Abstract—Using Raman spectroscopy (RS) approach in a spectral range of 1000–3000 cm–1 were used to study the conformational and structural changes that arise in the heme group and globin moiety of hemoglobin in human red blood cells at various temperatures and oxygen contents. In hypoxia, the hemoglobin conformation was shown to change as a result of the increasing contribution of hematoporphyrin pyrrole rings and vibrational motions of vinyl groups. Modifications were additionally detected in the contributions of symmetric and asymmetric vibrations of the CH2 and CH3 radicals of histidine (2850, 2860, and 2900 cm–1) and lysine (2880 and 2860 cm–1) residues. The mechanisms of oxygen binding are discussed for hemoglobin located in the submembrane region and cytoplasm of the cell.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Hypoxia is a known cause of pathological processes in tissues and depends on several factors, such as the blood flow rate, vascular diameters, interactions between red blood cells and endothelial cells, and the properties of hemoglobin (Hb) [1]. The rate of blood flow depends on deformability of red blood cells and the release of adenosine triphosphate, which stimulates purinergic receptors and triggers certain reaction cascades in vascular endothelial cells to activate the release of vasorelaxants [2]. Hb plays a key role in regulating the intracellular processes in red blood cells and acts in complex with the cytoplasmic domain of the band 3 protein to ensure oxygen fixation in the cell membrane [3, 4]. Because Hb is the only O2-binding protein in red blood cells, reversible association of deoxygenated Hb (deoxyHb) and subsequent steps of intracellular signaling are possible to consider as a mechanism that triggers O2-dependent processes in the red blood cell [5]. It is important to note that changes in the contents of other membrane-associated cytoplasmic proteins have been observed in hypoxia. The changes might underlie not only the mechanisms that sustain changes in membrane structural integrity and adenosine triphosphate release, but also the mechanisms that change the ordering of Hb molecules in the cell cytoplasm [6, 7].
It is clear that the conformational changes that heme and globin of Hb experience upon O2 transfer into the red blood cell are important to study in order to better understand the development of hypoxia. Combined dynamic light scattering and Raman spectroscopy (RS) is one of the methods suitable for evaluating simultaneously the changes in heme conformation and globin structure of Hb. The method is used to study the deformation of the methine groups of the porphyrin macrocycle in heme and to evaluate the contributions of vibrations of the CH group of amino-acid residues for Hb and plasma membrane proteins of the red blood cell (Table 1) [8–11].
In this work, we studied the conformational changes that heme and its nearest protein surroundings in globin experience in normobaric hypoxia and exposure to various temperatures.
MATERIALS AND METHODS
Study models included Hb solutions, red blood cell suspensions, and erythrocyte ghosts. Cells were isolated from blood samples of healthy donors (n = 10, age 20–40 years). Blood was collected from the cubital vein into Vacuette vacuum tubes (Greiner Bio-One, Austria) after overnight fasting. Heparin (20–50 units/mL blood) was used to prevent blood clotting. Blood samples were stored at 4°C after collection. Blood cells were pelleted by centrifugation at 1500 g at 4°C for 10 min, using a Laborfuge 400R laboratory centrifuge (Thermo Scientific, United States). The plasma and white blood cells were discarded and the pellet was washed three times with buffer A (145 mM NaCl, 5 mM KCl, 1 mM CaCl2, 4 mM Na2HPO4, 1 mM NaH2PO4, 1 mM MgSO4, 5 mM glucose, pH 7.0). The resulting erythrocyte suspension (Ht = 40%) was stored at 4°C and used within 3 h of preparation. To isolate Hb, a red blood cell suspension was supplemented with 10 volumes of a phosphate buffer (4 mM Na2HPO4, 1 mM NaH2PO4, pH 7.4), shaken thoroughly, and centrifuged at 6000 g at 4°C for 10 min. The supernatant was collected and stored at 4°C. The Hb concentration was inferred from the optical density at 415 nm, which was measured using a Hitachi 557 spectrophotometer (Hitachi, Japan).
Erythrocyte ghosts were prepared after hemolysis by washing the red blood cell pellet three times via centrifugation at 12 000 g at 4°C for 30 min. The ghost cells were estimated from the membrane protein concentration, which was measured by the Lowry assay [17] and was 4.9 ± 0.4 mg/mL.
