellaars DJ, Noga BR, Shefchyk SJ (1992) On the regulation of repetitive firing in lumbar motoneurones during fictive locomotion in the cat. Exp Brain Res 90:441–455
Cardona A, Rudomin P (1983) Activation of brainstem serotoninergic pathways decreases homosynaptic depression of monosynaptic responses of frog spinal motoneurons. Brain Res 280:373–378
Carstens E, Gilly H, Schreiber H, Zimmermann M (1987) Effects of midbrain stimulation and iontophoretic application of serotonin, noradrenaline, morphine and GABA on electrical thresholds of afferent C- and A-fibre terminals in cat spinal cord. Neuroscience 21:395–406
Ciranna L (2006) Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: implications in physiological functions and in pathology. Curr Neuropharmacol 4:101–114
Dityatev AE, Chmykhova NM, Dityateva GV, Babalian AL, Kleinle J, Clamann HP (2001) Structural and physiological properties of connections between individual reticulospinal axons and lumbar motoneurons of the frog. J Comp Neurol 430:433–447
El Manira A, Zhang W, Svensson E, Bussieres N (1997) 5-HT inhibits calcium current and synaptic transmission from sensory neurons in lamprey. J Neurosci 17:1786–1794
Enjin A, Leao KE, Mikulovic S, Le Merre P, Tourtellotte WG, Kullander K (2012) Sensorimotor function is modulated by the serotonin receptor 1d, a novel marker for gamma motor neurons. Mol Cell Neurosci 49:322–332. https://doi.org/10.1016/j.mcn.2012.01.003
Fink KB, Göthert M (2007) 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev 59:360–417. https://doi.org/10.1124/pr.107.07103
Grunnet M, Jespersen T, Perrier JF (2004) 5-HT1A receptors modulate small-conductance Ca2+-activated K+ channels. J Neurosci Res 78:845–854. https://doi.org/10.1002/jnr.20318
Hannon J, Hoyer D (2008) Molecular biology of 5-HT receptors. Behav Brain Res 195:198–213. https://doi.org/10.1016/j.bbr.2008.03.020
Harvey PJ, Li X, Li Y, Bennett DJ (2006) 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 96:1158–1170. https://doi.org/10.1152/jn.01088.2005
Helton LA, Thor KB, Baez M (1994) 5-hydroxytryptamine2A, 5-hydroxytryptamine2B, and 5-hydroxytryptamine2C receptor mRNA expression in the spinal cord of rat, cat, monkey and human. Neuroreport 5:2617–2620
Holohean AM, Hackman JC (2004) Mechanisms intrinsic to 5-HT2B receptor-induced potentiation of NMDA receptor responses in frog motoneurones. Br J Pharmacol 143:351–360. https://doi.org/10.1038/sj.bjp.0705935
Holohean AM, Hackman JC, Davidoff RA (1990a) Changes in membrane potential of frog motoneurons induced by activation of serotonin receptor subtypes. Neuroscience 34:555–564
Holohean AM, Hackman JC, Davidoff RA (1990b) An in vitro study of the effects of serotonin on frog primary afferent terminals. Neurosci Lett 113:175–180
Holohean AM, Hackman JC, Shope SB, Davidoff RA (1992) Serotonin1A facilitation of frog motoneuron responses to afferent stimuli and to N-methyl-d-aspartate. Neuroscience 48:469–477
Holz GGT, Shefner SA, Anderson EG (1985) Serotonin depolarizes type A and C primary afferents: an intracellular study in bullfrog dorsal root ganglion. Brain Res 327:71–79
Hoyer D, Hannon JP, Martin GR (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71:533–554
Jacobs BL, Azmitia EC (1992) Structure and function of the brain serotonin system. Physiol Rev 72:165–229
Jacobs BL, Fornal CA (1993) 5-HT and motor control: a hypothesis. Trends Neurosci 16:346–352
Jacobs BL, Martin-Cora FJ, Fornal CA (2002) Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev 40:45–52
Kalinina NI, Kurchavyi GG, Zaitsev AV, Veselkin NP (2016) Presynaptic serotonergic modulation of spontaneous and miniature synaptic activity in frog lumbar motoneurons. J Evol Biochem Physiol 52:359–368
Kjaerulff O, Kiehn O (2001) 5-HT modulation of multiple inward rectifiers in motoneurons in intact preparations of the neonatal rat spinal cord. J Neurophysiol 85:580–593
Kurchavyi GG, Kalinina NI, Vesselkin NP (2005) Effects of GABA and glycine on postsynaptic potentials of motoneurons of the frog Rana ridibunda. J Evol Biochem Physiol 41:647–659
Larkman PM, Kelly JS (1997) Modulation of IH by 5-HT in neonatal rat motoneurones in vitro: mediation through a phosphorylation independent action of cAMP. Neuropharmacology 36:721–733
Lopez-Garcia JA, King AE (1996) Pre- and post-synaptic actions of 5-hydroxytryptamine in the rat lumbar dorsal horn in vitro: implications for somatosensory transmission. Eur J Neurosci 8:2188–2197
McDearmid JR, Scrymgeour-Wedderburn JF, Sillar KT (1997) Aminergic modulation of glycine release in a spinal network controlling swimming in Xenopus laevis. J Physiol 503(Pt 1):111–117
Miles GB, Sillar KT (2011) Neuromodulation of vertebrate locomotor control networks. Physiology (Bethesda) 26:393–411. https://doi.org/10.1152/physiol.00013.2011