Oxygen displacement from red blood cell suspensions and Hb solutions was performed using a gas mixture consisting of nitrogen with 0.04% CO2 (PGS-servis, Russia). To displace oxygen, the gas mixture was blown through the gas phase over a sample at a flow rate of 0.1 L/min for 20 min [18]. Displacement was carried out with continuous agitation at room temperature. The oxygen displacement conditions used in this work ensured that the red blood cell suspensions and Hb solutions were in normobaric hypoxia after treatment, containing mostly deoxyHb, as determined by recording and analyzing their RS spectra (the technique is described below). Control (normoxic) samples of red blood cell suspensions and Hb solutions were prepared in the same conditions; however, air was used in place of the gas mixture. The samples were used to fill hematocrit capillary tubes with a cross-section diameter of 1 mm (Agat-Med, Russia). All manipulations of capillary tube filling were performed in a hermetic box continuously blown with the gas mixture (nitrogen containing 0.04% CO2). An excessive pressure of the hypoxic mixture was relieved using an air pressure relief valve with a threshold pressure of approximately 1 atm. Hematocrit capillary tubes placed in the box were preliminarily blown for 2 min. The partial pressure of oxygen in the gas mixture within the box was monitored using a KE-25 electrode (Figaro Engineering, Japan). The capillary tubes were hermetically sealed after filling and stored at 4°C for no more than 3 h.
Heme and globin conformations in Hb were studied by RS. RS spectra were recorded using a NTEGRA-SPECTRA confocal microscope–spectrometer (NT--MDT, Zelenograd, Russia) in a range of 1000–3000 cm–1. Measurements were performed with an increment of 0.8 cm–1. A Peltier-cooled CCD detector (–50°C, 5× objective, 0.15 aperture, grid with 600 grooves per mm) was employed; the laser power on a sample was 3 mW; the excitation wavelength was 532 nm; the time to record one spectrum was 15 s; three signal accumulation stages were included. The temperature was measured in a range of 22–36°C in the cell of the instrument; the duration of sample adaptation to a particular temperature was 5 min. The Raman spectra were processed using the Origin2017 program (OriginLab, United States). The baseline was subtracted and the spectrum smoothed during processing. A laser with a wavelength of 532 nm was chosen for excitation to minimize the contributions of signals from lipids and proteins of the red blood cell membrane to the Raman spectrum [11, 16, 17].
The contributions of various groups of the Hb molecule were estimated using the intensity ratios of bands observed in the Raman spectrum (Table 1) [8, 11, 18, 19].
The I1375/I1127 ratio characterized the contribution of vibrations of CH3 side groups of the pyrrole half-rings in hemoporphyrin. The vibrations are appreciable when the globin conformation changes in the immediate vicinity of heme. The ratio characterizes the extent of symmetric and asymmetric vibrations of the pyrrole half-rings; its changes characterize the change in Hb conformation from T (deoxyHb) to R (oxyhemoglobin, oxyHb).
The I1580/I1550 ratio characterized the contribution of vibrations of the methine bridges between pyrroles in hemoporphyrin. The vibrations are appreciable when the macrocycle is deformed. The ratio characterizes Hb affinity for ligands and, in particular, oxygen.
The I2800/I2880 ratio characterizes the contribution of symmetric relative to asymmetric vibrations of methylene groups in amino-acid residues.
The I2930/I2850 ratio characterized the contribution of symmetric terminal methylene group vibrations relative to symmetric vibrations of methylene groups of amino-acid residues. The ratio characterized the changes in polarity of amino-acid surroundings.
The ζ-potential of a red blood cell suspension reflected the changes in cell surface charge [20] and was measured using a Zetasizer Nano ZS instrument (Malvern Instruments, United Kingdom). A red blood cell suspension (1 mL, Ht = 40%) was added into a plastic cuvette with golden electrodes for ζ-potential recording. The suspension was agitated thoroughly to eliminate air bubbles and to prevent red blood cell accumulation in part of the cuvette. Measurements were performed at the cell temperature maintained at 25 or 36°C; the duration of sample adaptation to the temperature was 60 s; a total of 100 measurements were performed. The results were processed using Malvern software supplied with the instrument and MS Excel spreadsheets.
RESULTS AND DISCUSSION
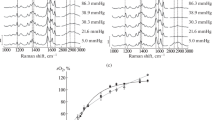
Our study showed that RS spectra obtained for Hb in solution and a red blood cell suspension have characteristic bands in the 1000–3000 cm–1 range (Fig. 1). In the absence of Hb, only bands in the 2800–3100 cm–1 region were observed in Raman spectra of erythrocyte ghosts and were due to vibrations of bonds involving CH groups of amino-acid residues (Figs. 1, 2a; Table 1) [16, 21]. The bands of the Raman spectrum 2800–3100 cm–1 range probably reflect symmetric and asymmetric vibrations of CH2 and CH3 radicals of histidine (2850, 2860, and 2900 cm–1) and lysine (2880 and 2860 cm–1) residues [16, 22–25].
The Raman spectra of heme and globin in (a) normoxia and (b) hypoxia were obtained for a red blood cell suspension and a Hb solution. The 1000–3000 cm–1 region was examined. The spectra were normalized to the 1220 cm−1 band according to the published method [9].
(a) Fragments of the Raman spectra of vibrations as obtained for a red blood cell suspension, a Hb solution, and a erythrocyte ghosts preparation in normoxia and hypoxia. The spectra were obtained for the 1000–3000 cm–1 region and were not normalized. (b) The ζ-potential of the red blood cell membrane in normoxia and hypoxia; (*) p ≤ 0.05.
We believe that an oxygen molecule is transferred from the environment into the red blood cell via several steps. Oxygen first enters the lipid bilayer of the plasma membrane and then is coordinated in the hemoporphyrin center of deoxyHb attached to the band 3 anion transport protein or AE1. Finally, oxyHb is desorbed in the cell together with the oxygen molecule, and protein–protein interactions within the cell change the heme capability to fix an oxygen molecule. Thus, conformational changes must arise first in the heme and then in the globin in the course of the above process.
To verify the assumption, heme conformational changes were monitored during oxygen displacement from the incubation medium by recording the Raman spectrum in a region of 1000–1800 cm–1 for isolated Hb and a red blood cell suspension. Characteristic differences were observed in amplitude changes of bands at 1355 and 1375 cm–1 in the 1300–1400 cm–1 region and bands at 1552 and 1580 cm–1 in the 1500–1650 cm–1 region (Fig. 3) [13, 22]. As an example, hypoxia-related changes observed in the 1500–1650 cm–1 region in the case of isolated Hb were greater than in the case of Hb contained in red blood cells (by 14.20 ± 0.52%). In the 1300–1400 cm–1 region, the Raman spectrum intensity of isolated Hb was 16.03 ± 0.45% higher than that of Hb contained in red blood cells. It is important to note that shifts of several bands in the 1300–1450 cm–1 region of the Raman spectrum were detected for Hb contained in red blood cells in hypoxia. Such shifts are characteristic of changes in the conjugation length of double bonds in the pyrrole rings of hemoporphyrin of heme.
The RS spectra of (a) a red blood cell suspension and (b) a Hb solution in normoxia and hypoxia and their difference spectra. The spectra were normalized to the maximum of the band at 1220 cm–1 (according to [9]).
The 2800–3000 cm–1 region of the Raman spectrum is a region of vibrations of CH bonds in globin amino-acid residues. Changes were observed in the contributions of bands at 2850 and 2880 cm–1 and were greater in the case of isolated Hb (by 13.20 ± 0.23%) than in the case of Hb contained within cells [12, 21] (Figs. 2, 3; Table 1). Changes observed in the Raman spectrum 200–500 cm–1 region are known to be due to the Fe–H is bond; the idea is supported by our data on changes in histidine contribution in the 2800–3000 cm–1 region [25, 27]. We note that the bands at 2850 and 2930 cm–1 in the isolated Hb spectra obtained in normoxia and hypoxia are shifted towards higher frequencies as compared with those observed for Hb contained in cells. The shift might be due to the changes that arise in globin conformation because Hb molecules are packed in a more ordered fashion in the cell as compared with a solution [21].
Globin conformational changes were induced by increasing the temperature of a Hb solution or a red blood cell suspension. Phase transitions of lipids are known to be absent in the plasma membrane of the red blood cell in a temperature range of 22–36°C [15, 19]. It is therefore possible that changes in the Raman spectrum of a red blood cell suspension in the 2800–3000 cm–1 range are associated exclusively with changes in proteins and, primarily, Hb [18, 27] (Table 2, Fig. 4). When the temperature was increased in normoxia (Fig. 5), substantial differences in conformational modulations of heme and globin were observed depending on the packing mode of Hb molecules (a red blood cell suspension vs. a Hb solution). The contribution of the pyrrole rings and vinyl groups decreased in the case of red blood cells, while the protein packing density substantially increased and the polarity of the surrounding decreased in the case of globin. As an example, the I1375/I1172 ratio was observed to change considerably in the Raman spectrum of a Hb solution when the temperature was increased, suggesting conformational changes for the pyrrole rings of heme (a decrease by 32.86 ± 0.45%), while such changes were not detected for a red blood cell suspension.