Mizutani H, Hori T, Takahashi T (2006) 5-HT1B receptor-mediated presynaptic inhibition at the calyx of Held of immature rats. Eur J Neurosci 24:1946–1954. https://doi.org/10.1111/j.1460-9568.2006.05063.x
Newton BW, Maley BE, Hamill RW (1986) Immunohistochemical demonstration of serotonin neurons in autonomic regions of the rat spinal cord. Brain Res 376:155–163
Ovsepian SV, Vesselkin NP (2004) Dual effect of GABA on descending monosynaptic excitatory postsynaptic potential in frog lumbar motoneurons. Neuroscience 129:639–646. https://doi.org/10.1016/j.neuroscience.2004.07.050
Ovsepian SV, Vesselkin NP (2006) Serotonergic modulation of synaptic transmission and action potential firing in frog motoneurons. Brain Res 1102:71–77. https://doi.org/10.1016/j.brainres.2006.04.035
Palacios JM (2016) Serotonin receptors in brain revisited. Brain Res 1645:46–49. https://doi.org/10.1016/j.brainres.2015.12.042
Pauwels PJ (1997) 5-HT 1B/D receptor antagonists. Gen Pharmacol 29:293–303
Perrier JF, Delgado-Lezama R (2005) Synaptic release of serotonin induced by stimulation of the raphe nucleus promotes plateau potentials in spinal motoneurons of the adult turtle. J Neurosci 25:7993–7999. https://doi.org/10.1523/JNEUROSCI.1957-05.2005
Perrier JF, Hounsgaard J (2003) 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J Neurophysiol 89:954–959. https://doi.org/10.1152/jn.00753.2002
Perrier JF, Alaburda A, Hounsgaard J (2003) 5-HT1A receptors increase excitability of spinal motoneurons by inhibiting a TASK-1-like K+ current in the adult turtle. J Physiol 548:485–492. https://doi.org/10.1113/jphysiol.2002.037952
Perrier JF, Rasmussen HB, Christensen RK, Petersen AV (2013) Modulation of the intrinsic properties of motoneurons by serotonin. Curr Pharm Des 19:4371–4384
Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL (2000) Synaptic control of motoneuronal excitability. Physiol Rev 80:767–852
Ridet JL, Tamir H, Privat A (1994) Direct immunocytochemical localization of 5-hydroxytryptamine receptors in the adult rat spinal cord: a light and electron microscopic study using an anti-idiotypic antiserum. J Neurosci Res 38:109–121. https://doi.org/10.1002/jnr.490380114
Sillar KT, Simmers AJ (1994) Presynaptic inhibition of primary afferent transmitter release by 5-hydroxytryptamine at a mechanosensory synapse in the vertebrate spinal cord. J Neurosci 14:2636–2647
Skydsgaard M, Hounsgaard J (1996) Multiple actions of iontophoretically applied serotonin on motorneurones in the turtle spinal cord in vitro. Acta Physiol Scand 158:301–310. https://doi.org/10.1046/j.1365-201X.1996.558326000.x
Tan HJ, Miletic V (1990a) Bulbospinal serotoninergic pathways in the frog Rana pipiens. J Comp Neurol 292:291–302. https://doi.org/10.1002/cne.902920211
Tan HJ, Miletic V (1990b) Electrophysiological properties of frog spinal dorsal horn neurons and their responses to serotonin: an intracellular study in the isolated hemisected spinal cord. Brain Res 528:344–348
Umemiya M, Berger AJ (1995) Presynaptic inhibition by serotonin of glycinergic inhibitory synaptic currents in the rat brain stem. J Neurophysiol 73:1192–1201
Woolston AM, Wedderburn JF, Sillar KT (1994) Descending serotonergic spinal projections and modulation of locomotor rhythmicity in Rana temporaria embryos. Proc Biol Sci 255:73–79. https://doi.org/10.1098/rspb.1994.0011
Wu SY, Wang MY, Dun NJ (1991) Serotonin via presynaptic 5-HT1 receptors attenuates synaptic transmission to immature rat motoneurons in vitro. Brain Res 554:111–121
Xie DJ, Uta D, Feng PY, Wakita M, Shin MC, Furue H, Yoshimura M (2012) Identification of 5-HT receptor subtypes enhancing inhibitory transmission in the rat spinal dorsal horn in vitro. Mol Pain 8:58. https://doi.org/10.1186/1744-8069-8-58
Xu C, Giuliano F, Sun XQ, Brisorgueil MJ, Leclerc P, Verge D, Conrath M (2007) Serotonin 5-HT2A and 5-HT5A receptors are expressed by different motoneuron populations in rat Onuf’s nucleus. J Comp Neurol 502:620–634. https://doi.org/10.1002/cne.21344
Zhang M (2015) Aromatic L-amino acid decarboxylase cells in the spinal cord: a potential origin of monoamines. Neural Regen Res 10:715–717. https://doi.org/10.4103/1673-5374.156960
Ziskind-Conhaim L, Seebach BS, Gao BX (1993) Changes in serotonin-induced potentials during spinal cord development. J Neurophysiol 69:1338–1349
Acknowledgements
This study was funded by Russian Foundation for Basic Research (RFBR, Grant number 15-04-05782).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and animal rights
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kalinina, N.I., Zaitsev, A.V. & Vesselkin, N.P. Presynaptic serotonin 5-HT1B/D receptor-mediated inhibition of glycinergic transmission to the frog spinal motoneurons. J Comp Physiol A 204, 329–337 (2018). https://doi.org/10.1007/s00359-017-1244-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-017-1244-y