In hypoxia (at a lower portion of oxyHb complexes in the sample), conformational changes in heme were due to the contributions of the pyrrole rings (I1375/I1172 increased by 21.07 ± 0.18%) and vibrations of vinyl groups (I1580/I1550 increased by 20.26 ± 0.17%) in a red blood cell suspension as well as in an isolated Hb solution (the changes were 17.60 ± 0.68 and 15.08 ± 0.53%, respectively). We note that changes in the heme moiety of Hb were greater in a red blood cell suspension than in an isolated Hb solution, suggesting an effect for a more tight packing of Hb molecules in the cell compared with a solution. The globin moiety of Hb contained in the cell showed an increase in the contribution of the polar surrounding of amino-acid residues (an increase in I2930/I2850) in hypoxia, while the protein packing density (I2850/I2880) remained unchanged [11]. Both packing density and the polarity of the globin surrounding were found to change when the globin moiety was tested in isolated Hb in similar conditions.
CONCLUSIONS
This work studied the molecular mechanisms of conformational changes in heme and globin within the red blood cell. The mechanism is sensitive to changes in the partial pressure of O2 and determines the complexation of Hb with the AE1 and Hb oligomerization in the cytoplasm. Hb has higher affinity for the red blood cell membrane as compared with oxyHb. The N-terminal region of the AE1 is accommodated in the central cavity of a Hb tetramer upon their binding, while the cavity becomes inaccessible as a result of Hb oxygenation. There is an anionic segment in the N-terminal region of the band 3 protein and the region enters the cationic central cavity of deoxyHb. The cavity is substantially narrowed upon O2 binding (O2 affinity for Hb decreases as a result) [27]. It is important to note that the hemoporphyrin conformation changes in hypoxia (a decrease in the portion of heme–O2 complexes) as a result of an increase in the contributions of the pyrrole rings of hemoporphyrin and vibrations of vinyl groups [9, 28]. Changes were observed for the contribution of histidine and lysine residues located close to the heme in globin. That is, the band at 2930 cm–1 shifted to 2940 cm–1, probably because conformational changes occurred in globin and the lysine contribution increased [21]. Thus, hypoxia is characterized by changes in the conformation and O2-binding potential of Hb hemoporphyrin. An increase in temperature must induce conformational changes in cell proteins and, primarily, globin. The contributions of vibrations of vinyl groups and the pyrrole rings of heme increased when the temperature was increased in hypoxia, but only in the case of Hb contained in cells (and not in the case of isolated Hb). We note that temperature-dependent changes in globin conformation in hypoxia may possibly be determined by the polar surrounding of amino acids (I2930/I2850) [11]. We associate these with structural changes that occur in membrane and cytoplasmic proteins because phase transitions of lipids have not been detected in the cell membrane at these temperatures [17]. The changes that occur in the conformation of heme and the arrangement of Hb amino-acid residues (e.g., histidines and lysines) located in its vicinity upon oxygen binding probably affect the localization of the anionic segment of the AE1, because the positions of amino-acid residues change or the negative charge density increases in the central cavity of Hb.
All of the above events take place in the plasma membrane of the red blood cell, and the surface charge of the membrane may be affected by the conformational changes that heme and globin experience upon oxygen binding [20]. In fact, the ζ-potential was estimated at –20.8 ± 0.5 mV in normoxia and 16.7 ± 1.3 mV in hypoxia (Fig. 2b). Thus, the surface potential of the membrane increases in hypoxia. The change might be determined by the interaction of deoxyHb with the membrane through the AE1 and possible structural changes in the membrane.
Hypoxia-dependent changes in affinity for membrane arise not only in Hb, but also in many other cytoplasmic proteins and are another element of the intracellular signaling system of the red blood cell [7, 18]. Depending on how ordered Hb molecules are in the cytoplasm compared with isolated Hb in solution, conformational changes occur in both heme (as a result of changes in vibrations of the pyrrole rings and vinyl group bonds) and globin (as a result of changes in its packing density).
REFERENCES
D. Holly and B. Glenn, Am. J. Physiol. Heart Circ. Physiol. 293, 2193 (2007)
M. R. Hardeman, P. T. Goedhart, and S. K. Shin, in Handbook of Hemorheology and Hemodynamics (IOS Press Ebooks, 2007), pp. 242–266.
J. A. Walder, R. Chatterjee, T. L. Steck, et al., J. Biol. Chem. 259 (16), 10238 (1984).
R. A. Reithmeier, J. R. Casey, A. C. Kalli, et al., Biochim. Biophys. Acta–Biomembr. 1858 (7), 1507 (2016
R. R. Tuck, J. D. Schmelzer, and P. A. Low, Brain 107 (3), 935 (1984)
O.G. Luneva, S. V. Sidorenko, O. O. Ponomarchuk, et al., Cell. Physiol. Biochem. 39 (1), 81 (2016)
S. V. Sidorenko, O. G. Luneva, T. S. Novozhilova, et al., Biochemistry, Suppl. Ser. A 12 (2), 114 (2018)
N. A. Brazhe, S. Abdali, A. R. Brazhe, et al., Biophys. J. 97 (12), 3206 (2009)
B. R. Wood and D. McNaughton, Biopolymers 67 (4–5), 1691 (2002)
G. V. Maksimov, N. V. Maksimova, A. A. Churin, et al., Biochemistry (Moscow) 66 (3), 295 (2001)
S. C. Goheen, L. J. Lis, O. Kucuk, et al., J. Raman Spectrosc. 24 (9), 275 (1993)
N. Parthasarathi, C. Hansen, S. Yamaguchi, et al., J. Am. Chem. Soc. 109 (13), 3865 (1987)
B. R. Wood, P. Caspers, G. J. Puppels, et al., Anal. Bioanal. Chem. 387 (5), 1691 (2007)
S. Nagatomo, M. Nagai, Y. Mizutani, et al., Biophys. J. 89 (2), 1203 (2005)
S. Choi, T. G. Spiro, K. C. Langry, et al., J. Am. Chem. Soc. 104 (16), 4345 (1982)
N. K. Howell, G. Arteaga, S. Nakai, et al., J. Agricult. Food Chem. 47 (3), 924 (1999)
H. Lowry, N. J. Rosebrough, A. L. Farr, et al., J. Biol. Chem. 193, 265 (1951)
S. V. Sidorenko, R. H. Ziganshin, O. G. Luneva, et al., J. Proteomics 184, 25 (2018)
I. P. Torres Filho, J. Terner, R. N. Pittman, et al., J. Appl. Physiol. 104 (6), 1809 (2008)
I. A. Tikhomirova, A. V. Murav’ev, L. A. Mikhailichenko, et al., Human Physiol. 32 (6), 748 (2006)
K. M. Marzec, D. Perez-Guaita, M. De Veij, et al., ChemPhysChem 15 (18), 3963 (2014)
B. R. Wood, L. Hammer, and D. McNaughton, Vib. Spectrosc. 38 (1-2), 78 (2005)
J. Surmacki, R. Musial, R. Kordek, et al., Mol. Cancer 12 (1), 48 (2013)
M. Nagai, N. Mizusawa, T. Kitagawa, et al., Biophys. Rev. 10 (2), 271 (2018)
J. M. Friedman, T. W. Scott, R. A. Stepnoski, et al., J. Biol. Chem. 258 (17), 10564 (1983)
S. Nagatomo, M. Nagai, and T. Kitagawa, J. Am. Chem. Soc. 133 (26), 10101 (2011)
S. N. Orlov, Byull. Sib. Med. 18 (2), 234 (2019)
A. A. Semenova, A. P. Semenov, E. A. Gudilina, et al., Mendeleev Commun. 26 (3), 177 (2016).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.
Statement of compliance with standards of research involving humans as subjects. All procedures performed in studies involving human participants were in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments. Informed consent was voluntarily provided by all individual participants involved in the study.
Additional information
Translated by T. Tkacheva
Abbreviations: Hb, hemoglobin; RS, Raman spectroscopy.
Rights and permissions
About this article
Cite this article
Slatinskaya, O.V., Luneva, O.G., Deev, L.I. et al. Conformational Changes that occur in Heme and Globin upon Temperature Variations and Normobaric Hypoxia. BIOPHYSICS 65, 213–221 (2020). https://doi.org/10.1134/S0006350920020220
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350920020220